Chapter 28 Humidification and inhalation therapy
The upper airway normally warms, moistens and filters inspired gas. When these functions are impaired by disease, or when the nasopharynx is bypassed by endotracheal intubation, artificial humidification of inspired gases must be provided.
PHYSICAL PRINCIPLES
Humidity, the amount of water vapour in a gas, may be expressed as:
PHYSIOLOGY
The nasal mucosa has a large surface area with an extensive vascular network which humidifies and warms inhaled gas more effectively than during mouth-breathing. Heating and humidification of dry gas are progressive down the airway, with an isothermic saturation boundary (i.e. 100% RH at 37°C or AH of 43 g/m3) just below the carina.1 Under resting conditions, approximately 250 ml of water and 1.5 kJ (350 kcal) of energy is lost from the respiratory tract in a day. A proportion (10–25%) is returned to the mucosa during expiration due to condensation.
The minimal moisture level to maintain ciliary function and mucus clearance is uncertain. Although reproducing an isothermic saturation boundary at the carina may be ideal, it does not seem essential in all situations. Mucus flow is markedly reduced when RH at 37°C falls below 75% (AH of 32 g/m3), and ceases when RH is 50% (AH of 22 g/m3).2 This suggests that an AH exceeding 33 g/m3 is needed to maintain normal function. At this AH level, inspired gas temperature does not appear to be important unless excessive.3 Mucociliary function is also impaired by upper respiratory tract infection, chronic bronchitis, cystic fibrosis, bronchiectasis, immotile cilia syndrome (including Kartagener’s syndrome), dehydration, hyperventilation, general anaesthetics, opioids, atropine and exposure to noxious gases. High fractional inspired oxygen concentrations (FiO2) may lead to acute tracheobronchitis, with depressed tracheal mucus velocity within 3 h.4 Inhaled β2-adrenergic agonists increase mucociliary clearance by augmenting ciliary beat frequency, and mucus and water secretion.5
CLINICAL APPLICATIONS OF HUMIDIFICATION
TRACHEAL INTUBATION
The need for humidification during endotracheal intubation and tracheostomy is unquestioned. As the upper airway is bypassed, RH of inspired gas falls below 50% with adverse effects, including:6
HEAT EXCHANGE
The respiratory tract is an important avenue to adjust body temperature by heat exchange. Humidification of gases reduces the fall in body temperature associated with anaesthesia and surgery.6 In this setting, active humidifiers are of no benefit over heat and moisture exchangers (HMEs).8 Excessive heat from humidification may produce mucosal damage, hyperthermia and overhumidification.9 However, if water content is not excessive, mucociliary clearance is unaffected up to temperatures of 42°C.3 Overhumidification may increase secretions and impair mucociliary clearance and surfactant activity, resulting in atelectasis.9
IDEAL HUMIDIFICATION
The basic requirements of a humidifier should include the following features:10
METHODS AND DEVICES
WATER BATH HUMIDIFIERS
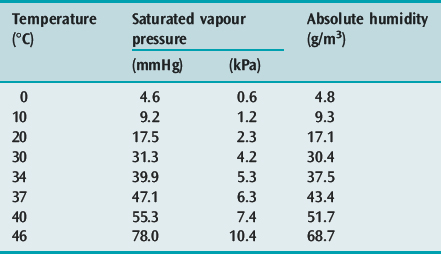
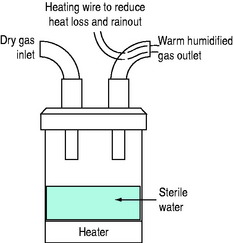
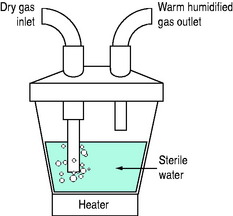
HOT-WATER HUMIDIFIERS (FigureS 28.1 and 28.2)
Inspired gas is passed over (i.e. blow-by humidifier, e.g. Fisher–Paykel, Fisher and Paykel Medical, New Zealand) or through (i.e. bubble or cascade humidifier, e.g. Bennett Cascade, Bennett Medical Equipment, USA) a heated-water reservoir. Gas leaving the reservoir theoretically contains high water content. The water bath temperature is thermostatically controlled (e.g. at 45–60°C) to compensate for cooling along the inspiratory tubing targeting an inspired RH of 100% at 37°C. A heated wire may be sited in the inspiratory tubing to maintain preset gastemperature and humidity (e.g. Fisher–Paykel humidifier). It is commonly believed that hot-water humidifiers do not produce aerosols, but microdroplets (mostly less than 5 μm diameter) have been reported with bubble humidifiers,11 and this may be a potential source of infection.
