Chapter 50 Human Blood Group Antigens and Antibodies
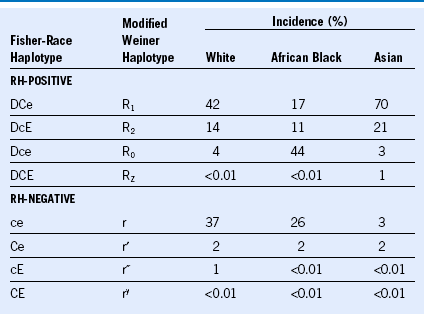
Table 50-1 Blood Group Systems, Antigens, Expression, and Disease Associations
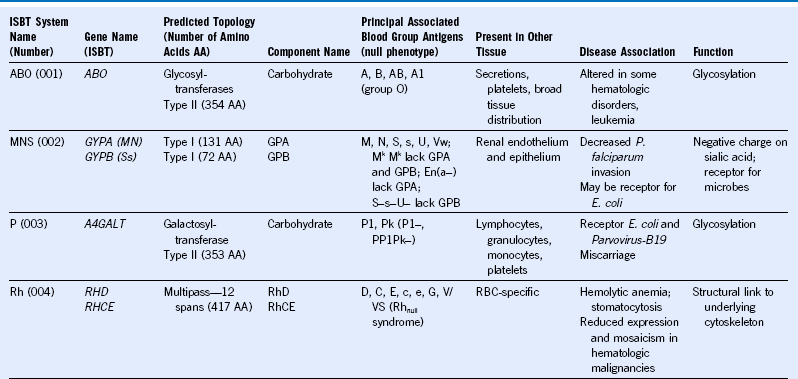
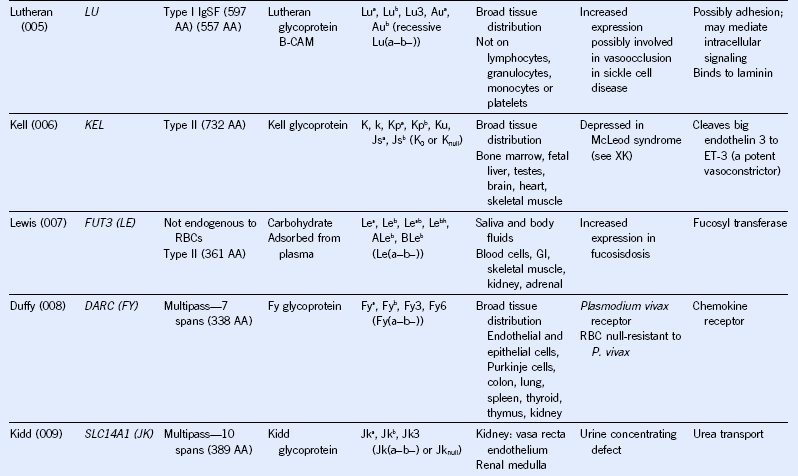
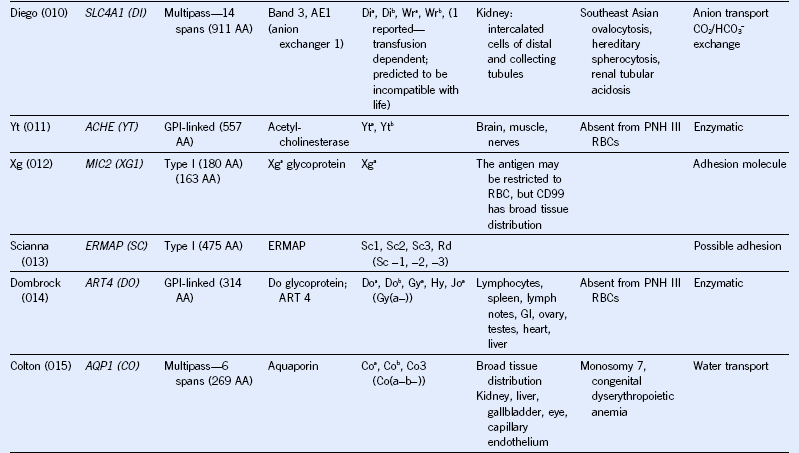
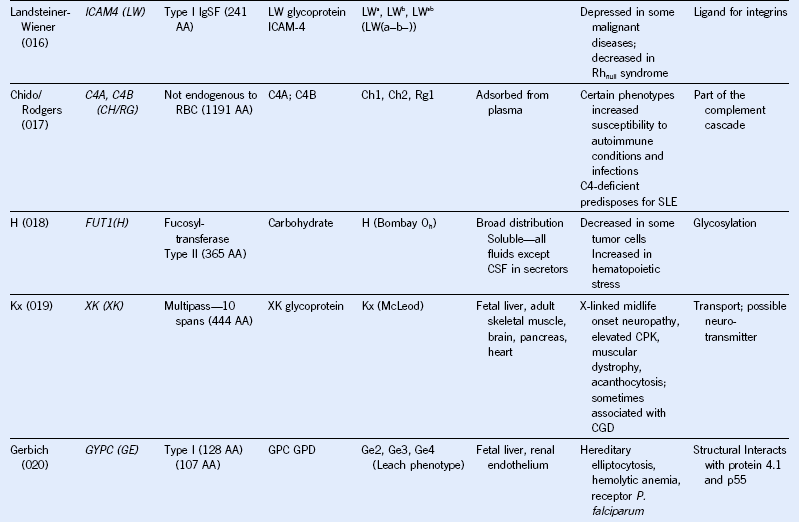
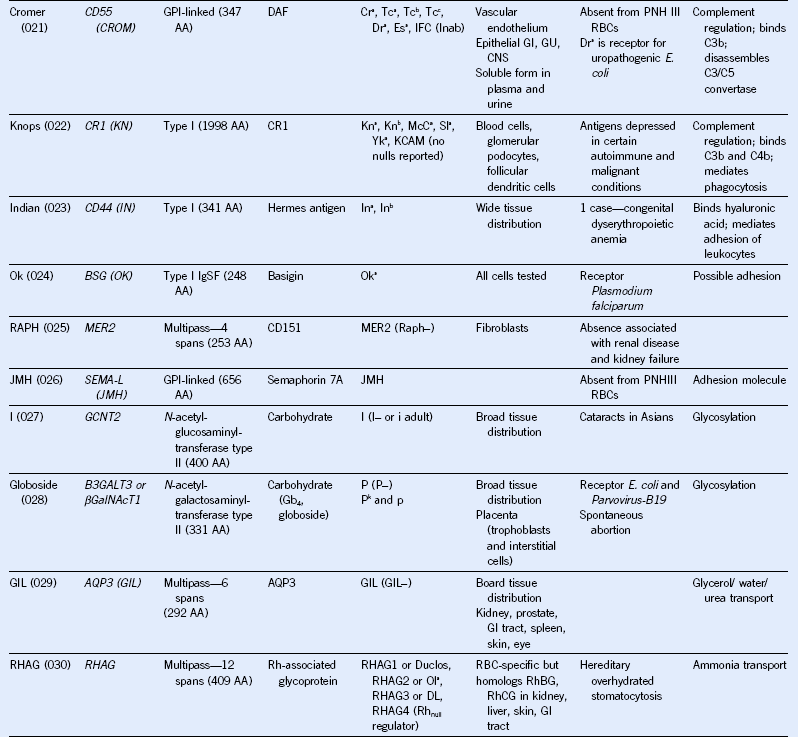
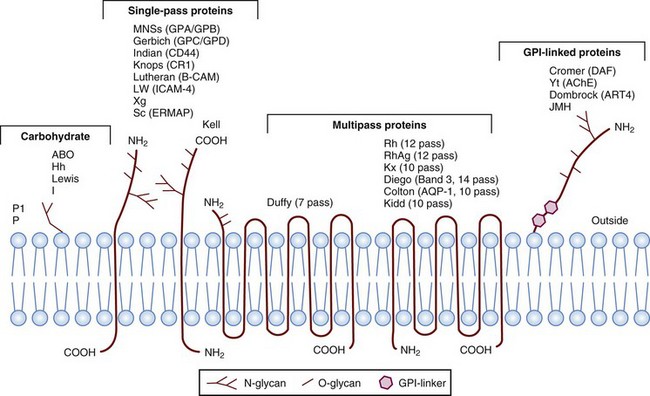
IgSF, Immunoglobulin super family; ISGN, International Society for Gene Nomenclature; type I, protein with a single pass through the RBC lipid bilayer with its amino terminus to the outside of the cell; type II, protein with a single pass through the RBC lipid bilayer with its amino terminus to the inside of the cell.
