The Human Parechoviruses: An Overview
In the summer of 1956, the first two members of the Parechovirus genus were isolated from Ohio children with diarrhea [1]. Human parechoviruses (HPeVs) types 1 and 2, as they would eventually become known, were originally designated as the 22nd and 23rd members (Harris and Williamson strains, respectively) of the “enteric cytopathic human orphan (ECHO) group” or echoviruses (E22 and E23, respectively) [2]. Over the next 50 years, the advent of molecular and genomic virology would provide tools for their in-depth characterization and the identification of other members of the Parechovirus genus [3]. This article reviews past and current medical literature concerning the virology, epidemiology, clinical significance, and diagnosis of the HPeV.
Historical background
The human Parechoviruses (HPeVs) constitute a genus within the Picornaviridae family of RNA viruses [3]. The family currently comprises 12 genera. Of these, the Enteroviruses, Hepatoviruses, and Parechoviruses are commonly pathogenic for humans. The traditional approach to the taxonomic classification of the Enteroviruses was based on schemes using pathogenicity in humans and animals, patterns of replication in cell culture, and neutralization testing [2,4,5]. The ultimate measure of antigenic relatedness of new viral isolates was based on the generation of antisera against a new serotype and reciprocal cross-neutralization testing using a complete panel of prototype enterovirus strains and antisera [5]. Although useful, this approach resulted in the inclusion of nonenteroviral isolates and misclassification of enterovirus strains within the four original species (Poliovirus, Coxsackievirus type A, Coxsackievirus type B, Echovirus) that constituted the genus Enterovirus.
This misclassification occurred with E22 and E23. Although originally classified as echoviruses, HPeV 1 and 2 exhibited growth characteristics that differed from those of other members of the species [1,6]. The development of experimental and computational methodologies to study the molecular biology and genomic analysis provided further evidence that E22 and E23 were atypical among members of the echovirus species, and other members of the genus in general [7–13]. The inability to hybridize subgenomic probes, pan-reactive with members from all species of the Enterovirus genus, to the genomes of E22 and E23 raised the possibility that their genomes differed significantly from those of other enteroviruses [7,8,13]. Ultimately, the elucidation of their genome sequences and their phylogenetic relationship to other enteroviruses confirmed that they where genetically distinct and merited classification into a novel genus: Parechovirus [3,10–12]. This genus currently consists of 16 types and, most likely, will continue to increase in number [14].
Virology
As with other Picornaviridae, the HPeVs are small (28 nm in diameter), nonenveloped RNA viruses [3,10,15,16]. The HPeV genome is a single-stranded, positive (messenger) sense RNA molecule approximately 7.35 kb in length (Fig. 1) [10–12,14,17–19]. The organization of the HPeV genome is consistent with that of other Picornaviridae [20]. A covalently linked protein, VPg (also designated 3B), is attached to the extreme 5’ end of the genome immediately preceding a long nontranslated region (NTR), constituting approximately 10% of the viral genome’s length. The 5’NTR precedes a single open reading frame (ORF) that is subdivided into three regions (P1–3) and codes for a polyprotein of approximately 2180 amino acids, depending on the type and strain [14,20]. The ORF is followed by a short 3’NTR and a terminal polyadenylated tail.
The 5’NTR of the Picornaviridae plays critical roles in viral replication and translation of the viral genome [20]. Multiple lines of evidence support the existence of higher-order structures, formed by short- and long-distance RNA–RNA interactions, within the picornaviral 5’NTR [20]. Experimental and computation data also support this for the HPeV [12,18,21]. A stem-loop structure (SLS) and pseudoknot at the extreme upstream end of the HPeV 5’NTR, shown to be essential for replication of the viral genome, have been computationally shown to exist in multiple HPeV types [18,22].
A series of SLSs, downstream of the replication-essential SLS, that constitute an internal ribosome entry site (IRES) exist in the HPeV [18,22]. The IRES is essential for efficient translation of the picornavirus genome [20]. The HPeV IRES seems to be more closely related to those of the Cardiovirus and Aphthovirus genera than to the Enteroviruses. Within the HPeV 5’NTR are conserved regions of nucleotide identity that are shared among members of the genus. These regions can be used to develop nucleic acid amplification tests (NAATs) for HPeV detection in clinical specimens.
The 3’NTR of the enteroviruses contains higher-order structures important in viral replication. Genomic analysis of HPeV types 1 through 5 revealed the existence of a predicted SLS in the 3’NTR [18], raising the possibility that it also plays a role in HPeV replication.
Translation of the ORF results in the generation of a large polyprotein that undergoes immediate posttranslational modification to yield the structural (capsid) and nonstructural proteins essential to HPeV replication and viral assembly (see Fig. 1) [20]. The structure and functions of all of the HPeV proteins have not been extensively studied. Although many have functions similar to enteroviral cognate proteins, differences are emerging. Unlike most of the Picornaviridae, in HPeV the P1 region codes for three rather than four capsid proteins (see Fig. 1). The capsid protein coding sequences are organized contiguously and without intervening stop codons as VP0 (1AB), VP3 (1C), VP1 (1D) in the 5’ to 3’ orientation [10–12,16–19]. The HPeV VP0 protein is not maturationally cleaved to form the VP2 and VP4 capsid proteins, as is typical of other enteroviruses, resulting in a capsid that is composed of three, rather than four, proteins in the mature virion [10–12,16–19]. The VP0 protein has been found to contain an SLS that may function as a cis-acting replication element (CRE) [18]. The CRE is critical for RNA replication in poliovirus where it is located within the 2C protein coding sequence. The C-terminal region of VP1 of several of the HPeV types contains an arginine–glycine-aspartic acid (RGD) motif that has been shown to be functional in binding to host cell integrins, which serve as receptors for some members of the genus [10,12,16,18,23,24]. The cell receptors used by HPeV lacking the RGD motif are currently unknown. The VP1 protein is also important for molecular typing of the HPeV [25,26]. The VP3 of HPeV characteristically varies from that of the enteroviruses because of the N-terminal extension of 28 to 34 amino acid residues [27].
The P2 and P3 regions code for seven nonstructural proteins (5’-2A, 2B, 2C, 3A, 3B, 3C, and 3D-3’) and their intermediates required for the viral lifecycle (see Fig. 1) [20]. The HPeV 2A protein seems to differ functionally from that of the enteroviruses in that it lacks proteolytic activity. Evidence indicates that it is incapable of mediating the cleavage between the 1D and 2A proteins [28,29]. In addition, the HPeV fail to shut off host cell protein synthesis [8,9,23], a function mediated in enteroviruses by 2A cleavage of eIF4G [20], which confers a replication advantage to the virus. The HPeV 2A protein also binds to RNA and may play a role in RNA replication [30]. The 2C protein of the HPeV also seems to differ from its cognate enterovirus protein. Like the enterovirus 2C, it localizes to endoplasmic reticulum membrane–associated replication complexes, supporting its role in viral replication. However, it is also associated with cellular structures not directly involved in replication [31,32]. The 2C protein also has AMP kinase activity, but the significance of this property is currently unknown.
Epidemiology
HPeVs have been reported worldwide [18,27,33–45]. When discussing the epidemiology of the HPeV, one must understand that until 2004 only 2 HPeV types were known to exist. Since then, 14 new types have been recognized [14]. Additionally, most of what is known about the epidemiology of the HPeV is based on cell culture detection of these agents, a methodology that has poor sensitivity for the detecting HPeV. Therefore, understanding of the epidemiology of the HPeV continues to evolve as new types are identified and molecular methodologies (eg, NAATs) are used for their detection.