Fisher–Paykel humidifier
Although these humidifiers are often regarded as providing optimal humidification in ventilated patients, their performance is lower than expected (36 mg/l with MR 850 with the outlet temperature set to 37°C and the delivery hose set at 40°C, and optimal conditions in bench studies, and up to 41.9 mg/l in practice).12 However, under conditions of high ambient temperature and/or high ventilator output temperature the inlet temperature to the humidification chamber can be high enough that the heater base operates at a low enough temperature that humidification is impaired,with reduction in inspired water content as low as 19 mg/l in bench studies, and 23.4 mg/l in practice.12 The automatic compensation now available on this humidifier helps compensate for poor performance, which can also be prevented by setting the chamber outlet and hose to 40°C. High minute ventilation is well tolerated, but a small decrease in water content output has also been found with higher respiratory rates.13
HEAT AND MOISTURE EXCHANGERS
Modern HMEs are popular intensive care unit (ICU) humidifiers due to their simplicity and increased efficiency. They all work on the basic principle of heat and moisture conservation during expiration, allowing inspired gas to be heated and humidified. HMEs may be hydrophobic or hygroscopic, and may also act as a microbial filter (HMEF). However, since nosocomial pneumonia is primarily due to aspiration of oropharyngeal secretions followed by secondary ventilator tubing colonisation, HMEFs have not been shown to reduce the frequency of nosocomial pneumonia, and the incidence is similar when HMEs and hot-water humidifiers are compared during prolonged ventilation.14,15
Modern HMEs are light with a small dead space (30–95 ml), but are varied in their level of humidification. Hygroscopic HMEs adsorb moisture on to a foam or paper-like material that is chemically coated (often calcium chloride or lithium chloride), and this tends to increase their efficiency (i.e. AH around 30 g/m3) compared to hydrophobic HMEs (i.e. AH 20–25 g/m3).16–21 The efficiency of older HMEs decreases with time;13 however, modern HMEs may retain their ability to humidify for at least 4 days with minimal change in resistance.22 Consequently, HMEs that achieve relatively high AH may be suitable for long-term mechanical ventilation in selected patients, particularly as the majority of reported HME complications (e.g. thick secretions and endotracheal tube occlusion) occurred with units of lower humidification levels.23–25 Nevertheless, HMEs increase dead space and imposed work of breathing, and cannot match the humidification offered by hot-water humidifiers, which remain the ‘gold standard’, particularly if secretions are thick or bloody, minute ventilation is high,15,19 humidification is necessary for more than 4 days26 or if used in children and neonates.
COMPLICATIONS OF HUMIDIFICATION
INADEQUATE HUMIDIFICATION
An AH exceeding 30 g/m3 is recommended in respiratory care.24 Inadequate humidification is usually only a problem with HMEs. With hot-water humidifiers, however, efficiency is reduced by increasing gas flow rates and rainout. A decrease of about 1°C occurs for each 10 cm of tubing beyond the end of the delivery hose (i.e. theY-connector and right-angled connector), and should be catered for. Inadequate humidification in high-frequency ventilation can be overcome by using superheated humidification of the entrained gas with a temperature thermistor built into the endotracheal tube.23,27
OVERHUMIDIFICATION
Overheating malfunction of hot-water humidifiers may cause a rise in core temperature, water intoxication, impaired mucociliary clearance and airway burns.9
IMPOSED WORK OF BREATHING
The work of breathing imposed by a humidifier, primarily resistive work, is an important additional respiratory load in ICU patients. Consequently their imposed work increases with inspiratory flow rate, and the progressive increase in water content of HMEs is also associated with increased resistance.28 The Fisher–Paykel humidifier imposes relatively low work compared to the Bennett cascade,29 and HMEs typically have a resistance of 2.5 cmH2O/l per second.19
INFECTION
Current evidence argues against humidifiers as important factors in nosocomial respiratory tract infection. Although water reservoirs represent a good culture medium for bacteria such as Pseudomonas species, it is rare to culture bacteria from humidifiers. Any such positive finding is usually preceded by colonisation of the circuit by the patient’s own flora within the first 24 h of use.30,31 Indeed, the incidence of nosocomial pneumonia is reported to be higher (due to outside contamination) if the circuit is changed too frequently (every 24 h32 or 48 h29).