Table 50-2 Uses of DNA-Based Genotyping Assays for Transfusion Medicine
Indirect Antiglobulin Test and Direct Antiglobulin Test
Table 50-3 Characteristics of Some Blood Group Alloantibodies (Listed in Approximate Order of Clinical Significance)
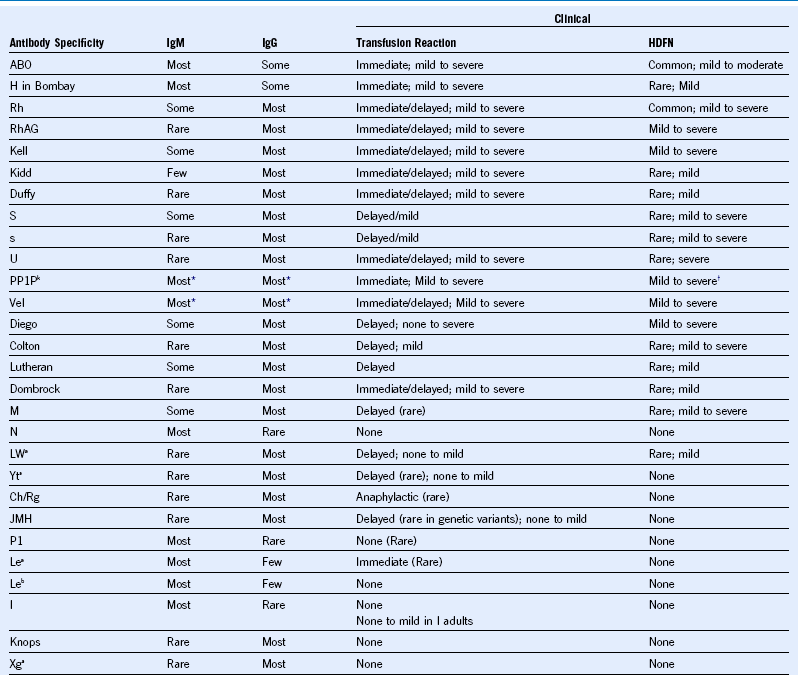
HDFN, Hemolytic disease of the fetus and newborn.
* Most examples of these antibodies are both IgM and IgG.
† Seldom hemolysis of fetal cells but high incidence of recurrent spontaneous abortions.
Rh Immune Globulin
Table 50-4 Approaches to Supplying Red Blood Cell Products to Prevent Alloimmunization in Patients with Sickle Cell Disease or Other Transfusion-Dependent Anemia
Transfusion Management of Patients With Warm Autoimmune Hemolytic Anemia
History: A transfusion history should be obtained to differentiate these results from a hemolytic transfusion reaction or hemolysis due to an alloantibody.
DAT: A direct antiglobulin test should be performed with anti-IgG and -C3. In clinically significant hemolysis, the DAT is usually strongly positive.
Eluate: If patient has been recently transfused (3-4 months), an eluate should be prepared from the patient cells to remove the antibody(ies); the eluate should be tested to determine specificity. The eluate is usually reactive with all cells when tested by the IAT with anti-IgG.
Phenotype: Type the patient’s RBCs for minor blood group antigens (Cc, Ee, K, Jka/b, Fya/b, Ss) if the patient has not been recently transfused. When possible, IgM typing reagents are used because the patient’s own antibody-coated RBCs may result in false-positive typing. Some laboratories are able to remove the IgG from the RBCs and perform a phenotype. Alternatively, genotyping for minor blood group antigens including Doa/b antigens (there is no serologic reagent) can be performed.
Adsorption: Adsorb the serum autoantibody onto the patient’s own RBCs to test for underlying alloantibody if the patient has not been recently transfused (3-4 months). If the patient has been recently transfused or if the low hematocrit results in insufficient autologous RBCs, perform alloadsorption with well-characterized RBCs (usually three with known antigen profiles). Test the adsorbed serum for underlying alloantibodies.
Crossmatch: Perform with neat and with adsorbed plasma. Crossmatch performed with neat plasma will usually be incompatible.
Communication: Inform ordering physician of reactivity and of delay in receiving crossmatched RBCs. Provide emergency-release RBCs if patient’s clinical situation warrants. Inform the physician that the patient may hemolyze transfused RBCs similar to hemolysis of his or her own RBCs.
Transfusion: Consider providing RBC units negative for minor antigens that the patient also lacks (consider matching for Cc, Ee, K, Jka/b, Fya/b, Ss). This potentially enables RBC units to be available before completion of the antibody identification testing. Transfusion with antigen matched units potentially allows continued transfusion without need for auto- or alloadsorption unless signs and symptoms of RBC destruction occur or there is a change in reactivity in antibody screening or the DAT.