The contribution of each of the 16 known HPeV types to the total burden of HPeV-related disease is yet unclear. Similarly, the worldwide distribution of each of the types is still being defined. Evaluation of longitudinal data spanning 39 years indicates that in the United States, HePV1 accounts for 1.8% (880/49,637) of all typed enterovirus isolates reported to the Centers for Disease Control and Prevention (CDC) [37,46]. During that period, HPeV 1 numbered among the top 15 isolates for 74% of all years. In contrast, HPeV 2 accounted for only 0.1% of all enteroviruses isolated. The contribution of HPeV 1 to the burden of enterovirus disease in the neonate seems to be similarly low: 0.8% (19/2356 typeable isolates) over a 21-year period [47]. However, because of multiple factors, these data may not represent the true burden of HPeV disease [37,46].
A report from Niigata, Japan documented that over a 15-year period, 41 HPeV isolates where identified using cell culture from stool, throat swabs, and cerebrospinal fluid (CSF) specimens [19]. In comparison, during that same period, 1437 enterovirus isolates were identified. Thus, approximately 2.8% of all enteroviruses isolated were HPeV. Typing showed that HPeV 1, 3, 4, and 6 constituted approximately 34%, 39%, 2%, and 24% of the total isolates, respectively. A second report from Japan found that 110 (2.2%) of 4976 stool specimens collected over a 9-year period from children with suspected viral infections were positive for HPeV [16]. HPeV 1 and 3 accounted for 57% (n = 63) and 40% (n = 44) of isolates, respectively. The remaining three isolates were HPeV 4 (n = 2) and HPeV 6 (n = 1).
A Swedish study also documented a low incidence of HPeV 2–related disease, with only five cases identified among 1000 enteroviruses over a 33-year period [34]. A Canadian report found that among 28 HPeV isolates identified over a 20-year period, types 1, 2, and 3 accounted for 71.4% (n = 20), 10.7% (n = 3), and 17.9% (n = 5), respectively [27].
The current data appear to indicate that overall the HPeV account for approximately 2% of all enteroviruses isolated by clinical laboratories using traditional methods. HPeV 1 is the most frequently identified member of the genus, followed by HPeV 3. Currently, other members seem to make minor contributions to the total burden of HPeV-related disease. However, as NAATs are used more frequently for their detection, this may change.
Evidence indicates that HPeV infections are acquired early in life and that geographic variations may contribute to the predominant serotypes initially encountered. A report summarizing data from the World Health Organization (WHO) Virus Unit from 1967 to 1974 documented that 61% of reported HPeV 1 infections occurred in infants younger than 1 year [48]. In the United States, most cases of HPeV 1 and 2 reported to the CDC have occurred in infants younger than 1 year (73% and 67.6%, respectively) [37]. Children younger than 5 years accounted for 95.6% and 88.2% of all HPeV 1 and 2 isolates, respectively [37].
Although available seroprevalence data are sparse and focus on a limited number of HPeV types, they too support that early infection with HPeV and geographic variation factor into what type is encountered [16,36,49–51]. Studies conducted in Finland documented that 72% to 89% of infants 2 years of age possessed antibodies to HPeV 1 [36,50]. A small study of Israeli infants showed that 89% of subjects had antibodies to HePV 1 through 3 at the same age [51]. The same study also showed that 70% of Canadian children younger than 5 years possessed antibodies to the same HPeV types [51].
Two studies conducted in Japan suggest that seroprevalence rates to HPeV 1 and HPeV 3 in children vary at different ages when adjusted for a similar definition of significant titers (>1:8) to the virus in question [16,49]. Among children aged 4 to 6 years from Hiroshima (n = 195) and Aichi (n = 207) Prefectures, the seroprevalence of antibodies to HPeV 1 and HPeV 3 was approximately equal (80% and 85%, respectively). However, the age at which seropositivity occurred to the specific serotypes varied between studies. In infants 7 to 12 months of age from Hiroshima Prefecture, 66% had antibodies to HPeV 1. In contrast, only 15% of infants of the same age from Aichi Prefecture showed presence of antibodies to HPeV 3. Among children aged 2 to 3 years from Hiroshima Prefecture, 80% had antibodies to HPeV 1. In comparison, only 45% of similarly aged children from Aichi Prefecture had antibodies to HPeV 3.
A Canadian study of 28 children, retrospectively identified as having HPeV infection, documented variations in the mean age at which infants had infections with various HPeV types [27]. The mean age at which HPeV 3 infection occurred was 0.7 months compared with 14.6 and 6.3 months for HPeV 1 and 2, respectively. In dramatic counterpoint to the previous study, a limited serologic survey of the prevalence of HPeV 3 among adults in the Milwaukee, Wisconsin area failed to detect any seropositive individuals [52].
A prospective, longitudinal study of HPeV infection, determined through stool culture, in 102 Norwegian infants followed up for 36 months documented that the cumulative incidence of infection was 86% by 24 months of age and 94% at 36 months [38]. The incidence rate of infection increased with increasing age: 11.6 versus 20 to 23 infections per 100 person months for infants aged 4 months and infants older than 15 months of age, respectively. The specific HPeV serotypes were not determined. Of the infants in one cohort, 55% had more than one HPeV infection during the period of observation, indicating that sequential HPeV infections are common [38].
Taken together, these studies indicate that for individual HPeV types, differences may exist regarding how soon after birth infants become infected. These differences may be a reflection of factors such as modes of transmission for a given HPeV type, prevalence of HPeV types in the community, and variations in patterns of exposure to HPeV types.
Although HPeV infections may occur throughout the year, they exhibit a strong seasonal epidemiology. Around the world, the peak incidence of infections occurs during the summer and fall months [27,35,38,44,50,53–61]. Several reports have documented a biennial pattern of HPeV 3 infection [40,56,57,62]. Other reports, however, have specifically failed to support this finding [19,38,42,59]. Cocirculation of multiple HPeV types within a community or geographic region is common [38,41,42,44,55,59,60,62,63]. HPeVs have been associated with community [58] and nosocomial outbreaks in pediatric and neonatal units [34,35,45,64,65].
Clinical syndromes
Transmission of the HPeV seems to occur via the fecal–oral and respiratory routes. Fecal–oral transmission is favored by high viral titers in stool. In one report, the median HPeV titer was 502,380 copies/mL (range, 3,170–503,377,290 copies/mL) [55]. Reports of neonates with HPeV infections occurring in the first 2 days of life may support the possibility of in utero transmission [45,56].
After infection, the virus is shed from the gastrointestinal and upper respiratory tracts [38,54,66]. The duration of shedding from these sites is still poorly characterized but may range from less than 2 weeks to 5 months [38,66] in stool and 1 to 3 weeks in the upper respiratory tract [54,66]. The median duration of HPeV shedding in stool has been shown to be 51 days [38]. Approximately 10% of infected children shed virus in stool for more than 3 months.
The detection of HPeV from the stool of healthy, asymptomatic infants, and high seroprevalence rates among infants and children in the general population support that many, if not most, infections are subclinical [16,38,45,55]. A comparison of symptoms from longitudinally followed infants showed that no symptom (cough, sneezing, fever [>38°C], diarrhea) was significantly associated with HPeV detection in stool when compared with children not shedding virus [38]. Additionally, given the high prevalence of HPeV infections, severe infections are probably rare.