Many HMEs are also effective bacterial filters, with efficiencies usually greater than 99.9 977%,15 i.e. less than 23 out of 1 million bacteria will pass through – filters that can exclude all virus particles are not currently available. However, provided that fresh circuits are used for each new patient, filtration does not alter the incidence of ventilator-associated pneumonia or mortality.33
AEROSOL DELIVERY
NEBULISERS34
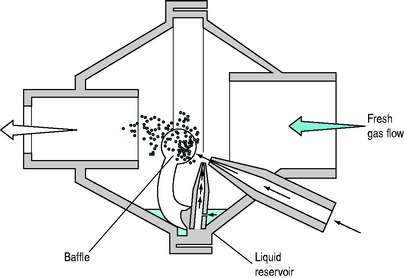
The most common jet nebulisers are sidestream nebulisers. These use an extrinsic gas flow through a narrow orifice, to create a presure gradient that draws the drug mixture from a liquid reservoir (i.e. Bernoulli’s principle; Figure 28.3). The gas is then directed at a baffle to reduce the mean particle size. This extrinsic gas flow (usually 3–10 l/min) adds to the inspiratory flow, and may increase patient tidal volume unless the ventilator automatically compensates, or the preset tidal volume is adjusted. Further, this additional gas flow may impair ventilator triggering since it may prevent the development of a negative pressure or necessary reduction in continuous flow needed. Mainstream nebulisers employ inspiratory gas flow to actuate nebulisation. These are commonly large-volume water nebulisers that entrain air to achieve fresh gas flow rates of 20–30 l/min.
Nebuliser aerosol deposition is highly variable, with significant rainout in the ventilatory circuit, endotracheal tube and large conducting airways. In a bench study delivery ranged from 6% of nebuliser charge to 37% depending upon humidification and breath activation; parallel in-vivo data were similar but found a 20-fold difference in drug delivery.35 Numerous factors have been shown to improve aerosol deposition, but only some of these are commonly practised. For example, although heating and humidification reduce aerosol deposition by ∼50% due to increase in both droplet size and rainout, regular circuit disconnection and exclusion of humidification prior to nebulisation are not practical. However, placement of the nebuliser in the inspiratory limb of the circuit less than 30 cm from the endotracheal tube allows this tubing to act as a spacer or aerosol-holding chamber. This reduces aerosol velocity and losses due to impaction. Inspiratory activation of the nebuliser increases deposition by two- and threefold under dry and humidified conditions respectively;35 use of a large chamber fill, tidal volume of 500 ml or more and minimisation of turbulent inspiratory flow (low flow rate, prolonged inspiratory time) also augment aerosol delivery.31,36
METERED-DOSE INHALERS
MDIs suspend micronised crystals of drug in a propellant gas under high pressure, allowing a relatively fixed volume (i.e. dose) to be delivered with each actuation (e.g. 90 μg for salbutamol and 18 μg for ipratropium).37 Aerosol delivery is approximately 4–6% in ventilated adults,32 but increases to 11% when a spacer chamber is used, which is similar to optimal no-spacer values reported in the ambulant population.38 Similar to nebulisers, absence of humidification, inspiratory limb position, inspiratory activation and minimisation of turbulence by lowering inspiratory flow rate and prolonging inspiratory time will increase aerosol delivery. It is important to avoid use of an elbow adapter, because this has been associated with dramatic reductions in aerosol delivery and efficacy. Further hazards of MDIs include a low risk of reaction to chlorofluorocarbons, and the risk of necrotising inflammation of the airway mucosa when large doses of drug are administered with an MDI and catheter system due to oleic acid which is present in some MDI formulations.39
CLINICAL APPLICATIONS OF INHALATION THERAPY
MUCOLYTICS
The role of mucolytics to reduce viscosity of secretions in critically ill patients is unknown. Oral mucolytics such as acetylcysteine reduce exacerbations of chronic bronchitis and chronic obstructive pulmonary disease (COPD), although this appears to be isolated to subjects not receiving inhaled steroids.40 Aerosolised acetylcysteine has also been used as a mucolytic, but may cause bronchospasm. Case reports of recombinant human DNase appear promising when standard regimens fail to clear thick secretions.36
BRONCHODILATOR THERAPY
Optimal aerosol delivery and bronchodilator response are extremely important in critically ill patients with severe airflow limitation, and this can be achieved using either a nebuliser or MDI technique provided that care is taken to optimise performance. The response can be judged clinically, and in ventilated patients monitored by changes in peak-to-plateau airway pressure gradient, calculated airways resistance and intrinsic PEEP. Numerous studies show effective bronchodilatation with β2-agonists and anticholinergic agents such as ipratropium in ventilated patients,31 and their combination is more effective than a single agent alone.36 When compared with systemic corticosteroids, inhaled steroids are effective in severe asthma41 and in exacerbations of COPD.42
