Chapter 258 Human Papillomaviruses
Epidemiology
The most common manifestation of HPV is latent infection, defined by the detection of HPV DNA in the absence of any detectable HPV-associated lesion. Approximately 20% of sexually active adolescents have detectable HPV at any given time and have normal cytologic findings and no detectable lesions. External genital warts are much less common, occurring in <1% of adolescents. The most common clinically detected lesion in adolescent women is the cervical lesion termed low-grade squamous intraepithelial lesion (LSIL) (Table 258-1). This lesion appears to occur in 25-30% of adolescents infected with HPV. LSILs are considered benign cellular changes associated with HPV infection. As with HPV DNA detection, most LSILs regress spontaneously in young women and do not require any intervention or therapy. Less commonly, HPV can induce more severe cellular changes, termed high-grade squamous intraepithelial lesions (HSILs) (Chapter 547).
Table 258-1 BETHESDA SYSTEM FOR REPORTING CERVICAL/VAGINAL CYTOLOGY
| DESCRIPTIVE DIAGNOSIS OF EPITHELIAL CELL ABNORMALITIES | EQUIVALENT TERMINOLOGY |
|---|---|
| SQUAMOUS CELL | |
| Atypical squamous cells of undetermined significance (ASC-US) | Squamous atypia |
| Atypical squamous cells, cannot exclude HSIL (ASC-H) | |
| Low-grade squamous intraepithelial lesion (LSIL) | Mild dysplasia, condylomatous atypia, HPV-related changes, koilocytic atypia, cervical intraepithelial neoplasia (CIN) 1 |
| High-grade squamous intraepithelial lesion (HSIL) | Moderate dysplasia, CIN 2, severe dysplasia, CIN 3, carcinoma in situ |
| GLANDULAR CELL | |
| Endometrial cells, cytologically benign, in a postmenopausal woman | |
| Atypical glandular cells of undetermined significance | |
| Endocervical adenocarcinoma | |
| Endometrial adenocarcinoma | |
| Extrauterine adenocarcinoma | |
| Adenocarcinoma, not otherwise specified | |
Some infants may acquire papillomaviruses during passage through an infected birth canal, leading to recurrent respiratory papillomatosis. Cases also have been reported after cesarean section. The maximum incubation period for emergence of clinically apparent lesions (genital warts or laryngeal papillomas) after perinatally acquired infection is unknown but appears to be 6 mo (Chapter 382.2).
Genital warts appearing in later childhood may result from sexual abuse with HPV transmission during the abusive contact. Genital warts may represent a sexually transmitted infection even in some very young children. Their presence is cause to suspect that possibility. A child with genital warts should therefore be provided with a complete evaluation for evidence of possible abuse (Chapter 37.1), including the presence of other sexually transmitted infections (Chapter 114). Presence of genital warts in a child does not confirm sexual abuse, because perinatally transmitted genital warts may go undetected until the child is older. Typing for specific genital HPV types in children is not helpful in diagnosis or to confirm sexual abuse status, because the same genital types occur in both perinatal transmission and abuse. Nonetheless, the type detected in the infant is not always the same as the mother’s type, suggesting other sources of HPV acquisition.
Clinical Manifestations
The clinical findings in HPV infection depend on the site of epithelial infection.
Skin Lesions
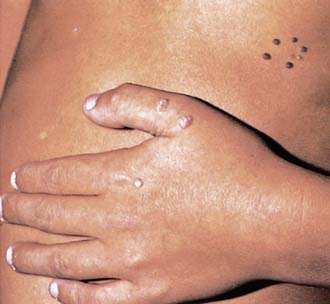
The typical HPV-induced lesions of the skin are proliferative, papular, and hyperkeratotic. Common warts are raised circinate lesions with a keratinized surface (Fig. 258-1). Plantar and palmar warts are practically flat. Multiple warts are common and may create a mosaic pattern. Flat warts appear as small (1-5 mm), flat, flesh-colored papules.
Genital Warts
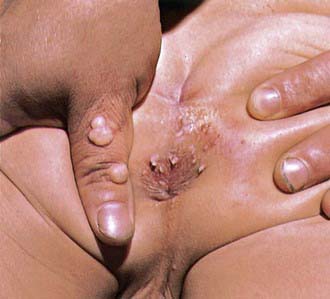
Genital warts may be found throughout the perineum around the anus, vagina, and urethra, as well as in the cervical, intravaginal, and intra-anal areas (Fig. 258-2). Intra-anal warts occur predominantly in patients who have had receptive anal intercourse, in contrast with perianal warts, which may occur in men and women without a history of anal sex. Although rare, lesions caused by genital genotypes can also be found on other mucosal surfaces, such as conjunctivae, gingiva, and nasal mucosa. They may be single or multiple lesions and are frequently found in multiple anatomic sites. External genital warts can be flat, dome-shaped, keratotic, pedunculated, and cauliflower-shaped and may occur singly, in clusters, or as plaques. On mucosal epithelium, the lesions are softer. Depending on the size and anatomic location, lesions may be pruritic and painful, may cause burning with urination, may be friable and bleed, or may become superinfected. Adolescents are frequently disturbed by the development of genital lesions. Other rarer lesions caused by HPV of the external genital area include Bowen disease, bowenoid papulosis, squamous cell carcinomas, Buschke-Löwenstein tumors, and vulvar intraepithelial neoplasias (VINs).