Central nervous system infections
The HPeVs have been linked to various central nervous system (CNS) syndromes, including acute flaccid paralysis [16,19,33], encephalitis [57,61,67–69], and meningitis [33,59,61,69].
Meningitis
Detection of HPeV from permissive (upper respiratory and gastrointestinal tracts) [19,42,53,58,62] and nonpermissive (CSF, blood) sites [40,57,58] supports that the HPeVs and, in particular HPeV 3, contribute to the burden of viral meningitis worldwide. A retrospective study of CSF specimens previously found to be negative for enteroviruses from 761 Dutch children 5 years of age, collected from 2004 to 2006, showed that 4.6% had HPeV genome present (typing was not attempted) [57]. Of these, 97% were younger than 24 months, with infants younger than 28 days constituting 46% of all positive children. The prevalence of HPeV-positive specimens fluctuated annually: 8.2%, 0.4% and 5.7% for 2004, 2005 and 2006, respectively.
A Scottish study examined CSF samples from 1480 individuals collected from 2006 to 2008 [40]. In 14 (0.9%) individuals, HPeV was detected by reverse transcription-polymerase chain reaction (RT-PCR); all were type 3. The prevalence of positive specimens varied by year: 0.7% in 2006, 0% in 2007, and 7.2% in 2008. All 14 patients were younger than 3 months. The frequency of detection for subjects in this age cohort was 2.9%.
A retrospective analysis of 780 CSF nucleic acid extracts from United States children, previously shown to be negative for enteroviruses, documented that 7.4% (n = 58) harbored HPeV genome [61]. Of these subjects, 81% were 2 months of age or younger and 26% were 1 month of age or younger. Genotyping showed HPeV 3 in 52 and HPeV 1 in 1. The prevalence of positive specimens varied significantly by year of the study: 1.8% in 2006, 16.9% in 2007, and 0% in 2008.
A review of archival CSF specimens from 397 Spanish infants younger than 12 months, obtained to exclude CNS infection, found that 2.3% (n = 9) were positive for the HPeV genome [59]. All but one of the infants were younger than 2 months and 4 infants (44%) were younger than 30 days. In 8 subjects, genotyping showed that the HPeV was type 3. Lastly, during an HPeV 3 epidemic in Hiroshima, Japan, 4% of CSF specimens evaluated were positive [58].
These reports indicate that HPeV, and particularly HPeV 3, may be detectable in 2.3% to 7.4% of all infants undergoing CSF evaluation for possible infection. Most cases occurred in infants younger than 3 months. As point of comparison, several of the studies documented that enterovirus was detected in 5.3% to 25.7% of CSF samples evaluated [40,59,61]. Considerable annual variation was observed (0%–16.9%) in the prevalence of HPeV-positive CSF samples. Despite the presence of other HPeV types circulating in the community, HPeV 3 was the overwhelmingly dominant cause of HPeV-related meningitis [40].
Review of clinical and laboratory data from 96 infants (including 2 with proven or possible encephalitis) from the three largest reports of patients with unequivocal HPeV 3 CSF infection indicate that fever is present in more than 95% of infants [57,59,61]. Irritability was also reported in more than 95% of cases. In all reports, males accounted for most cases (55%–71%). An exanthem was reported in 17% to 60% of infants, and was characterized as maculopapular in nature in one report [61]. Gastrointestinal signs (emesis, diarrhea, distention) were reported in approximately one-quarter to half of cases. Respiratory symptoms (“rhinorrhea, cough, tachypnea, apnea, wheezing, and/or abnormalities on radiograph of the thorax”) were present in 36% in one series [57]. Conspicuously absent in all reports were findings indicating increased intracranial pressure (bulging fontanelle) or meningeal irritation (nuchal stiffness, Kernig or Brudzinski signs).
The CSF cytochemical evaluation showed that most patients had no or minimal abnormalities [57,59,61]. Only 12% of 67 infants had a white blood cell count above the norm for age [59,61], and in only 2 was the pleocytosis greater than 25 cells/mm3. Hypoglycorrhachia was reported in only 2 infants and the CSF protein was increased above age-adjusted normal values in only 7.5% [59,61]. Similar CSF findings to those described in the previous large series have been reported in case reports of HPeV meningitis [70].
Ninety-eight percent of infants were hospitalized. Of the two infants not admitted, one was observed in the emergency room for 24 hours and the other discharged home [57,59,61]. Antibiotics were administered to 82% to 100% of infants. In a cohort of infants from the United States, 17% also received acyclovir [61]. Although 54% of infants in one report were felt to have a sepsis-like clinical presentation, only one required admission to an intensive care unit [57]. The mean duration of hospitalization varied from 3.6 to 7.4 days [57,59,61]. No deaths were reported in any of the studies [57,59,61]. Long-term outcome data were not provided.
Acute flaccid paralysis
The HPeVs have been etiologically linked to cases of acute flaccid paralysis [16,19,33,71]. The original identification of HPeV 3 was from a 1-year-old female with fever, diarrhea, and transient paralysis [16]. Other HPeV types associated with acute flaccid paralysis include 1 and 6 [19,33].
The sole recorded outbreak of HPeV-related acute flaccid paralysis occurred in Jamaica and was the result of HPeV 1 [33]. Two adults (26 and 27 years) and 4 children (21 months and 4, 7, and 13 years) were involved. Three children were fully immunized against polio and the fourth had received two doses of oral polio vaccine. All had acute onset of flaccid paralysis with inability to walk and one child also had involvement of the muscles of respiration and required mechanical ventilation. Two of three older children reported paraesthesias of extremities. Cranial nerve involvement occurred in one child. All children had areflexia and two had signs of meningeal irritation. CSF pleocytosis was present in only one child, but protein was increased in all. Although all children recovered, two required admission to the intensive care unit and three had lower extremity weakness as sequelae.
A case involving a 10-month-old male, reported as encephalomyelitis with residual paralysis, may have been acute flaccid paralysis [71]. The clinical presentation consisted of fever, emesis, generalized hypotonia, and decreased tendon reflexes. No pleocytosis was observed on examination of the CSF. Type 1 HPeV genome was detected in the CSF. Recovery was complicated by residual upper extremity paralysis.
Encephalitis
Reports of HPeV-related encephalitis have exclusively involved neonates and young infants [56,57,61,68,69]. HPeV types 1 and 3 have been isolated from confirmed cases, with HPeV 3 dominating [68,69].
Evaluation of cases for which sufficient information is provided indicates that most cases have occurred in full-term neonates, with onset of symptoms occurring in the first 2 weeks of life [56,68,69]. Among cases in prematurely born infants, the onset of illness was later: at 50 to 90 days of life [68]. Seizures constituted the predominant clinical finding (82%). In one-third, recurrent seizures persisted for more than 24 hours. Fever and irritability were each present in 73% of infants. Rash was present in approximately two-thirds and apnea in 55%. Approximately one-third had hypotension that required inotropic support. Additional findings included diarrhea, hypertonia, and photophobia. CSF evaluation was abnormal in only one patient and showed moderate pleocytosis [68].
All infants showed abnormalities on neuroimaging using MRI or cranial ultrasound [68,69]. MRI detected white matter changes consisting of high signal intensity and punctate lesions in 90%. These consisted of high signal intensity and punctate lesions. MRI detected white matter changes in two infants without seizures [68,69]. Severe periventricular echogenicity was detected with cranial ultrasound in all infants.