BRONCHODILATOR DOSING
Standard doses of salbutamol are 2.5 mg administered by a nebuliser or four puffs of an MDI (360 μg), and this can be expected to elicit a bronchodilator response that is slightly shorter-lasting than is usual in ambulatory subjects. Consequently, dosing should be 3–4-hourly. Ipatropium is usually administered as 0.5 mg by a nebuliser or 4 puffs of an MDI. Higher or more frequent drug dosing is often effective when there is severe, reversible airflow obstruction, and continuous nebulisation of a β2-agonist may be used in acute severe asthma until there has been a clinical response. If the patient is moribund with minimal ventilation or unable to tolerate nebulisation, parenteral administration should be considered; however, under most circumstances there is no particular benefit with this route.43
NON-INVASIVE POSITIVE-PRESSURE VENTILATION (NIPPV)
Optimal bronchodilator therapy for patients requiring NIPPV, particularly for acute exacerbations of COPD, is unknown. It is preferable not to interrupt NIPPV for this purpose and both nebulisers and MDIs appear effective during NIPPV.36 Despite the potential for increased loss of nebulised drug in a high-gas-flow continuous positive-airways pressure circuit, good bronchodilator response with salbutamol has been reported in this setting.44
DELIVERY OF ANTIBIOTICS AND ANTIVIRAL AGENTS
Aerosolised antibiotics have been contentious for many years; however, the potential to achieve high concentrations of antibiotics at the site of infection while minimising adverse effects remains appealing. Numerous studies in patients with cystic fibrosis have shown a reduction in sputum volume and density of bacteria, and improved lung function with reduced risk of hospitalisation when inhaled aminoglycosides are used.45,46 Similar results, including a reduction of markers of airway inflammation, have been reported with bronchiectasis,47,48 and in mechanically ventilated patients with chronic respiratory failure.49 Although there has been concern that aerosolised antibiotics will promote the development of resistant organisms,50 this study examined whole ICU prophylaxis with polymyxin, rather than treatment, and other studies using aminoglycosides have not reported the development of resistance.39,51 Other antimicrobials that have been safely nebulised include vancomycin, amphotericin B and pentamidine for Pneumocystis carinii pneumonia. However, inhaled amphotericin may result in bronchospasm.
In animal models of bronchopneumonia inhaled amikacin52 and ceftazidime53 achieved 3–30 times increases in lung tissue concentrations, and better sterilisation of lung when compared to intravenous administration. Using either inhaled gentamicin or vancomycin there appears to be clinical benefit when inhaled antibiotics are added to systemic therapy in ventilator-associated pneumonia.54 Aerosolised ribavarin is effective for respiratory syncytial virus infection, resulting in a shorter period of ventilation and hospitalisation.55 However, ribavarin deposition in the circuit may cause valve malfunction, and concerns of teratogenicity in health care personnel have dictated its use with care.43
SPUTUM INDUCTION
Nebulised 3% saline is effective in sputum induction for diagnosing P. carinii pneumonia in patients with the acquired immunodeficiency syndrome (AIDS), thereby often obviating the need for bronchoscopy.56 Sputum induction has been used to diagnose a number of other infections, and appears to be safe in patients with severe airflow limitation.57
SURFACTANT THERAPY
Surfactant preparations have been delivered by instillation and as an aerosol in neonates with respiratory distress syndrome, and in adults with the acute respiratory distress syndrome. Aerosolised surfactant achieves a more uniform distribution and avoids problems of instilling liquid into injured lungs. However, large amounts are needed for lung deposition, and preferential distribution occurs to less damaged lung areas that receive better ventilation.58
1 Hedley RM, Allt-Graham J. Heat and moisture exchangers and breathing filters. Br J Anaesth. 1994;73:227-236.
2 Forbes AR. Humidification and mucus flow in the intubated trachea. Br J Anaesth. 1973;45:874-878.
3 Forbes AR. Temperature, humidity and mucus flow in the intubated trachea. Br J Anaesth. 1974;46:29-34.
4 Sackner MA, Landa J, Hirsch J, et al. Pulmonary effects of oxygen breathing: a six-hour study in normal man. Ann Intern Med. 1975;82:40-43.
