Chapter 564 Hormones and Peptides of Calcium Homeostasis and Bone Metabolism
Parathyroid hormone (PTH) and vitamin D are the principal regulators of calcium homeostasis (Chapters 48 and 694). Calcitonin and PTH-related peptide (PTHrP) are important primarily in the fetus.
Parathyroid Hormone
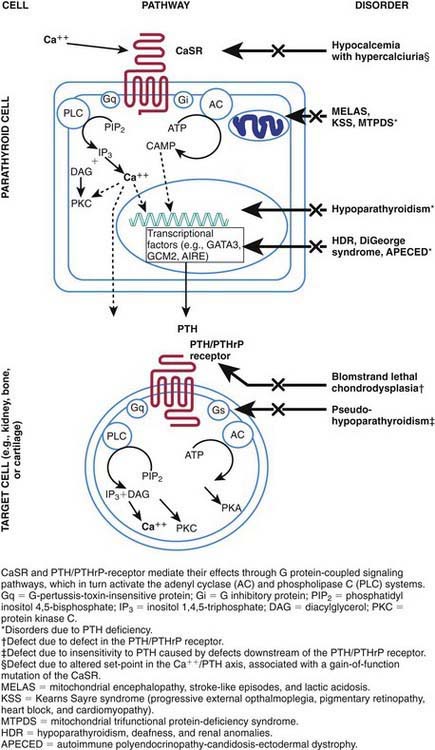
When serum levels of calcium fall, the signal is transduced through the calcium-sensing receptor, and secretion of PTH increases (Fig. 564-1). PTH stimulates activity of 1α-hydroxylase in the kidney, enhancing production of 1,25-dihydroxycholecalciferol, also written 1,25(OH)2D3. The increased level of 1,25(OH)2D3 induces synthesis of a calcium-binding protein (calbindin-D) in the intestinal mucosa, with resultant absorption of calcium. PTH also mobilizes calcium by directly enhancing bone resorption, an effect that requires 1,25(OH)2D3. The effects of PTH on bone and kidney are mediated through binding to specific receptors on the membranes of target cells and through activation of a transduction pathway involving a G-protein coupled to the adenylate cyclase system (Chapter 566).
The calcium-sensing receptor regulates the secretion of PTH and the reabsorption of calcium by the renal tubules in response to alterations in serum calcium concentrations. The gene for the receptor is located on chromosome 3q13.3-q21 and encodes a cell surface protein that is expressed in parathyroid glands and kidneys and belongs to the family of G protein–coupled receptors. In the normally functioning calcium-sensing receptor, hypocalcemia induces increased secretion of PTH and hypercalcemia depresses PTH secretion. Loss-of-function mutations cause an increased set point with respect to serum calcium, resulting in hypercalcemia and in the conditions of familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Acquired hypocalciuric hypercalcemia may be due to autoantibodies to the calcium-sensing receptor and manifests with hypercalcemia and hyperparathyroidism. Gain-of-function mutations result in depressed secretion of PTH in response to hypocalcemia, leading to the syndrome of familial hypocalcemia with hypercalciuria (see Fig. 564-1).
Parathyroid Hormone–Related Peptide
PTHrP, like PTH, activates PTH receptors in kidney and bone cells and increases urinary cyclic adenosine monophosphate (cAMP) and renal production of 1,25(OH)2D3. It is produced in almost every type of cell of the body, including every tissue of the embryo at some stage of development. PTHrP is critical for normal fetal development. Inactivating mutations of the receptor for PTH/PTHrP result in a lethal bone disorder characterized by short limbs and markedly advanced bone maturation known as Blomstrand chondrodysplasia (see Fig. 564-1). PTHrP appears to have a paracrine or autocrine role because serum levels are low except in a few clinical situations. Cord blood contains levels of PTHrP that are 3-fold higher than in serum from adults; it is produced by the fetal parathyroid glands and appears to be the main agent stimulating maternal-fetal calcium transfer. PTHrP appears to be essential for normal skeletal maturation of the fetus, which requires 30 g of calcium during a normal gestation. During pregnancy, maternal absorption of calcium increases from about 150 mg daily to 400 mg during the second trimester.
Ardeshirpour L, Cole DE, Carpenter TO. Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev. 2007;5(Suppl 1):584-598.
Canaff L, Zhou X, Mosesova I, et al. Glial cells missing-2 (GCM2) transactivates the calcium-sensing receptor gene: effect of a dominant-negative GCM2 mutant associated with autosomal dominant hypoparathyroidism. Hum Mutat. 2009;30(1):85-92.
Jacabus CH, Holick MF, Shao G, et al. Hypervitaminosis D associated with drinking milk. N Engl J Med. 1992;326:1173-1177.
Mornet E. Hypophosphatasia: the mutations in the tissue-nonspecific alkaline phosphatase gene. Hum Mutat. 2000;15(4):309-315.
Mitchell DM, Juppner H. Regulation of calcium homeostasis and bone metabolism in the fetus and neonate. Curr Opin Endocrinol Diabetes Obes. 2010;17:25-30.
Stanley S, Shaw NJ. Is it my calcium, doctor? Arch Dis Child Educ Pract Ed. 2009;94:169-176.