Diagnosis
Screening for cervical cancer begins with cytology, which is either performed by Papanicolaou (Pap) smear or liquid-based cytology. Screening guidelines, which were updated in 2009 by the American Society for Colposcopy and Cervical Pathology (ASCCP) and American College of Obstetrics and Gynecology, recommend to start screening at age 21 yr. Screening earlier is more likely to result in unnecessary referrals for colposcopy, because most lesions in this group are likely to be LSILs and therefore to regress. Annual cytology is recommended until at least 3 normal cytology results are obtained. After this, the recommended frequency interval varies between 1-3 yr. The recommended terminology used for cytologic evaluation is based on the Bethesda system (see Table 258-1). Terminology used for histology is based on the World Health Organization recommendation using cervical intraepithelial neoplasia (CIN) 1, 2, and 3 (see Table 258-1). Although the purpose of screening is to identify CIN 3+ lesions, the majority of these lesions are found in women who were referred for atypical squamous cells of undetermined significance (ASCUS) or LSILs on cytology. On the other hand, few CIN 3 or cancers exist in adolescent populations. Cytologic evaluation of cervical cells is a screening test and not confirmatory. New guidelines often take years before their uptake. If cytology is obtained in an adolescent, the 2006 ASCCP guidelines for management of abnormal cytology and histology should be followed. For adolescents, ASCUS and LSIL are treated the same. The current recommendation for adolescents with ASCUS or LSIL is to repeat cytology every 12 mo for up to 24 mo. For persistent ASCUS or LSILs at 2 yr or 24 mo of follow-up, referral for colposcopy is recommended. Adolescents with HSIL at any visit should be referred for colposcopy and biopsy. In adult women, HSIL can be treated without histologic confirmation. However, this approach should be avoided in adolescents because HSIL is often misdiagnosed in this group.
In adult women older than 21 yr, high-risk HPV testing can be used to assist in ASCUS triage. The latter recommendation was based on the observations that adult women with ASCUS and a positive HPV test result for high-risk types are more likely to have CIN 2/3 than women with a negative HPV test result. HPV testing in adolescents, whether used for ASCUS triage or follow-up, is not recommended because of the high prevalence of HPV. If HPV testing is performed inadvertently, it is recommended to ignore the HPV test result. Once the LSIL is confirmed by histology (i.e., CIN 1), treatment of CIN 1 is not recommended; women can be followed with cytology at 12-mo intervals. An adolescent in whom histology confirms CIN 2/3 or CIN 2 can be followed by colposcopy and cytology at 6-mo intervals if the patient is compliant. If CIN 2/3 continues to persistent at 2 yr of follow-up, treatment is recommended. CIN 3 in adolescents is recommended to be treated as in adults. These guidelines and updates can be found at www.asccp.org.
Treatment
Most common (plantar, palmar, skin) warts eventually resolve spontaneously (Chapter 659). Symptomatic lesions should be removed. Removal includes a variety of self-applied therapies, including salicylic acid preparations and provider-applied therapies (cryotherapy, laser therapy, electrosurgery). Genital warts in children and adolescents are benign and usually remit, but only over an extended period. It is recommended that genital lesions be treated if the patient or the parent requests therapy. As for common warts, treatment is categorized into self-applied and provider-applied. No one therapy has been shown to be more efficacious than any other. Provider-applied therapies include surgical treatments (electrosurgery, surgical excision, laser surgery) and office-based treatment (cryotherapy with liquid nitrogen or a cryoprobe, podophyllin resin 10-25%, and bi- or tri-chloroacetic acid). Office-based treatments are usually applied once a week for 3-6 wk. Podophyllin resins have lost favor to other methods because of the variability in preparations. Intralesional interferon is no more effective than other therapies and is associated with significant adverse effects; this therapy is reserved for treatment of recalcitrant cases.
American Academy of Pediatrics, Committee on Child Abuse and Neglect. Guidelines for the evaluation of sexual abuse of children. Pediatrics. 1991;87:254-260.
Beutner KR, Reitano MV, Richwald GA, et al. External genital warts: report of the American Medical Association consensus conference: AMA expert panel on external genital warts. Clin Infect Dis. 1998;27:796-806.
Bjorge T, Engeland A, Luostarinen T, et al. Human papillomavirus infection as a risk factor for anal and perianal skin cancer in a prospective study. Br J Cancer. 2002;87:61-64.
Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630-631.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
Ho GYF, Birman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-428.
Moore K, Cofer A, Elliot L, et al. Adolescent cervical dysplasia: histologic evaluation, treatment, and outcomes. Am J Obstet Gynecol. 2007;197:141.e1-141.e6.
Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125-1131.
Moscicki AB, Ellenberg JH, Crowley-Nowick P, et al. Risk of high-grade squamous intraepithelial lesion in HIV-infected adolescents. J Infect Dis. 2004;190:1413-1421.
Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995-3002.
Moscicki AB, Schiffman M, Kjaer S, et al. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl 3):S42-S51.
Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277-284.
Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364(9446):1678-1683.
Muñoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161-2170.
Rintala MAM, Grénman SE, Puranen MH, et al. Transmission of high-risk human papillomavirus (HPV) between parents and infant: a prospective study of HPV in families in Finland. J Clin Microbiol. 2005;43:376-381.
Siegfried E, Rasnick-Conley J, Cook S, et al. Human papillomavirus screening in pediatric victims of sexual abuse. Pediatrics. 1998;101:43-47.
Sinclair KA, Woods CR, Kirse DJ, et al. Anogenital and respiratory tract human papillomavirus infections among children: age, gender, and potential transmission through sexual abuse. Pediatrics. 2005;116:815-825.
Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621-632.
Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine. 2006;24:5571-5583.
Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;1(191):731-738.
Wright TCJr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.