5 LaFortuna CL, Fazio F. Acute effect of inhaled salbutamol on mucociliary clearance in health and chronic bronchitis. Respiration. 1985;45:111-113.
6 Chalon J, Patel C, Ali M, et al. Humidity and the anesthetized patient. Anesthesiology. 1974;50:195-198.
7 Circeo LE, Heard SO, Griffiths E, et al. Overwhelming necrotizing tracheobronchitis due to inadequate humidification during high-frequency jet ventilation. Chest. 1991;100:268-269.
8 Linko K, Honkavaara P, Niemenen MT. Heated humidification after major abdominal surgery. Eur J Anaesthesiol. 1984;1:285-291.
9 Shelley MP, Lloyd GM, Park GR. A review of the mechanisms and methods of humidification of inspired gases. Intens Care Med. 1988;14:1-9.
10 Chamney AR. Humidification requirements and techniques. Anaesthesia. 1969;24:602-617.
11 Rhame FS, Streifel A, McComb C, et al. Bubbling humidifiers produce microaerosols which can carry bacteria. Infect Control. 1986;7:403-407.
12 Lellouche F, Taillé S, Maggiore SM, et al. Influence of ambient and ventilator output temperatures on performance of heated-wire humidifiers. Am J Respir Crit Care Med. 2004;170:1073-1079.
13 Schumann S, Stahl CA, Moller K, et al. Moisturizing and mechanical characteristics of a new counter-flow type heated humidifier. Br J Anaesth. 2007;98:531-538.
14 Dreyfuss D, Djedaini K, Gros K, et al. Mechanical ventilation with heated humidifiers or heat and moisture exchangers: effects on patient colonization and incidence of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;151:986-992.
15 Kollef M, Shapiro S, Boyd V, et al. A randomized clinical trial comparing an extended-use hygroscopic condenser humidifier with heated-water humidification in mechanically ventilated patients. Chest. 1998;113:759-767.
16 Jackson C, Webb AR. An evaluation of the heat and moisture exchange performance of four ventilator circuit filters. Intens Care Med. 1992;18:246-2468.
17 Mebius C. Heat and moisture exchangers with bacterial filters: a laboratory evaluation. Acta Anaesthesiol Scand. 1992;36:572-576.
18 Martin C, Papazian L, Perrin G, et al. Performance evaluation of three vaporizing humidifiers and two heat and moisture exchangers in patients with minute volumes > 10 l/min. Chest. 1992;102:1347-1350.
19 Shelley M, Bethune DW, Latimer RD. A comparison of five heat and moisture exchangers. Anaesthesia. 1986;41:527-532.
20 Sottiaux T, Mignolet G, Damas P, et al. Comparative evaluation of three heat and moisture exchangers during short-term postoperative mechanical ventilation. Chest. 1993;104:220-224.
21 Unal N, Kanhai JKK, Buijk SLCE, et al. A novel method of evaluation of three heat-moisture exchangers in six different ventilator settings. Intens Care Med. 1998;24:138-146.
22 Thomachot L, Boisson C, Arnaud S, et al. Changing heat and moisture exchangers after 96 hours rather than 24 hours: a clinical and microbiological evaluation. Crit Care Med. 2000;28:714-720.
23 Misset B, Escudier B, Rivara D, et al. Heat and moisture exchanger vs heated humidifier during long-term mechanical ventilation: a prospective randomized study. Chest. 1990;100:160-163.
24 Martin C, Perrin G, Gevaudan MJ, et al. Heat and moisture exchangers and vaporizing humidifiers in the intensive care unit. Chest. 1990;97:144-149.
25 Cohen IL, Weinberg PF, Fein IA, et al. Endotracheal tube occlusion associated with the use of heat and moisture exchangers in the intensive care unit. Crit Care Med. 1988;16:277-279.
26 AARC clinical practice guideline. Humidification during mechanical ventilation. Respir Care. 1992;37:887-890.
27 Gluck E, Heard S, Patel C, et al. Use of high frequency ventilation in patients with ARDS: a preliminary report. Chest. 1993;103:1413-1420.
28 Ploysongsang Y, Branson R, Rashkin MC, et al. Pressure flow characteristics of commonly used heat-moisture exchangers. Am Rev Respir Dis. 1988;138:675-678.
29 Oh TE, Lin ES, Bhatt S. Resistance of humidifiers, and inspiratory work imposed by a ventilator-humidifier circuit. Br J Anaesth. 1991;66:258-263.