No deaths occurred [68,69]. Although long-term neurologic outcome data were not available for all patients, only 64% were neurologically normal at the time of the clinical report. Neurologic sequelae included epilepsy, cerebral palsy, visual impairment, learning disability, and mild distal hypotonia [68]. Repetitive seizures and a discontinuous background detected on amplitude-integrated electroencephalogram were found to correlate with poor neurodevelopmental outcome [68].
Neonatal disease
As with enteroviral infections in neonates, most HPeV infections in this age group are likely asymptomatic or result in benign, self-limited febrile illness [72,73]. However, similar to their viral cousins, HPeVs have the potential to cause severe life-threatening disease in the newborn [52,56].
Undifferentiated febrile illness
The HPeV have frequently been isolated from the stools of neonates and infants with undifferentiated febrile illness [42,70,74]. A small but well-documented case series has been published describing the clinical, laboratory, and radiologic findings in three neonates, aged 7 to 21 days, with HPeV 3 infection presenting as a febrile illness without a discernable cause [70]. Fever (≥39° C) and irritability were presenting complaints in all infants. Rhinorrhea in association with cough and poor feeding were reported in one infant each. All had exposure histories significant for contact with family members with upper respiratory tract infections before the onset of illness. At admission, one infant each was tachypneic and tachycardic. An erythematous rash, present at admission or developing the next day, occurred in all infants. CSF and chest radiographs were normal in all. After admission, all infants developed worsened respiratory status consisting of tachypnea and oxygen desaturation singly or in combination; prompting admission to the intensive care unit. All infants became afebrile by the fourth day after admission. HPeV 3 was detected from nasopharyngeal aspirates of all infants. Given the similarity of the clinical and CSF findings of these infants to those described previously with meningitis, one or all may have had CNS infection. However, CSF was not evaluated for the presence of HPeV using NAAT in any patient.
The clinical findings described in this case series are similar to those described in neonates with enterovirus infections [75]. However, additional studies with larger numbers of patients are necessary to fully characterize this syndrome.
Hemorrhage-hepatitis syndrome
A report of severe HPeV infections in neonates included two infants with hepatitis and associated thrombocytopenia [56]. Although specific details are not provided, the latter two infants may have had hemorrhage-hepatitis syndrome [47], a condition often associated with echovirus 11 infections in neonates [75]. A well-documented case of HPeV 3–related hemorrhage-hepatitis syndrome has been reported [69]. Onset of illness occurred at 24 days of age with fever and irritability. Admission aspartate and alanine aminotransferases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST], respectively) levels and platelet count were initially normal. On the fourth day of hospitalization the ALT and AST levels increased to 5421 U/L and 1207 U/L, respectively. The infant developed prolonged prothrombin and partial thromboplastin times and thrombocytopenia. Although none of the infants died, one required a liver transplant at 3 years of age as a result of severe liver necrosis [56].
Necrotizing enterocolitis
Neonatal HPeV 1 infection has been associated with necrotizing enterocolitis [35,45]. In an outbreak of gastroenteritis involving 19 infants being cared for in a neonatal intensive care unit, 7 with bloody diarrhea had abdominal radiograph findings of early necrotizing enterocolitis. One was found to have pneumatosis intestinalis.
In addition to the constellation of signs and symptoms associated with the specific clinical syndromes discussed previously, manifestations reported in single or combined association with HPeV infection in neonates include fever, apnea, tachypnea, respiratory distress, rhinorrhea, conjunctivitis, and rash [45,70].
Gastrointestinal disease
Diarrhea is a component of the symptom complex of almost every syndrome associated with HPeV infection. Multiple studies, varying in stringency, controls, and clinical venues, have examined the role of HPeV in the origin of acute gastroenteritis in children [41,42,44,55,60,63] and adults [55].
Using RT-PCR, a study conducted in Germany examined the presence of HPeV in the stool of patients of all ages with acute enteritis [55]. Only specimens negative for rota-, adeno-, astro-, noro-, and enteroviruses were included. No HPeVs were detected in a cohort of 118 patients comprised of hospitalized young children and older children and adults evaluated in the context of enteritis outbreaks. A second cohort consisted of 499 nonhospitalized subjects with community-acquired enteritis and 39 controls without enteritis [55]. Eight subjects in the former group and one control subject were found to have HPeV. All positive subjects were children, and all but one was younger than 2 years. The prevalence of HPeV among the enteritis and control groups was not statistically significant: 1.6% (8/499) versus 2.6% (1/39), respectively. Prevalence was 11.6% (7/60) when only children younger than 2 years of age were included. The authors concluded that the HPeVs were not responsible for epidemic enteritis but rather were causes of endemic disease [55].
An RT-PCR evaluation for rota-, adeno-, saporo-, noro- and astroviruses in 82 fecal specimens collected from Thai children with acute gastroenteritis found HPeV genome in 12 (14.6%) [44]. Patients in whom HPeV was identified ranged in age from 6 to 24 months. Typing was possible in 9 and revealed types 1 (n = 5), 2 (n = 1), 3 (N = 2), and 4 (n = 1). Clinical findings and venue of collection were not provided.
A study conducted in Sri Lankan children hospitalized with acute gastroenteritis examined 362 fecal specimens for the presence of HPeV genome using RT-PCR [60]. All specimens positive for HPeV were also tested for the presence of rota-, adeno-, saporo-, noro-, and astroviruses. HPeV was detected in 8.3% (n = 30). Among the 27 specimens that were typed, HPeV types 1 (n = 11), 3 (n = 1), 4 (n = 5), 5 (n = 3), 10 (n = 5), and 11 (n = 2) were identified. Coinfection with other viral enteric pathogens was found in 67%. The 10 patients solely infected with HPeV ranged in age from 2 to 26 months. Fever and emesis were present in 30% and 40% of patients, respectively. Respiratory symptoms (wheezing, cough, and coryza) were present in 30% [60].
A study of Japanese children aged 2 months to 15 years with acute gastroenteritis examined stool specimens collected at pediatric clinics for the presence of HPeV genome using RT-PCR [63]. Of 477 stool specimens, 247 were found to be negative for rota-, adeno-, saporo-, noro-, and astroviruses and were tested for HPeV genome using RT-PCR. HPeV was detected in 8.1% (n = 20) of samples. Typing was possible in 17 specimens, and identified HePV 1 (n = 15) and HPeV 3 (n = 2). Patients with HPeV ranged in age from 5 to 51 months. Fever and emesis were present in 30% and 15%, respectively. Respiratory symptoms consisting of cough and coryza were noted in 10% and 5% of patients, respectively.
A study designed to identify novel HPeVs evaluated stool samples from 335 Brazilian children aged 6 years or younger evaluated as outpatients or hospitalized for acute diarrhea lasting 13 days or less with associated dehydration [41]. RT-PCR detected HPeV in 16.1% (n = 54) of samples. Typing was possible in only 11 samples and identified types 1, 5, 6, and 8. HPeV 1 was the most frequently identified (n = 7). Information regarding coinfection with other diarrheal agents and clinical data were not provided.
Based on available information, HPeVs seem to be associated with endemic cases of diarrheal illness restricted to infants and young children. This finding is not surprising given the high prevalence of seropositivity (>80%–85%) to various HPeV types found in older children and adults, as discussed previously. In addition to diarrhea, fever, vomiting, or respiratory symptoms may be present in up to one-third of cases. The studies summarized earlier seem to indicate that no significant differences exist in the prevalence of HPeV infection in children between industrialized (8.2%–11.6%) [55,63] and developing (8.3%–14.6%) nations [44,60]. The finding of significant coinfection (66.7%) with other enteric viral pathogens [60] raises questions as to whether dual infections result in more severe disease.
