Chapter 74 Hodgkin’s Lymphoma
Hodgkin’s lymphoma (HL) was first described by the British physician Thomas Hodgkin in 1832, when he reported six cases of pathologic enlargement of the lymph nodes and spleen at Guy’s Hospital.1 Attempts to treat the disease using various chemical or surgical means were unsuccessful until around the turn of the century, when the effectiveness of x-rays in shrinking the disease was first demonstrated. The crude, low-energy x-ray equipment available at the time allowed only temporary reduction of the enlarged lymph nodes. The development of kilovoltage equipment in the 1920s and the subsequent pioneering work of Gilbert, a Swiss radiotherapist, paved the way for the definitive treatment of patients with Hodgkin’s lymphoma.2 Vera Peters at the Ontario Institute of Radiotherapy first reported the curability of early-stage Hodgkin’s lymphoma in 1950, using high doses of fractionated radiation therapy.3 The availability of modern high-energy radiation therapy equipment in the late 1950s and early 1960s allowed the delivery of higher doses of radiation to deep-seated tumors with fewer limitations caused by reactions in the superficial tissues. The introduction of effective combination chemotherapy further improved the treatment outcome of Hodgkin’s lymphoma, especially in patients with unfavorable prognostic features or advanced-stage disease.4 Over the last 3 decades, there have been continued improvements in radiation therapy techniques allowing more uniform and better targeted dose delivery, the development of more effective and less toxic multiagent chemotherapy regimens, advances in radiographic imaging technology, and the refinement of prognostic factors that allow better tailoring of treatments. Hodgkin’s lymphoma, a previously fatal illness, has now become one of the most curable forms of malignant disease.
Etiology and Epidemiology
Hodgkin’s lymphoma is a relatively uncommon neoplasm, with approximately 8000 new cases in the United States each year, representing less than 1% of all cancer diagnoses.5 The incidence, patient age, and gender distribution of Hodgkin’s lymphoma vary, depending on the geographic location. The age-incidence curve in developed countries is characterized by a bimodal distribution.6,7 There is an initial peak in young adults at around age 25 years and a second peak occurring at age 60 to 70 years, in which a male predominance is observed. The majority of the cases seen in young adulthood are of a nodular sclerosis (NS) histologic type, and many of the factors that have been associated with the development of Hodgkin’s lymphoma in these patients appeared to be a reflection of delayed exposure to infectious agents and/or higher socioeconomic status. These include early birth order, small sibship size, growing up in single-family houses, having few playmates, and having parents with a high level of education.8–10 In contrast, in economically disadvantaged parts of the world, Hodgkin’s lymphoma is relatively rare among young adults.7 The mixed cellularity (MC) subtype is the predominant histologic subtype in developing countries, with an initial peak in childhood for boys and a late peak in older patients.
Several of the epidemiologic and clinical features of the disease are suggestive of infectious causes, and there has been increasing evidence that the Epstein-Barr virus (EBV) may be involved in the pathogenesis of Hodgkin’s lymphoma in at least a subset of cases. Patients with a history of infectious mononucleosis, in which EBV is the causative agent, are at an approximately threefold increased risk for Hodgkin’s lymphoma, and particularly EBV-associated Hodgkin’s lymphoma.7,11,12 Elevated levels of the IgG and IgA immunoglobulins against the EBV capsid antigen have been demonstrated months to years prior to clinical Hodgkin’s lymphoma development.13,14 In about one-third to half of cases of classical Hodgkin’s lymphoma occurring in Western populations, the monoclonal EBV genome can be detected in the Reed-Sternberg cells.15,16 EBV positivity is predominantly associated with the MC subtype,17 which is more common among young children and older adults, and is less frequently associated with NS cases seen mostly in young adults in the developed world.