30 Craven DE, Goularte TA, Make BJ. Contaminated condensate in mechanical ventilator circuits: a risk factor for nosocomial pneumonia. Am Rev Respir Dis. 1984;129:625-628.
31 Dreyfuss D, Djedaini K, Weber P, et al. Prospective study of nosocomial pneumonia and of patient and circuit colonisation during mechanical ventilation with circuit changes every 48 hours versus no change. Am Rev Respir Dis. 1991;143:738-743.
32 Craven DE, Connolly MG, Lichtenberg DA, et al. Contamination of mechanical ventilators with tubing changes every 24 or 48 hours. N Engl J Med. 1982;306:1505-1509.
33 Lacherade J-C, Auburtin M, Cerf C, et al. Impact of humidification systems on ventilator-associated pneumonia: a randomized multicenter trial. Am J Respir Crit Care Med. 2005;172:1276-1282.
34 O’Doherty MJ, Thomas SHL. Nebuliser therapy in the intensive care unit. Thorax. 1997;52(Suppl 2):S56-S59.
35 Miller DD, Amin MM, Palmer LB, et al. Aerosol delivery and modern mechanical ventilation in vitro/in vivo evaluation. Am J Respir Crit Care Med. 2003;168:1205-1209.
36 Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med. 1997;156:3-10.
37 Manthous CA, Hall JB. Administration of therapeutic aerosols to mechanically ventilated patients. Chest. 1994;106:560-571.
38 Dhand R. Inhalation therapy in invasive and noninvasive mechanical ventilation. Curr Opin Crit Care. 2007;13:27-38.
39 Spahr-Schopfer IA, Lerman J, Cutz E, et al. Proximate delivery of a large experimental dose from salbutamol MDI induces epithelial airway lesions in intubated rabbits. Am J Respir Crit Care Med. 1994;150:790-794.
40 Poole PJ, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;3:CD001287.
41 Rodrigo GJ. Rapid effects of inhaled corticosteroids in acute asthma. an evidence-based evaluation. Chest. 2006;130:1301-1311.
42 Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulised budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med. 2002;165:698-703.
43 McFadden ER. Acute severe asthma. Am J Respir Crit Care Med. 2003;168:740-759.
44 Parkes SN, Bersten AD. Aerosol delivery and bronchodilator efficacy during continuous positive airway pressure delivered by face mask. Thorax. 1997;52:171-175.
45 Ramsey BW, Dorkin HL, Eisenberg JD, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993;328:1740-1746.
46 Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in cystic fibrosis. Cystic fibrosis inhaled tobramycin study group. N Engl J Med. 1999;340:23-30.
47 Lin H-C, Cheng H-F, Wang C-H, et al. Inhaled gentamicin reduces airway neutrophil activity and mucus secretion in bronchiectasis. Am J Respir Crit Care Med. 1997;155:2024-2029.
48 Barker AF, Couch L, Fiel SB, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. 2000;162:481-485.
49 Palmer LB, Smaldone GC, Simon SR, et al. Aerosolized antibiotics in mechanically ventilated patients: delivery and response. Crit Care Med. 1998;26:31-39.
50 Feeley TW, DuMoulin GC, Hedley-White J, et al. Aerosol polymyxin and pneumonia in seriously ill patients. N Engl J Med. 1975;293:471-475.
51 Burns JL, Van Dalfsen JM, Shawar RM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis. 1999;179:1190-1196.
52 Goldstein I, Wallet F, Nicolas-Robin A, et al. Lung deposition and efficiency of nebulised amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med. 2002;166:1375-1381.
53 Tonnellier M, Ferrari F, Goldstein I, et al. Intravenous versus nebulised ceftazidime in ventilated piglets with and without experimental bronchopneumonia: comparative effects of helium and nitrogen. Anesthesiology. 2005;102:995-1000.
54 Palmer LB, Baram D, Duan T, et al. Ventilator associated pneumonia (VAP) and clinical pulmonary infection score (CPIS): effects of aerosolized antibiotics (AA). Am J Respir Crit Care Med. 2006;173:A525.
55 Committee on Infectious Diseases. Use of ribavarin in the treatment of respiratory syncytial virus infection. Pediatrics. 1993;92:501-504.
56 Bigby TD, Margolskee D, Curtis JL, et al. The usefulness of induced sputum in the diagnosis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986;133:515-518.
57 Vlachos-Mayer H, Leigh R, Sharon RF, et al. Success and safety of sputum induction in the clinical setting. Eur Respir J. 2000;16:997-1000.
58 Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;147:218-233.