Respiratory disease
Multiple reports have described respiratory symptoms alone or in combination with other syndrome complexes in patients in whom HPeV has been detected through culture, serology, or NAATs [45,48,55,57,60,63,64,70]. Data from the WHO Virus Unit indicated that approximately one-quarter of cases of HPeV 1 presented with respiratory disease [48]. A 15-year review of HPeV-related cases from a university center in Japan showed that 14.6% of 41 cases were respiratory tract infections [19]. HPeV types identified included 1 (n = 2), 3 (n = 3), and 6 (n = 1). A series of outbreaks of HPeV 1–related respiratory tract disease were reported from a neonatal unit in the United States. Although the report included 64 premature infants, only 28% had virologic or serologic criteria sufficient to support acute HPeV infection [64].
Although these studies seem to support a role for HPeV as the etiologic agents of respiratory tract disease, a large Scottish study raises questions concerning the causal role of at least some types [54]. This study evaluated 3844 respiratory specimens, collected from 2200 individuals, for the presence of traditional respiratory viral pathogens (respiratory syncytial virus, influenza viruses A and B, parainfluenza viruses 1 through 3, adenovirus and bocavirus), and HPeV using NAAT. HPeVs were detected in only 34 specimens (0.9% sample prevalence) collected from 27 individuals (1.2% person prevalence).Of those in whom any respiratory virus was identified (n = 930), HPeV accounted for 2.9% of cases. All HPeV infections occurred in children aged 6 months to 5 years of age, 89% were younger than 3 years of age. Typing was possible in 33 specimens; only HPeV types 1 (n = 25) and 6 (n = 8) were identified. Coinfection with one or more of the traditional respiratory viral pathogens was noted in 76% of children in whom HPeV was detected. The authors concluded that the findings failed to provide evidence supporting the proposed role of HPeV as a cause of respiratory disease in children [54]. The observed incidence of HPeV infections in children younger than 5 years was within the expected frequency of the general population. Lastly, the clinical profile, in terms of nonspecific and upper and lower respiratory tract symptoms, of those in whom HPeV was detected coincided more closely to the profiles of individuals with negative samples.
Sudden unexplained infant death
Reports of pediatric fatalities associated with HPeV infection are rare [16,17,52,76]. The HPeVs have been recovered from autopsy specimens obtained from pediatric decedents diagnosed with sudden unexplained infant death [52]. A review of autopsy reports from 1263 infants and children found that viruses were identified in 34% (n = 426). HPeVs were identified in 24 (5.6%). Typing identified 20 as HPeV 1; 3 as HPeV 3; and 1 as HPeV 6. The isolate of HPeV 6 was obtained from an infant who died of blunt force trauma and one HPeV 3 isolate came from an infant who died of asphyxiation secondary to loss of tracheal airway. In these instances, HPeV was not deemed causal in the deaths. The remaining HPeV 3 isolates were obtained from two female infants aged 4 weeks and 4 months, respectively. Both had viral-like upper respiratory tract symptoms at the time of death or 1 week preceding it. HPeV 3 was isolated from lung and in swabs of the nasopharynx and colon. The authors concluded that HPeV 3 may have contributed to their deaths.
Miscellaneous clinical associations
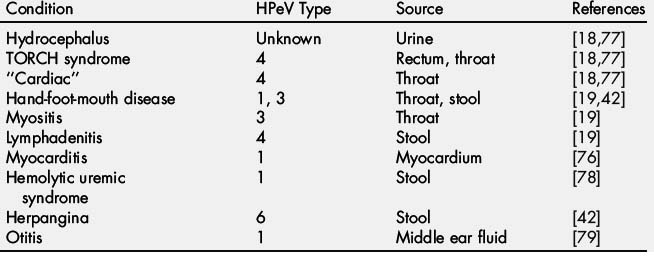
Two reports document the isolation of HPeV types 6 and 5 from the CSF and stool from children with Reye syndrome [18,19,77]. HPeVs have been isolated from infants and children with a wide array of illnesses and syndromes (Table 1). A causal link may exist between the HPeV and those conditions. However, in most of the reports, the HPeVs were detected from sites where prolonged shedding is known to occur and not from the involved organ system or tissue. Additionally, reports fail to detail whether other infectious agents, known to be associated with the syndrome, were conclusively excluded. Further adding to the complexity of ascribing a causal relationship between HPeV and an illness is the codetection of a second agent, known to be linked to the disease in question [42,54,63].
Diagnosis
Several approaches (NAAT, cell culture, or serology) have been used to diagnose HPeV infections. Routine use of these tests in the clinical laboratory is generally limited by the lack of commercially available reagents.
Nucleic acid amplification
Currently, RT-PCR is the preferred methodology for detecting HPeV because of its sensitivity and ability to detect all known HPeV types in a clinically meaningful time frame. Although the HPeVs are related to the enteroviruses, RT-PCR assays used for detecting the latter do not detect HPeVs because of sequence heterogeneity within the 5’NTR of members of the two genera.
Detection of the HPeV using RT-PCR has relied on amplification of portions of the HPeV 5’NTR flanked by stretches of conserved nucleic acid sequence. The initially described assays detected the amplified region using agarose gel electrophoresis or liquid hybridization [36,54,80–82]. The advent of real-time amplification systems has led to the development of several highly sensitive HPeV real-time RT-PCR (rRT-PCR) assays capable of simultaneous amplification and detection of amplification products [54,83–85]. One- and two-step rRT-PCR assays have been described for HPeV detection [54,83,85]. In a two-step RT-PCR, the conversion of the HPeV RNA genome target area into a complimentary DNA (cDNA) copy (ie, reverse transcription) is performed initially. An aliquot of the cDNA reaction is then transferred to a second reaction vessel for amplification of the target region (ie, PCR). The one-step RT-PCR combines the cDNA synthesis and PCR in a single reaction vessel.
rRT-PCR assays have been shown to be significantly more sensitive than cell culture for detecting the HPeV. One-step HPeV rRT-PCRs and a two-step rRT-PCR have been developed that show approximately 100- to 1000-fold greater sensitivity than culture for detection of HPeV [83,85]. Using stool specimens, one report documented that cell culture failed to identify HPeV in 64% of specimens found to be positive using rRT-PCR [86]. A two-step rRT-PCR assay using clinical specimens (CSF, blood, stool, and throat swabs) showed superior detection of the HPeV rRT-PCR over cell culture [87]. All patients with positive CSF specimens (n = 3) also had positive results from blood. Fourteen patients who had positive HPeV results from blood or CSF also had positive results from feces or throat. The rRT-PCR detected 19 additional positive specimens (16/159 feces and 3/57 throat swabs) that were missed by culture.
Optimum diagnostic RT-PCR assays targeting the 5’NTR for detection of HPeV are not type-specific. They are designed for increased sensitivity and to broadly detect all known HPeV types from clinical specimens. Type-specific HPeV identification relies on phylogenetic evaluation of the VP1 coding region, and is considered to be the criterion standard for HPeV typing [26,86]. HPeV strains of the same genotype share 77% or more nucleotide sequence identity or 87% or more amino acid identity of VP1 [26]. A recent report described an assay for amplification of the complete VP1 sequences from HPeV 1 through 7 and 9 through 14 for use in genotyping [26]. The VP1 assays are less sensitive than the rRT-PCR assays targeting the 5’NTR region, and hence some of low-titer clinical specimens may be potentially missed by the VP1 assays [26,86]. Although genotyping is not necessary for clinical purposes, it may be a useful adjunct to diagnosis for epidemiologic purposes and to gain a better understanding of genotype–disease association.