The observation of familial aggregation of cases of Hodgkin’s lymphoma suggests that genetic susceptibility as well as environmental exposure may contribute to the development of Hodgkin’s lymphoma. A fivefold increased risk has been demonstrated in first-degree relatives, and siblings of young adults with Hodgkin’s lymphoma have a sevenfold increased risk.18 The excess risk appears to be more pronounced in same-sex siblings, which may be related to more shared environmental exposure.19 In a twin study of young adults with Hodgkin’s lymphoma, monozygotic twins of patients had an almost 100-fold increased risk,20 whereas no increased risk for dizygotic twins was observed, supporting the contribution of heritable factors to the development of the disease. In particular, follow-up twin studies have suggested that persons with genetically determined lower interleukin-6 (IL-6) levels may be less susceptible to young adult Hodgkin’s lymphoma.21 In addition, a genome screen of families at high risk for Hodgkin’s lymphoma has provided evidence for a susceptibility gene on several chromosomes, particularly chromosome 4.22 Finally, a number of specific human leukocyte antigen (HLA) haplotypes have been identified to be associated with an increased risk of Hodgkin’s lymphoma.22–24 Because the immune response is genetically determined by the HLA type, patients with these HLA haplotypes may have increased susceptibility to certain infections, which in turn may lead to the increased Hodgkin’s lymphoma risk as well as increased susceptibility to autoimmune conditions. In fact, an elevated risk of Hodgkin’s lymphoma has been found in patients with personal histories of several autoimmune conditions.25
Biologic Characteristics and Molecular Biology
The malignant cells in classical Hodgkin’s lymphoma, the Hodgkin/Reed-Sternberg (HRS) cells, are large, uninucleated or multinucleated cells that usually make up only 1% of the cells present in the tissue sample, with most of the tumor consisting of a variety of non-neoplastic inflammatory cells and fibrosis. Results of molecular single-cell studies have shown that in over 90% of cases, HRS cells have monoclonal immunoglobulin gene rearrangements that are characteristic of mature B lymphocytes, and somatically mutated VH genes that are specific markers for germinal center B cells and their descendants, supporting a germinal center or postgerminal center B-cell origin.25,26 Unlike normal B cells that have undergone successful maturation through the germinal cells, however, HRS cells characteristically show an absence of immunoglobulin gene expression. This has been attributed to mutations in the coding or regulatory regions,27 lack of expression of transcription factors that are responsible for activation of the promoters and enhancers,28 epigenetic silencing of the immunoglobulin heavy-chain transcription,29 and constitutive expression of Notch1 and STAT5.30,31 Despite their inability to express immunoglobulin receptors, HRS cells are resistant to apoptosis, which normally removes immunoglobulin-negative B cells that have traversed the germinal center.32
There is increasing evidence connecting the prevention of apoptosis and survival of HRS cells to the activation of the nuclear factor kappa B (NFκB) transcription factor-signaling pathway. Constitutive NFκB is required for proliferation and survival of Hodgkin’s lymphoma tumor cells.28 The cause of the constitutive activation of NFκB is probably multifactorial and may include amplification of the REL gene,33 mutations in NFκB inhibitors,34 and somatic mutations in the novel tumor suppressor gene TNFAIP3.35 There is an inverse relationship between EBV infection and inactivation of A20, the protein encoded by the TNFAIP3 gene, suggesting that they may represent alternative pathways of pathogenesis.35
Molecular profiling experiments using Hodgkin’s lymphoma-derived cell lines with suppressed and unsuppressed NFκB activity have been performed in order to better understand the NFκB signaling pathway and to identify its target genes. One of the regulators of apoptosis that is expressed in dependence of NFκB is cIAP2, a direct inhibitor of caspase 3, suggesting that HRS cells are protected from caspase 3-induced apoptosis by cIAP2.29 Another NFκB-dependent regulator of apoptosis is CD95, which has been shown to be up-regulated in HRS cells. Unlike the other NFκB-dependent regulators, however, CD95 is known to trigger apoptosis rather than prevent it. The resistance of HRS cells to CD95-mediated apoptotic cell death may be due to functional inhibition of death receptor pathways by cellular FADD-like IL1B-converting enzyme inhibitory proteins (c-FLIP), which is one of the most strongly NFκB-regulated genes.30 The contribution of NFκB signaling to the development of Hodgkin’s lymphoma is further supported by epidemiologic data showing that regular aspirin use is associated with a reduced risk of developing Hodgkin’s lymphoma, presumably through inhibition of NFκB transcription.31 Continued efforts in the elucidation of the NFκB pathway in the pathogenesis of Hodgkin’s lymphoma may have important therapeutic implications through pharmacologic down-regulation of NFκB activity and its target genes, and increasing the susceptibility of HRS cells to apoptosis. However, bortezomib, a proteasome inhibitor that inhibits NFκB activity, does not appear to be clinically active against Hodgkin’s lymphoma.
