Chapter 130 HSCT from Alternative Sources and Donors
Unrelated Donor Transplants
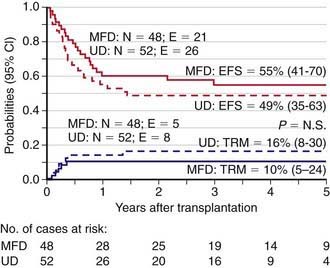
Initially, HLA polymorphism and the limitations of conventional (i.e., serological) HLA-typing techniques unfavorably affected the accuracy of matching, thus increasing rejection rates and the incidence of acute and chronic graft versus host disease (GVHD). Consequently, because the event-free survival of recipients of an unrelated donor allograft was worse than that observed when the donor was a compatible sibling transplant, there was no consensus on the use of unrelated donor transplants for nonmalignant diseases, such as thalassemia. DNA-based (i.e., high-resolution molecular) techniques for HLA typing have revealed an impressive number of new alleles within antigens that were previously defined by serology. Matching by high-resolution DNA typing reduces the risk of immune complications, namely graft rejection and GVHD, but also decreases the chance of finding a suitable donor. Nevertheless, the advent of both high-resolution molecular HLA class I and II loci-typing coupled with progress in the prophylaxis and treatment of GVHD has resulted in a reduction of transplantation-related mortality and improved outcomes. Indeed, outcomes from a fully matched unrelated volunteer donor are now similar to those of HSCT from an HLA-identical sibling, as indicated by results of unrelated donor transplantation in children with acute lymphoblastic leukemia (ALL) in the 2nd complete remission, juvenile myelomonocytic leukemia, or thalassemia (Fig. 130-1).
Umbilical Cord Blood Transplants
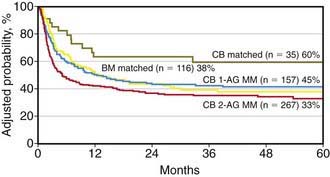
UCB transplantation (UCBT) is a viable option for children who need allogeneic HSCT (Fig. 130-2). Several hundred children have been cured through transplantation with either related or unrelated UCB units. UCBT offers the advantages of absence of risks to donors, reduced risk of transmitting infections, and, for transplants from unrelated donors, immediate availability of cryopreserved cells, with the median time from start of search to transplantation being only 3-4 wk. In comparison to bone marrow transplantation (BMT), advantages of UCBT include lower incidence and severity of GVHD, easier procurement and prompter availability of cord blood cells, and potential to use donors that have HLA disparities with the recipient. Despite these advantages, UCBT patients may be exposed to an increased risk of early fatal complications, mainly due to a lower engraftment rate of donor hematopoiesis, delayed kinetics of neutrophil recovery, and lack of adoptive transfer of pathogen-specific memory T-cells. Transfer of donor-derived, memory T cells significantly contributes to early immunologic reconstitution of children after unmanipulated allogeneic bone marrow or peripheral blood stem cell transplantation.
Donor Versus Recipient NK-Cell Alloreactivity
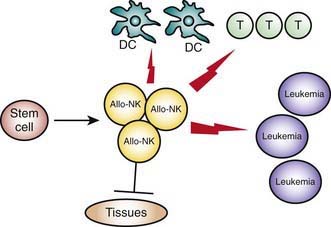
Haplo-HSCT trials demonstrate that MHC class I mismatches, which generate an alloreactive NK-cell response in the graft versus host direction, eradicate leukemia cells, improve engraftment, and protect from T cell–mediated GVHD (Fig. 130-3). Lack of an NK-alloreactive donor is the strongest independent risk factor for leukemia relapse after adjustment for disease status at transplant. The potential for donor versus recipient NK-cell alloreactivity, which can be predicted by standard HLA typing, is recommended when selecting the donor of choice from among the mismatched family members.
Autologous Hematopoietic Stem Cell Transplantation
Autologous HSCT is employed primarily to prevent relapse in patients with AML who achieve complete remission after induction therapy and also for selected children with relapsed lymphomas and selected solid tumors (Table 130-1).
American Academy of Pediatrics Section on Hematology/Oncology and Section on Allergy/Immunology. Cord blood banking for potential future transplantation. Pediatrics. 2007;119:165-170.
Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in 104 patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447-3454.
Ball LM, Bernardo ME, Roelofs H, et al. Co-transplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haplo-identical hematopoietic stem cell transplantation. Blood. 2007;110:2764-2767.
Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematological malignancy. Blood. 2005;105:1343-1347.
Comoli P, Basso S, Zecca M, et al. Preemptive treatment of EBV-related post-transplant lymphoproliferative disorders after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007;7:1648-1655.
Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947-1954.
Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069-2081.
Frassoni F, Gualandi F, Podestà M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831-839.
Gluckman E, Rocha V, Arcese W, et al. on behalf of the Eurocord Group: Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397-407.
Gluckman E, Rocha V, Ionescu I, et al. on behalf of Eurocord-Netcord and EBMT. Results of unrelated cord blood transplant in Fanconi anemia patients: risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant. 2007;13:1073-1082.
Hwang WY, Samuel M, Tan D, et al. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444-453.
Kurtzberg J, Prasad VK, Carter SL, et al. COBLT Steering Committee. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318-4327.
Lee SJ, Klein J, Haagenson M, et al. High resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576-4583.
MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410-2415.
Martin PL, Carter SL, Kernan NA, et al. Results of the Cord Blood Transplantation Study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12:184-194.
Montagna D, Maccario R, Locatelli F, et al. Emergence of anti-tumor cytolytic T cells is associated with maintenance of hematological remission in children with acute myeloid leukemia. Blood. 2006;108:3843-3850.
Moretta A, Locatelli F, Moretta L. Human NK cells: from HLA class I-specific killer Ig-like receptors to the therapy of acute leukemias. Immunol Rev. 2008;224:58-69.
Rocha V, Locatelli F. Searching for alternative donors of haematopoietic stem cell for paediatric patients. Bone Marrow Transplant. 2008;41:207-214.
Ruggeri L, Aversa F, Martelli MF, et al. Haploidentical transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202-218.
Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097-2100.
Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433-440.
Saarinen-Pihkala UM, Gustafsson G, Ringden O, et al. No disadvantages in outcome using matched unrelated donors as compared with matched sibling donors for bone marrow transplantation in children with acute lymphoblastic leukemia in second remission. J Clin Oncol. 2001;19:3406-3414.
Stern M, Ruggeri L, Mancusi A, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990-2995.
Strother D, Ashley D, Kellie SJ, et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiation: results of a collaborative study. J Clin Oncol. 2001;19:2696-2704.
Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111-122.
Velardi A. Role of KIRs and KIR ligands in hematopoietic transplantation. Curr Opin Immunol. 2008;20:581-587.
Wagner JE, Eapen M, MacMillan ML, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109:2256-2262.