Currently only a limited number of clinical and reference laboratories in the United States offer RT-PCR testing for HPeV [88]. In the absence of RT-PCR assays approved by the U.S. Food and Drug Administration for HPeV detection, clinical laboratories that elect to develop in-house RT-PCR assays for detection or genotyping should carefully validate assays intended for clinic use. Validation of RT-PCR detection systems is complex and should include careful selection of the primers and probes for amplification, analysis of the polymerase enzymes, amplification conditions, platforms, and specimen types to be tested.
Cell culture
The HPeV can produce enterovirus-like cytopathic effect on appropriate cell lines [86]. However, not all HPeV types replicate equally on the same primary cells or cell lines. A recent study documented that HT29 (primary colorectal adenocarcinoma) cells recovered 75% of all cultivable HPeV types 1, 4, and 6 from stool specimens [86]. HPeV 1 and 4 could also be cultured using tm (tertiary cynomolgus monkey kidney), Vero (African green monkey kidney), A549 (human lung adenocarcinoma), and RD (rhabdomyosarcoma) cells. In contrast, HPeV 3 grew only on Vero and A549 cells. An earlier study noted tm cells could also detect HPeV 3 isolates from stool specimens [89]. Thus, optimal recovery of HPeV requires the use of multiple cell lines.
The use of cell culture for clinical detection of HPeV is limited by several factors. As indicated, the detection of HPeV using traditional cell culture methodologies lacks sensitivity because not all cell lines support their growth equally [36,86]. Furthermore, many of the primary cells or cell lines required for optimum detection of the HPeV are not part of routine cell culture systems used in clinical laboratories. Even using the widest combination of cells known to support the growth of HPeV, many types grow poorly, if at all, in cell culture (eg, HPeV types 11 to 14). The ability to propagate the HPeV in cell culture is also dependant on viral load in the specimen being tested, so that specimens containing lower viral titers may fail to yield HPeV [86]. Cell culture detection of HPeV may take several days, hence limiting its clinical usefulness in the acute management of the patient. Lastly, the lack of commercial immunostaining reagents for confirmation and type-specific identification of isolated HPeV strains necessitates the use of neutralization assays with a panel of standardized serotype-specific antiserum pools; a process that is laborious, time-consuming, and limited by the availability of reagents. All of these limitations have constrained the use of appropriate cell culture HPeV detection to research, reference, and a few commercial laboratories.
Specimen selection and interpretation of results
Specimen types selected for testing should be based on disease condition and organ system involved. Stool, throat swabs, and CSF are good choices for detecting gastrointestinal, respiratory, and CNS infections, respectively. Similar to RT-PCR detection of enteroviruses using blood, limited data suggest that additional testing of blood may improve the diagnostic yield for detecting CNS infection.
Several important concepts should be borne in mind when interpreting RT-PCR or cell culture results. Because HPeV are excreted from the throat and gastrointestinal tract for prolonged periods [38,66], their detection in these sites should not be taken as prima facie proof of a causal role in illnesses being evaluated. Their presence may represent shedding from an infection that occurred weeks earlier and have no relationship to the medical problem being evaluated. The same is not the case for HPeV detected in CSF and blood, which their detection is considered diagnostic. Additionally, detection of an HPeV in the stool or throat specimen of a neonate may be considered supportive evidence for the cause of an HPeV-related syndrome.
Serology
Serologic assays can be used to detect HPeV-specific antibody responses in infected individuals. However, the time required to perform them, lack of standardization, and commercial reagents make them impractical for clinically relevant use. Therefore, they are generally restricted to use as epidemiologic research tools. Enzyme immunoassays (EIAs) using synthetic peptides derived from the capsid proteins VP0 and VP3 have been developed [51,90]. An EIA using a recombinant VP3 protein showed 96% sensitivity and 100% specificity in detecting HPeV antibodies in previously characterized sera from HPeV 1–positive adults. Antibodies to VP0 and VP1 have been useful for immunostaining of HPeV-infected cells in cell culture. The immunogenic epitope on VP3 is suited for use as an antigen in EIAs to determine seroimmunity. As with other viral infections, demonstration of either HPeV-specific IgM or a fourfold rise in HPeV IgG antibodies in paired acute and convalescent sera collected 3 to 4 weeks apart is considered indicative of acute infection. Currently available serologic assays cannot differentiate antibodies elicited by infection with different HPeV serotypes and, therefore, are unable to determine HPeV type-specific infection.
Prevention and control
No vaccine is currently available for the prevention of HPeV disease. The HPeVs are spread primarily by the lack of adequate hand hygiene. Hand washing prevents their spread because their major mode of transmission is via the fecal–oral route. During nursery outbreaks, cohorting infected neonates may be effective in limiting the outbreak. For patients hospitalized with HPeV-related syndromes, infection control measures similar to those for the enteroviruses, consisting of standard precautions, are sufficient [91].
Summary
The HPeVs have emerged as increasingly recognized causes of several important clinical syndromes in neonates and young infants. Understanding of their epidemiology and the ability to diagnose infections caused by these agents have been greatly improved through the development of NAATs. Although much has been learned, additional well-designed studies are necessary to better define the epidemiology and clinical syndromes associated with the HPeV and their contribution to the burden of childhood disease.
References
[1] R. Wigand, A.B. Sabin. Properties of ECHO types 22, 23 and 24 viruses. Arch Gesamte Virusforsch. 1961;11:224-247.
[2] Enteric cytopathogenic human orphan (ECHO) viruses. Science. 1955;122:1187-1188.
[3] N.J. Knowles, T. Hovi, A.M.Q. King, et al. Overview of taxonomy. In: E. Ehrenfeld, E. Domingo, R.P. Roos, editors. The picornaviruses. Washington, DC: American Society for Microbiology Press; 2010:19-32.
[4] Committee on the enteroviruses. The enteroviruses. Am J Public Health Nations Health. 1957;47:1556-1566.
[5] Committee on enteroviruses. Classification of human enteroviruses. Virology. 1962;16:501-504.
[6] D.N. Shaver, A.L. Barron, D.T. Karzon. Distinctive cytopathology of ECHO viruses type 22 and 23. Proc Soc Exp Biol Med. 1961;106:648-652.
[7] H.A. Rotbart, M.J. Levin, L. Villareal. Use of subgenomic poliovirus DNA hybridization probes to detect the major subgroup of enteroviruses. J Clin Microbiol. 1984;20:1105-1108.
[8] B.A. Coller, N.M. Chapman, M.A. Beck, et al. Echovirus 22 is an atypical enterovirus. J Virol. 1990;64:2692-2701.
[9] B.G. Coller, S.M. Tracy, D. Etchison. Cap-binding complex protein p220 is not cleaved during echovirus 22 replication in HeLa cells. J Virol. 1991;65:3903-3905.
[10] T. Hyypia, C. Horsnell, M. Maaronen, et al. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci U S A. 1992;89:8847-8851.
[11] M.S. Oberste, K. Maher, M.A. Pallansch. Complete sequence of echovirus 23 and its relationship to echovirus 22 and other human enteroviruses. Virus Res. 1998;56:217-223.
[12] F. Ghazi, P.J. Hughes, T. Hyypia, et al. Molecular analysis of human parechovirus type 2 (formerly echovirus 23). J Gen Virol. 1998;79:2641-2650.