Pathology
Since the 1930s, a number of pathologic classification systems for Hodgkin’s lymphoma have been developed. The Rye classification system, introduced at a conference in Rye, New York, in 1966, divided cases of Hodgkin’s lymphoma into lymphocyte predominance (LP), nodular sclerosis (NS), mixed cellularity (MC), and lymphocyte depletion (LD) disease.32 This system was widely adopted in the next 25 years and was subsequently modified in the revised European-American classification of malignant lymphomas (REAL) and later in the World Health Organization (WHO) classification33,34 (Table 74-1). In the current classification system, HL is specifically recognized as a lymphoma. Based on morphologic, immunophenotypical, and clinical characteristics, it is divided into two distinct entities: classical Hodgkin’s lymphoma and nodular lymphocyte predominant Hodgkin’s lymphoma (NLPHD). Table 74-2 compares the morphologic and immunophenotypical features of the malignant cells of these two entities. The diagnosis of HL is based on morphologic assessment with identification of HRS cells or their variants, along with immunohistochemical studies.
TABLE 74-1 The WHO Histologic Classification of Hodgkin’s Lymphoma34
TABLE 74-2 Comparison of Morphologic and Immunophenotypical Characteristics of Malignant Cells of Classical Hodgkin’s Disease (HD) and Nodular Lymphocyte Predominance Hodgkin’s Disease (NLPHD)
| Classical HD (HRS Cells) | NLPHD (L/H Cells) | |
|---|---|---|
| Nuclei | Mononucleated and multinucleated, monolobulated and multilobulated | Mononucleated, multilobulated |
| Nucleoli | Large | Multiple, small |
| CD30 | Positive | Negative |
| CD15 | Positive in majority of cases | Negative |
| CD45 | Negative | Positive |
| CD20 | Negative in majority of cases | Positive |
| CD79a | Negative in majority of cases | Positive |
Classical Hodgkin’s Lymphoma
The NS subtype accounts for approximately 70% of classical Hodgkin’s lymphomas, affecting predominantly young adults. Morphologically, it is characterized by the presence of one or more sclerotic bands radiating from a thickened lymph node capsule. The British National Lymphomas Investigation subclassified NS Hodgkin’s lymphoma into two grades based on the percentage of nodules showing lymphocyte depletion or increased number of anaplastic-appearing HRS cells.35,36 The prognostic value of this grading system with modern therapy is unclear, however.
The MC subtype is more frequently seen in developing countries, accounting for over half of the cases, whereas in the more developed parts of the world, it makes up approximately 25% of classical Hodgkin’s lymphoma cases. Morphologically, HRS cells are seen scattered in a diffuse inflammatory background with the absence of nodular sclerosing fibrosis. In contrast to the NS and lymphocyte-rich classic Hodgkin’s disease (LRCHD), EBV positivity is much more frequent in the MC subtype.17
LRCHD accounts for approximately 5% of classic Hodgkin’s lymphoma cases and has a male predominance and older median age at presentation.33 It is characterized by a background infiltrate of small, mature, predominantly B lymphocytes with rare HRS cells and variants. It can resemble NLPHD morphologically, and immunohistochemical studies of the malignant cells are essential to make the distinction. The prognosis may be slightly better than for other subtypes of classic Hodgkin’s disease.33
The lymphocyte depletion subtype has an increased number of HRS cells and is depleted in lymphocytes. Many cases that were previously classified as lymphocyte depletion Hodgkin’s lymphoma are now determined to be anaplastic or large-cell non-Hodgkin’s lymphoma based on immunohistochemical studies.37 Reliable clinical data on this subtype are limited given its rarity and the uncertainty concerning its diagnosis.
Nodular Lymphocyte Predominance Hodgkin’s Lymphoma
NLPHD makes up 5% of Hodgkin’s lymphoma cases. The malignant cells of NLPHD are the lymphocytic-predominant (LP) cells, which are also known as popcorn cells due to their characteristic appearance. The pathogenesis of LP cells is probably distinct from that of HRS cells but probably shares constitutive NFκB activity.38 Unlike classic Hodgkin’s lymphoma, the neoplastic cells of NLPHD typically lack the expression of CD15 and CD30 markers but are consistently positive for CD20 and CD45.38 A nodular pattern is seen, usually completely or partially replacing the lymph node. The nodules tend to be large and closely packed, and L&H cells are typically seen within or around the nodules. There are usually large numbers of CD57-positive small lymphocytes in the nodules, often with ringing around the L&H cells. In about 3% to 5% of cases, transformation to diffuse large B-cell lymphoma may occur.