[13] P. Auvinen, G. Stanway, T. Hyypia. Genetic diversity of enterovirus subgroups. Arch Virol. 1989;104:175-186.
[14] Picornaviridae study group. Human parechoviruses. Available at: http://www.picornastudygroup.com/types/parechovirus/hpev.htm Accessed April 13, 2011
[15] R.M. Jamison. Morphology of echovirus 22. J Virol. 1969;4:904-906.
[16] M. Ito, T. Yamashita, H. Tsuzuki, et al. Isolation and identification of a novel human parechovirus. J Gen Virol. 2004;85:391-398.
[17] Y. Abed, G. Boivin. Molecular characterization of a Canadian human parechovirus (HPeV)-3 isolate and its relationship to other HPeVs. J Med Virol. 2005;77:566-570.
[18] M. Al-Sunaidi, C.H. Williams, P.J. Hughes, et al. Analysis of a new human parechovirus allows the definition of parechovirus types and the identification of RNA structural domains. J Virol. 2007;81:1013-1021.
[19] K. Watanabe, M. Oie, M. Higuchi, et al. Isolation and characterization of novel human parechovirus from clinical samples. Emerg Infect Dis. 2007;13:889-895.
[20] VR. Racaniello. Picornaviridae: the viruses and their replication. In: D.M. Knipe, P.M. Howley, editors. Field’s virology. Philadelphia: Lippincott, Williams & Wilkins; 2007:795-838.
[21] L.A. Seal, R.M. Jamison. Evidence for secondary structure within the virion RNA of echovirus 22. J Virol. 1984;50:641-644.
[22] A.S. Nateri, P.J. Hughes, G. Stanway. In vivo and in vitro identification of structural and sequence elements of the human parechovirus 5’ untranslated region required for internal initiation. J Virol. 2000;74:6269-6277.
[23] G. Stanway, N. Kalkkinen, M. Roivainen, et al. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J Virol. 1994;68:8232-8238.
[24] J. Seitsonen, P. Susi, O. Heikkilä, et al. Interaction of alphaVbeta3 and alphaVbeta6 integrins with human parechovirus 1. J Virol. 2010;84:8509-8519.
[25] M.S. Oberste, W.A. Nix, K. Maher, et al. Improved molecular identification of enteroviruses by RT-PCR and amplicon sequencing. J Clin Virol. 2003;26:375-377.
[26] W.A. Nix, K. Maher, M.A. Pallansch, et al. Parechovirus typing in clinical specimens by nested or semi-nested PCR coupled with sequencing. J Clin Virol. 2010;48:202-207.
[27] Y. Abed, G. Boivin. Human parechovirus infections in Canada. Emerg Infect Dis. 2006;12:969-975.
[28] T. Schultheiss, S.U. Emerson, R.H. Purcell, et al. Polyprotein processing in echovirus 22: a first assessment. Biochem Biophys Res Commun. 1995;217:1120-1127.
[29] E. Martinez-Salas, M.D. Ryan. Translation and protein processing. In: E. Ehrenfeld, E. Domingo, R.P. Roos, editors. The picornaviruses. Washington, DC: American Society for Microbiology Press; 2010:141-161.
[30] O. Samuilova, C. Krogerus, T. Pöyry, et al. Specific interaction between human parechovirus nonstructural 2A protein and viral RNA. Biol Chem. 2004;279:37822-37831.
[31] C. Krogerus, D. Egger, O. Samuilova, et al. Replication complex of human parechovirus 1. J Virol. 2003;77:8512-8523.
[32] C. Krogerus, O. Samuilova, T. Pöyry, et al. Intracellular localization and effects of individually expressed human parechovirus 1 non-structural proteins. J Gen Virol. 2007;88:831-841.
[33] J.P. Figueroa, D. Ashley, D. King, et al. An outbreak of acute flaccid paralysis in Jamaica associated with echovirus type 22. J Med Virol. 1989;29:315-319.
[34] A. Ehrnst, M. Eriksson. Echovirus 23 Observed as a nosocomial infection in infants. Scand J Infect Dis. 1996;28:205-206.
[35] E. Birenbaum, R. Handsher, J. Kuint, et al. Echovirus type 22 outbreak associated with gastro-intestinal disease in a neonatal intensive care unit. Am J Perinatol. 1997;14:469-473.
[36] P. Joki-Korpela, T. Hyypiä. Diagnosis and epidemiology of echovirus 22 infections. Clin Infect Dis. 1998;27:129-136.
[37] N. Khetsuriani, A. Lamonte-Fowlkes, S. Oberste, et al. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ. 2006;55:1-20.
[38] G. Tapia, O. Cinek, E. Witsø, et al. Longitudinal observation of parechovirus in stool samples from Norwegian infants. J Med Virol. 2008;80:1835-1842.
[39] T.L. Shan, W. Guo, L. Cui, et al. The first detection of human parechovirus infections in China. J Clin Virol. 2009;45:371-372.
[40] H. Harvala, I. Robertson, T. Chieochansin, et al. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as identified by direct typing of cerebrospinal fluid samples. J Infect Dis. 2009;199:1753-1760.
[41] J.F. Drexler, K. Grywna, A. Stöcker, et al. Novel human parechovirus from Brazil. Emerg Infect Dis. 2009;15:310-313.
[42] M. Ito, T. Yamashita, H. Tsuzuki, et al. Detection of human parechoviruses from clinical stool samples in Aichi, Japan. J Clin Microbiol. 2010;48:2683-2688.
[43] A. Boros, M. Uj, P. Pankovics, et al. Detection and characterization of human parechoviruses in archived cell cultures, in Hungary. J Clin Virol. 2010;47:379-381.
[44] N.T.K. Pham, Q.D. Trinh, P. Khamrin, et al. Diversity of human parechoviruses isolated from stool samples collected from Thai children with acute gastroenteritis. J Clin Microbiol. 2010;48:115-119.
[45] S. Ljubin-Sternak, E. Juretić, M. Šantak, et al. Clinical and molecular characterization of a parechovirus type 1 outbreak in neonates in Croatia. J Med Virol. 2011;83:137-141.
[46] Centers for Disease Control and Prevention. Nonpolio enterovirus and human parechovirus surveillance—United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2010;59:1577-1580.
[47] N. Khetsuriani, A. Lamonte, M.S. Oberste, et al. Neonatal enterovirus infections reported to the national enterovirus surveillance system in the United States, 1983–2003. Pediatr Infect Dis J. 2006;25:889-893.
[48] N.R. Grist, E.J. Bell, F. Assaad. Enteroviruses in human disease. Prog Med Virol. 1978;24:114-157.
[49] S. Takao, Y. Shimazu, S. Fukuda, et al. Seroepidemiological study of human parechovirus 1. Jpn J Infect Dis. 2001;54:85-87.
[50] S. Tauriainen, M. Martiskainen, S. Oikarinen, et al. Human parechovirus 1 infections in young children–no association with type 1 diabetes. J Med Virol. 2007;79:457-462.
[51] Y. Abed, D. Wolf, R. Dagan, et al. Development of a serological assay based on a synthetic peptide selected from the VP0 capsid protein for detection of human parechoviruses. J Clin Microbiol. 2007;45:2037-2039.
[52] G. Sedmak, W.A. Nix, J. Jentzen, et al. Infant deaths associated with human parechovirus infection in Wisconsin. Clin Infect Dis. 2010;50:357-361.
[53] K.S. Benschop, J. Schinkel, R.P. Minnaar, et al. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin Infect Dis. 2006;42:204-210.