Clinical Manifestations, Patient Evaluation, and Staging
Clinical Presentation
Patients may also present exclusively with constitutional symptoms in the absence of any physical findings. These symptoms, also known as B symptoms, include fever, unexplained weight loss, and drenching night sweats. Severe, generalized pruritus, not classified as a B symptom, is noted in about 10% to 15% of patients and has been associated with a poorer prognosis.39 Alcohol-induced pain, typically at the site of lymphadenopathy or bony involvement, can be a presenting symptom in some patients.40
Staging System
The Ann Arbor staging classification (Table 74-3), developed in 1971, is a four-stage system formulated to provide prognostic information and to guide therapeutic decisions. It does not reflect other important prognostic factors such as bulky disease or multiple sites of involvement, however.41 The availability of improved imaging techniques has also changed its applicability. In 1988, a meeting was held in the Cotswolds, England, where revisions to the Ann Arbor staging system were made42 (Table 74-4). The following main changes were made: (1) The use of computed tomography (CT) scanning is allowed to assess disease involvement below the diaphragm. (2) For stage II disease, the number of anatomic nodal sites is indicated by a subscript (e.g., stage II3). (3) For stage III disease, upper and lower abdominal involvement was subdivided as III1 and III2, respectively. (4) Bulky disease is denoted by X, defined as more than one-third widening of the mediastinum at the T5 to T6 level or more than 10 cm maximum dimension of the nodal mass. (5) Unconfirmed/uncertain complete remission (CRu) was introduced to denote the presence of a residual imaging abnormality but the absence of pathologically confirmed residual disease.
TABLE 74-3 Ann Arbor Staging Classification for Hodgkin’s Lymphoma
| Stage | Definitions |
|---|---|
| Stage I | Involvement of single lymph node region (I) or of single extralymphatic organ or site (IE) |
| Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm alone (II) or with involvement of limited, contiguous extralymphatic organ or tissue (IIE) |
| Stage III | Involvement of lymph node regions on both sides of the diaphragm (III), which may include the spleen (IIIS) or limited, contiguous extralymphatic organ or site (IIIE), or both (IIISE) |
| Stage IV | Diffuse or disseminated foci of involvement of one or more extralymphatic organs or tissues, with or without associated lymphatic involvement |
The absence or presence of fever, night sweats, and or unexplained weight loss of 10% or more of body weight in the 6 months preceding admission are to be denoted in all cases by the suffix letters A or B, respectively. The clinical stage (CS) denotes the stage as determined by all diagnostic examinations and a single biopsy only. If a second biopsy of any kind has been obtained, whether negative or positive, the term pathologic stage (PS) is used.
TABLE 74-4 The Cotswolds Staging Classification for Hodgkin’s Lymphoma
| Stage | Definitions |
|---|---|
| Stage I | Involvement of a single lymph node region or lymphoid structure (e.g., spleen, thymus, Waldeyer’s ring) or involvement of a single extralymphatic site (IE) |
| Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm (hilar nodes, when involved on both sides, constitute stage II disease); localized contiguous involvement of only one extranodal organ or site and lymph node region(s) on the same side of the diaphragm (IIE) The number of anatomic regions involved should be indicated by a suffix (e.g., II3) |
| Stage III | Involvement of lymph node regions on both sides of the diaphragm (III), which may also be accompanied by involvement of the spleen (IIIS) or by localized contiguous involvement of only one extranodal organ site (IIIE) or both (IIISE) III1: With or without involvement of splenic, hilar, celiac, or portal nodes III2: With involvement of para-aortic, iliac, and mesenteric nodes |
| Stage IV | Diffuse or disseminated involvement of one or more extranodal organs or tissues, with or without associated lymph node involvement |
| Designations Applicable to Any Disease Stage | |
| A | No symptoms |
| B | Fever (temperature >38° C), drenching night sweats, unexplained weight loss >10% of body weight within the prior 6 months |
| X | Bulky disease (a widening of the mediastinum by more than one-third of the presence of a nodal mass with a maximal dimension >10 cm) |
| E | Involvement of a single extranodal site that is contiguous or proximal to the known nodal site |
| CS | Clinical stage |
| PS | Pathologic stage (as determined by staging laparotomy) |
Patient Evaluation and Staging Workup