[54] H. Harvala, I. Robertson, E.C. McWilliam Leitch, et al. Epidemiology and clinical associations of human parechovirus respiratory infections. J Clin Microbiol. 2008;46:3446-3453.
[55] S. Baumgarte, L. Kleber de Souza Luna, K. Grywna, et al. Prevalence, types, and RNA concentrations of human parechoviruses, including a sixth parechovirus type, in stool samples from patients with acute enteritis. J Clin Microbiol. 2008;46:242-248.
[56] M.A. Verboon-Maciolek, T.G. Krediet, L.J. Gerards, et al. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008;27:241-245.
[57] K.C. Wolthers, K.S. Benschop, J. Schinkel, et al. Human parechoviruses as an important viral cause of sepsis like illness and meningitis in young children. Clin Infect Dis. 2008;47:358-363.
[58] M. Yamamoto, K. Abe, K. Kuniyori, et al. Epidemic of human parechovirus type 3 in Hiroshima City, Japan in 2008. Jpn J Infect Dis. 2009;62:244-245.
[59] L. Piñeiro, D. Vicente, M. Montes, et al. Human parechovirus in infants with systemic infection. J Med Virol. 2010;82:1790-1796.
[60] N.T. Pham, S. Takanashi, D.N. Tran, et al. Human parechovirus infection in children hospitalized with acute gastroenteritis in Sri Lanka. J Clin Microbiol. 2011;49:364-366.
[61] R. Selvarangan, M. Nzabi, S.B. Selvaraju, et al. Human parechovirus 3 causing sepsis-like illness in children from Midwestern United States. Pediatr Infect Dis. 2011;30(3):238-242.
[62] S. van der Sanden, E. de Bruin, H. Vennema, et al. Prevalence of human parechovirus in The Netherlands in 2000 to 2007. J Clin Virol. 2008;46:2884-2889.
[63] N.T. Pham, W. Chan-It, P. Khamrin, et al. Detection of human parechovirus in stool samples collected from children with acute gastroenteritis in Japan during 2007–2008. J Med Virol. 2011;83:331-336.
[64] S. Berkovich, J. Pangan. Recoveries of virus form premature outbreaks of respiratory disease: the relation of ECHO virus type 22 to disease of the upper and lower respiratory tract in the premature infant. Bull N Y Acad Med. 1968;44:377-387.
[65] M. Barbi Guidotti, D. Cappellini, C.C. Pedretti, et al. Spread of echovirus 23 in a neonatal pathology unit. Ann Sclavo. 1982;24:76-84.
[66] D. Pajkrt, K.S. Benschop, B. Westerhuis, et al. Clinical characteristics of human parechoviruses 4–6 infections in young children. Pediatr Infect Dis J. 2009;28:1008-1010.
[67] M. Koskiniemi, R. Paetau, K. Linnavuori. Severe encephalitis associated with disseminated echovirus 22 infection. Scand J Infect Dis. 1989;21:463-466.
[68] M.A. Verboon-Maciolek, F. Groenendaal, C.D. Hahn, et al. Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol. 2008;64:266-273.
[69] R.E. Levorson, B.A. Jantausch, B.L. Wiedermann, et al. Human parechovirus-3 infection: emerging pathogen in neonatal sepsis. Pediatr Infect Dis J. 2009;28:545-547.
[70] G. Boivin, Y. Abed, F.D. Boucher. Human parechovirus 3 and neonatal infections. Emerg Infect Dis. 2005;11:103-107.
[71] V. Legay, J.J. Chomel, E. Fernandez, et al. Encephalomyelitis due to human parechovirus type 1. J Clin Virol. 2002;25:193-195.
[72] J.A. Jenista, K.R. Powell, M.A. Menegus. Epidemiology of neonatal enterovirus infection. J Pediatr. 1984;104:685-690.
[73] J. Modlin. Update on enterovirus infections in infants and children. Adv Pediatr Infect Dis. 1996;12:155-180.
[74] K.S. Benschop, J. Schinkel, M.E. Luken, et al. Fourth human parechovirus serotype. Emerg Infect Dis. 2006;12:1572-1575.
[75] M. Abzug. Presentation, diagnosis, and management of enterovirus infections in neonates. Paediatr Drugs. 2004;6:1-10.
[76] H.M. Maller, D.F. Powars, R.E. Horowitz, et al. Fatal myocarditis associated with ECHO virus, type 22, infection, in a child with apparent immunological deficiency. J Pediatr. 1967;71:204-210.
[77] D. Schnurr, M. Dondero, D. Holland, et al. Characterization of echovirus 22 variants. Arch Virol. 1996;141:1749-1756.
[78] S. O’Regan, P. Robitaille, J.G. Mongeau, et al. The hemolytic uremic syndrome associated with ECHO 22 infection. Clin Pediatr (Phila). 1980;19:125-127.
[79] S. Tauriainen, S. Oikarinen, K. Taimen, et al. Temporal relationship between human parechovirus 1 infection and otitis media in young children. J Infect Dis. 2008;198:35-40.
[80] M.S. Oberste, K. Maher, M.A. Pallansch. Specific detection of echoviruses 22 and 23 in cell culture supernatants by RT-PCR. J Med Virol. 1999;58:178-181.
[81] V. Legay, Chomel, B. Lina. Specific RT-PCR procedure for the detection of human parechovirus type 1 genome in clinical samples. J Virol Methods. 2002;102:157-160.
[82] P. Jokela, P. Joki-Korpela, M. Maaronen, et al. Detection of human picornaviruses by multiplex reverse transcription-PCR and liquid hybridization. J Clin Microbiol. 2005;43:1239-1245.
[83] C.E. Corless, M. Guiver, R. Borrow, et al. Development and evaluation of a ’real-time’ RT-PCR for the detection of enterovirus and parechovirus RNA in CSF and throat swab samples. J Med Virol. 2002;67:555-562.
[84] K. Benschop, R. Molenkamp, A. van der Ham, et al. Rapid detection of human parechoviruses in clinical samples by real-time PCR. J Clin Virol. 2008;41:69-74.
[85] W.A. Nix, K. Maher, E.S. Johansson, et al. Detection of all known parechoviruses by real-time PCR. J Clin Microbiol. 2008;46:2519-2524.
[86] K. Benschop, R. Minnaar, G. Koen, et al. Detection of human enterovirus and human parechovirus (HPeV) genotypes from clinical stool samples: polymerase chain reaction and direct molecular typing, culture characteristics, and serotyping. Diagn Microbiol Infect Dis. 2010;68:166-173.
[87] G.T. Noordhoek, J.F. Weel, E. Poelstra, et al. Clinical validation of a new real-time PCR assay for detection of enteroviruses and parechoviruses, and implications for diagnostic procedures. J Clin Virol. 2008;41:75-80.
[88] M.L. Landry. The molecular diagnosis of parechovirus infection: has the time come? Clin Infect Dis. 2010;50:362-363.
[89] M. de Vries, K. Pyrc, R. Berkhout, et al. Human parechovirus type 1, 3, 4, 5, and 6 detection in picornavirus cultures. J Clin Microbiol. 2008;46:759-762.
[90] A. Alho, J. Marttila, J. Ilonen, et al. Diagnostic potential of parechovirus capsid proteins. J Clin Microbiol. 2003;41:2294-2299.
[91] J.D. Siegel, E. Rhinehart, M. Jackson, et al. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings 2007. Available at: www.cdc.gov/hicpac/pub.html Accessed April 13, 2010