An adequate surgical biopsy for pathologic assessment by an experienced hematopathologist is essential in the initial diagnosis of Hodgkin’s lymphoma. Careful history taking and physical examination are needed for all patients. Particular attention should be placed on the presence and duration of constitutional symptoms, as well as other symptoms that may be indicative of the extent and bulkiness of local disease. On physical examination, all nodal groups should be thoroughly palpated, with clear documentation of the extent of disease involvement. Baseline blood work, some of which has been shown to be of prognostic value in patients with Hodgkin’s lymphoma, should be obtained. Studies should include a complete blood count with differential, sedimentation rate, and serum albumin levels.43 A fluorine-18-fluorodeoxyglucose positron emission tomography (FDG-PET) scan, which has been shown to be more sensitive than CT scanning and results in up-staging of 15% to 25% of patients, is now considered part of the standard staging workup for Hodgkin’s lymphoma.44–50 A separate diagnostic CT scan is not necessary if it was done as part of an integrated PET/CT scan. The performance of bone marrow biopsy should be limited to patients with advanced-stage disease or those with constitutional symptoms given the low yield in patients with early-stage, favorable-prognosis disease of less than 1%.51,52 The recommended patient evaluation and staging studies for newly diagnosed Hodgkin’s lymphoma are listed in Table 74-5.
TABLE 74-5 Patient Evaluation and Staging in Hodgkin’s Lymphoma
| History |
CT, computed tomography; MUGA, multiple gated acquisition (scanning); PET, positron emission tomography.
Prognostic Factors
For patients with early-stage Hodgkin’s lymphoma, several prognostic factors, largely based on patients treated with radiation therapy alone, have been identified. These include B symptoms, sedimentation rate, disease bulk, number of sites of disease, patient age, and histologic subtype. Cooperative groups have used varying combinations of these factors to stratify patients into favorable versus unfavorable disease in clinical trials. Examples of various prognostic classification systems used by cooperative groups are shown in Table 74-6.
TABLE 74-6 Prognostic Classification Systems for Clinical Stage I to II Hodgkin’s Lymphoma
| Institution | Prognostic Classification |
|---|---|
| European Organization for Research and Treatment of Cancer |
High Risk:
ESR, erythrocyte sedimentation rate; LP, lymphocyte predominance subtype; NS, nodular sclerosis subtype.
For patients with advanced-stage disease, Hasenclever and colleagues43 developed the International Prognostic Score (IPS), using data from over 5000 patients with advanced-stage Hodgkin’s lymphoma, mostly treated with doxorubicin-based combination chemotherapy. Seven factors were found to have similar independent prognostic values in predicting freedom from progression (FFP) and overall survival (OS). These included hypoalbuminemia (<4 g/dL), anemia (<10.5 g/dL), male sex, age 45 years or older, stage IV disease, leukocytosis (>15,000/µL), and lymphocytopenia (<600/µL or <8% of the white cell count, or both). The 5-year FFP ranged from 42% in patients with an IPS score of 5 or higher to 84% in patients with a score of 0 (Table 74-7). Patients with five or more of the adverse factors account for only 7% of the study population, however. The IPS has been used in trials for patient selection and patient stratification, and it may also have a role in guiding tailored therapy based on relapse risk in advanced-stage patients.
TABLE 74-7 International Prognostic Scoring System for Advanced-Stage Hodgkin’s Lymphoma*
| Prognostic Score | 5-yr Freedom from Progression | 5-yr Overall Survival |
|---|---|---|
| 0 | 84% | 89% |
| 1 | 77% | 90% |
| 2 | 67% | 81% |
| 3 | 60% | 78% |
| 4 | 51% | 61% |
| >5 | 42% | 56% |
* Each of the following factors carries a score of 1: hypoalbuminemia, anemia, male sex, age ≥45 years, stage IV disease, leukocytosis, and lymphocytopenia.
More recently, an early response to PET has been identified as a powerful prognostic tool in Hodgkin’s lymphoma. In a study by Gallamini and associates53 that included 260 patients with stage II to IVB Hodgkin’s lymphoma, the 2-year progression-free survival (PFS) for patients with positive versus negative PET results after two cycles of chemotherapy (PET2) were 12.8% and 95%, respectively (p <.0001). On multivariate regression analysis that included PET2 status and IPS as a continuous variable, IPS lost its prognostic value and only PET2 status had significant independent prognostic value for PFS rates (hazard ratio [HR], 38.3; p <.0001). Trials have been designed investigating the role of response-adapted therapy based on PET findings.
Primary Therapy for Early-Stage Hodgkin’s Lymphoma
Early-stage disease is found in about 60% of all cases of Hodgkin’s lymphoma. Historically, the primary therapy for early-stage Hodgkin’s lymphoma had been extended-field radiation therapy (EFRT) alone, with the addition of chemotherapy in the presence of unfavorable prognostic factors such as large mediastinal adenopathy, constitutional symptoms, high number of involved sites, and/or elevated sedimentation rate. Since the introduction of Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine (ABVD), a more effective and less toxic combination chemotherapy regimen than mechlorethamine, vincristine, procarbazine, and prednisone (MOPP), there has been a shift to the use of combined-modality therapy in early-stage patients, which yields long-term cure rates of 80% to 90%. Randomized studies have shown a significantly higher freedom from treatment failure (FFTF) rate with combined-modality therapy than with radiation therapy alone.54–56 Notably, in the European Organization for Research and Treatment of Cancer (EORTC) H8F trial comparing three cycles of MOPP/ABV plus IFRT versus subtotal nodal irradiation alone, at a median follow-up of 92 months, the significant difference in event-free survival (EFS) translated into a significant overall survival difference between the two arms at 10 years (97% vs. 92%; p = .001).55
Because of the excellent survival response in this relatively young group of patients, late effects of therapy have been increasingly recognized.* Most of the current trials in early-stage Hodgkin’s lymphoma focus on treatment reduction and modification. The key questions addressed by the trials on the use of combined-modality therapy for early-stage Hodgkin’s lymphoma include the following: (1) What is the optimal combination chemotherapy regimen? (2) How many cycles of chemotherapy are needed? (3) What is the appropriate radiation field size and dose? (4) Can radiation therapy be eliminated?
Optimal Combination Chemotherapy Regimen
Investigators have explored alternatives or modifications of the ABVD regimen to limit toxicity in favorable patients or to improve efficacy in unfavorable patients. Examples include vinblastine, bleomycin, and methotrexate (VBM),65–68 methotrexate, vinblastine, and prednisone (MVP),66 Adriamycin (doxorubicin), cyclophosphamide, etoposide, vincristine, bleomycin, and prednisolone (VAPEC-B),69 Adriamycin (doxorubicin) and vinblastine (AV),54 Novantrone (mitoxantrone), Oncovin (vincristine), vinblastine, and prednisone (NOVP),70 and epirubicin, bleomycin, vinblastine, and prednisone (EBVP II, adminstered monthly).71 Most of these regimens showed promising results, at least in patients with favorable-prognosis disease, with relapse-free survival (RFS) of about 90%. The Stanford V regimen (nitrogen mustard, Adriamycin (doxorubicin), vincristine, vinblastine, etoposide, bleomycin, and prednisone, followed by radiation therapy to initial nodal involvement in selected cases), a short but intensive 12-week regimen, was originally developed for patients with advanced-stage disease or bulky, early-stage disease.72,73 The Stanford group recently reported the results on 87 patients with nonbulky, stage I to IIA disease treated with an abbreviated 8-week course of Stanford V therapy followed by IFRT to 30 Gy.74 At a median follow-up of 9 years, the FFP and OS were 94% and 96%, respectively. In the most recent version (Stanford V-C), the nitrogen mustard is replaced by cyclophosphamide and the radiation dose is reduced from 30 Gy to 20 Gy. The German Hodgkin’s Study Group (GHSG) HD13 trial compared two cycles of ABVD, AVD, ABV, and AV, all followed by 30 Gy of IFRT in clinical stage I to II patients without risk factors. The two arms without dacarbazine (AV and ABV) were closed early due to higher than expected relapse rates, but the final results of this trial are pending at this time.
Attempts to use less intensive chemotherapy in patients with unfavorable-prognosis disease have been disappointing. In the EORTC H7U study, comparing unfavorable-prognosis clinical stage I to II patients treated with six cycles of EBVP II and IFRT versus six cycles of MOPP/ABV and IFRT, the 10-year EFS (68% vs. 88%; p <.0001) and 10-year OS (79% vs. 87%; p = .0175) were significantly lower in the EBVP II arm.75 In order to improve the treatment outcome in patients with unfavorable features, trials had been conducted to determine whether these patients may benefit from the regimen containing bleomycin, etoposide, Adriamycin (doxorubicin), cyclophosphamide, Oncovin (vincristine), procarbazine, and prednisone (BEACOPP), originally developed for patients with advanced-stage disease. Both the EORTC H9U and the GHSG HD11 studies compared four to six cycles of ABVD with four cycles of baseline BEACOPP, followed by IFRT to 20 to 30 Gy. No significant differences in 4-year EFS or OS rates were observed between BEACOPP and ABVD in the EORTC H9U trial.76 In the GHSG HD11 trial, at a median follow-up of 82 months, a significantly higher 5-year FFTF in the four cycles of baseline BEACOPP arm over the four cycles of ABVD arm, if followed by 20 Gy of IFRT (86.8% vs. 81.1%; 95% CI of difference, 0.1% to 11.3%). There was no significant difference between BEACOPP and ABVD if followed by 30 Gy of IFRT, however.77 Patients treated with baseline BEACOPP had a higher rate of severe toxicity than patients treated with ABVD (73.8% vs. 51.5%; p <.001). The GHSG HD14 trial tested increasing dose intensity using dose-escalated BEACOPP in this population. This trial randomized patients with unfavorable clinical stage I to II disease to four cycles of ABVD versus two cycles of dose-escalated BEACOPP and two cycles of ABVD, followed by IFRT to 30 Gy. Preliminary results showed a significantly superior 3-year FFTF in the dose-escalated BEACOPP-containing arm (96% vs. 90%) but no differences in OS.78
Optimal Duration of Chemotherapy
Whether the number of cycles of chemotherapy can be shortened in patients with favorable-prognosis disease was addressed by the GHSG HD10 trial, in which clinical stage I to II patients without risk factors were randomized to four cycles or two cycles of ABVD, followed by 30-Gy or 20-Gy IFRT. At a median follow-up of 7.5 years, there were no significant differences between four or two cycles of chemotherapy in 8-year FFTF (88.4% vs. 85.7%) or 8-year OS (94.6% vs. 94.4%).79 In patients with unfavorable-prognosis disease, the optimal number of cycles of chemotherapy was one of the study questions in two successive EORTC trials using different chemotherapy regimens. In the EORTC-H8U trial, patients were randomized to six cycles of MOPP/ABV followed by IFRT, four cycles of MOPP/ABV followed by IFRT, or four cycles of MOPP/ABV followed by EFRT.80 At a median follow-up of 92 months, there was no significant difference in 5-year EFS among the three treatment groups (84%, 88%, and 87%). The OS at 10 years was also not significantly different (88%, 85%, and 84%).55 In the previously described EORTC-H9U trial, randomizing patients to six cycles of ABVD, four cycles of ABVD, or four cycles of BEACOPP, all followed by IFRT, at a median follow up of 57 months, there was no difference in 4-year EFS among the three arms (91%, 87%, and 90%; p = .38).76
Optimal Radiation Field Size
Several randomized trials have compared EFRT and IFRT after chemotherapy for early-stage Hodgkin’s lymphoma. In the EORTC H8U trial, two of the three arms compared four cycles of MOPP/ABV followed by either IFRT or EFRT, and no significant differences in failure-free or OS rates were detected at a median follow-up of 92 months.55 Similarly, the GHSG HD8 trial for unfavorable early-stage patients found no differences in 5-year FFTF and OS between EFRT or IFRT after two cycles of cyclophosphamide, Oncovin (vincristine), procarbazine, and prednisone (COPP) and ABVD at a median follow-up of 54 months.81 An Italian trial randomized 136 patients with clinical stage I unfavorable Hodgkin’s lymphoma and clinical stage IIA favorable and unfavorable Hodgkin’s lymphoma to either EFRT or IFRT after four cycles of ABVD.82 At a median follow-up of 116 months, no differences in 12-year FFP or OS were observed. Three cases of secondary malignant disease were reported, all of which were in the EFRT arm.
An involved field encompasses not only the involved nodes but also the other lymph nodes within the same lymph node region, as denoted by the Rye classification for staging (Fig. 74-1). Guidelines for IFRT to specific sites have been detailed by Yahalom and Mauch.83 For example, in a patient presenting with an enlarged cervical lymph node, an involved field would include the entire ipsilateral cervical chain and the supraclavicular region because these nodes are considered to be within one region. Similarly, an involved field for a patient with groin disease would encompass the inguinal and femoral nodes. In a patient with mediastinal involvement, the field would cover the mediastinal, hilar, subcarinal, and medial supraclavicular nodes. Although the hilar nodes are scored separately traditionally, the hilar and subcarinal nodes are included in the mediastinal field. In addition, the medial supraclavicular nodes are included in order to cover the upper mediastinum (top of T1). Of note, IFRT was developed in the two-dimensional treatment planning era, in which the field design was based largely on bony landmarks, with inclusion of a considerable volume of normal tissue in the irradiated field.
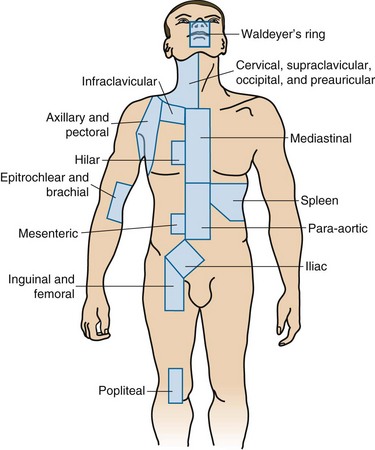
Figure 74-1 The anatomic definitions of separate lymph node regions based on the Rye classification for staging.
Although IFRT is considered the standard treatment field at this time as part of combined-modality therapy, there are data suggesting that relapse sites in Hodgkin’s lymphoma after chemotherapy alone are largely limited to the initially involved node.84 This has led to the concept of involved-node radiotherapy (INRT). The definition of INRT is in evolution and varies somewhat among groups. In general, the clinical target volume (CTV) is based on the prechemotherapy and postchemotherapy nodal volumes, with exclusion of normal displaced structures based on the postchemotherapy scan. The use of PET scanning in the treatment position is encouraged but is not mandated. In the European Organization for Research and Treatment of Cancer/Groupe d’Etudes des Lymphomes de l’Adulte (EORTC-GELA) H10 and H11 trials for early-stage favorable and unfavorable Hodgkin’s lymphoma, INRT was adopted in both the standard and experimental arms. The EORTC-GELA group defines the planning target volume (PTV) as a 1-cm isotropic expansion of the CTV to allow for organ motion and setup variations.85 In addition, four-dimensional planning and intensity-modulated radiation therapy (IMRT) were allowed.
Investigators from British Columbia reported results of their experience with INRT for early-stage Hodgkin’s lymphoma patients. At a median follow-up of 50 months, no local recurrences were observed. However, the INRT employed in this study encompassed the initially involved lymph nodes with a margin ranging from 1.5 cm to 5 cm to the field edge and therefore included a larger volume than that of the INRT as defined by the European groups.86 In addition, two-dimensional planning was performed in some of the patients.
The GHSG is planning a randomized trial for patients with risk factors (HD17) comparing IFRT and INRT. The GHSG defines the PTV as a 2-cm axial and 3-cm cranial caudal expansion of the CTV (if necessary, it can be reduced to 1 to 1.5 cm if there is close proximity to critical structures) and for mediastinal disease, a 1-cm axial and 2-cm cranial caudal expansion.78 This is the only randomized trial directly comparing IFRT with INRT, the results of which may provide information on the effectiveness of INRT after chemotherapy in preserving local control while limiting acute and long-term side effects. In the meantime, efforts to standardize the definition of INRT using modern image guidance and conformal radiotherapy technique are essential.
Optimal Radiation Dose
The appropriate radiation dose after chemotherapy in early-stage Hodgkin’s lymphoma has been explored by three trials.71,87,88 The EORTC H9F trial is a three-arm trial in which all patients receive six cycles of EBVP II.71 After a complete response, patients were randomized to receive no further treatment, 36-Gy IFRT, or 20-Gy IFRT. Patients with a partial response all received 36 Gy of IFRT with or without a 4-Gy boost. A complete response or complete response unconfirmed was achieved in 619 patients. At a median follow-up of 33 months, the 4-year EFS rates in the three arms of 36-Gy IFRT, 20-Gy IFRT, and no radiotherapy were 87%, 84%, and 70%, respectively. The difference in treatment results between the two doses of radiotherapy was not significant. However, the no-radiotherapy arm was closed early because stopping rules were met (>20% of events) (see later discussion).







