Chapter 74 Hodgkin’s Lymphoma
Hodgkin’s lymphoma (HL) was first described by the British physician Thomas Hodgkin in 1832, when he reported six cases of pathologic enlargement of the lymph nodes and spleen at Guy’s Hospital.1 Attempts to treat the disease using various chemical or surgical means were unsuccessful until around the turn of the century, when the effectiveness of x-rays in shrinking the disease was first demonstrated. The crude, low-energy x-ray equipment available at the time allowed only temporary reduction of the enlarged lymph nodes. The development of kilovoltage equipment in the 1920s and the subsequent pioneering work of Gilbert, a Swiss radiotherapist, paved the way for the definitive treatment of patients with Hodgkin’s lymphoma.2 Vera Peters at the Ontario Institute of Radiotherapy first reported the curability of early-stage Hodgkin’s lymphoma in 1950, using high doses of fractionated radiation therapy.3 The availability of modern high-energy radiation therapy equipment in the late 1950s and early 1960s allowed the delivery of higher doses of radiation to deep-seated tumors with fewer limitations caused by reactions in the superficial tissues. The introduction of effective combination chemotherapy further improved the treatment outcome of Hodgkin’s lymphoma, especially in patients with unfavorable prognostic features or advanced-stage disease.4 Over the last 3 decades, there have been continued improvements in radiation therapy techniques allowing more uniform and better targeted dose delivery, the development of more effective and less toxic multiagent chemotherapy regimens, advances in radiographic imaging technology, and the refinement of prognostic factors that allow better tailoring of treatments. Hodgkin’s lymphoma, a previously fatal illness, has now become one of the most curable forms of malignant disease.
Etiology and Epidemiology
Hodgkin’s lymphoma is a relatively uncommon neoplasm, with approximately 8000 new cases in the United States each year, representing less than 1% of all cancer diagnoses.5 The incidence, patient age, and gender distribution of Hodgkin’s lymphoma vary, depending on the geographic location. The age-incidence curve in developed countries is characterized by a bimodal distribution.6,7 There is an initial peak in young adults at around age 25 years and a second peak occurring at age 60 to 70 years, in which a male predominance is observed. The majority of the cases seen in young adulthood are of a nodular sclerosis (NS) histologic type, and many of the factors that have been associated with the development of Hodgkin’s lymphoma in these patients appeared to be a reflection of delayed exposure to infectious agents and/or higher socioeconomic status. These include early birth order, small sibship size, growing up in single-family houses, having few playmates, and having parents with a high level of education.8–10 In contrast, in economically disadvantaged parts of the world, Hodgkin’s lymphoma is relatively rare among young adults.7 The mixed cellularity (MC) subtype is the predominant histologic subtype in developing countries, with an initial peak in childhood for boys and a late peak in older patients.
Several of the epidemiologic and clinical features of the disease are suggestive of infectious causes, and there has been increasing evidence that the Epstein-Barr virus (EBV) may be involved in the pathogenesis of Hodgkin’s lymphoma in at least a subset of cases. Patients with a history of infectious mononucleosis, in which EBV is the causative agent, are at an approximately threefold increased risk for Hodgkin’s lymphoma, and particularly EBV-associated Hodgkin’s lymphoma.7,11,12 Elevated levels of the IgG and IgA immunoglobulins against the EBV capsid antigen have been demonstrated months to years prior to clinical Hodgkin’s lymphoma development.13,14 In about one-third to half of cases of classical Hodgkin’s lymphoma occurring in Western populations, the monoclonal EBV genome can be detected in the Reed-Sternberg cells.15,16 EBV positivity is predominantly associated with the MC subtype,17 which is more common among young children and older adults, and is less frequently associated with NS cases seen mostly in young adults in the developed world.
The observation of familial aggregation of cases of Hodgkin’s lymphoma suggests that genetic susceptibility as well as environmental exposure may contribute to the development of Hodgkin’s lymphoma. A fivefold increased risk has been demonstrated in first-degree relatives, and siblings of young adults with Hodgkin’s lymphoma have a sevenfold increased risk.18 The excess risk appears to be more pronounced in same-sex siblings, which may be related to more shared environmental exposure.19 In a twin study of young adults with Hodgkin’s lymphoma, monozygotic twins of patients had an almost 100-fold increased risk,20 whereas no increased risk for dizygotic twins was observed, supporting the contribution of heritable factors to the development of the disease. In particular, follow-up twin studies have suggested that persons with genetically determined lower interleukin-6 (IL-6) levels may be less susceptible to young adult Hodgkin’s lymphoma.21 In addition, a genome screen of families at high risk for Hodgkin’s lymphoma has provided evidence for a susceptibility gene on several chromosomes, particularly chromosome 4.22 Finally, a number of specific human leukocyte antigen (HLA) haplotypes have been identified to be associated with an increased risk of Hodgkin’s lymphoma.22–24 Because the immune response is genetically determined by the HLA type, patients with these HLA haplotypes may have increased susceptibility to certain infections, which in turn may lead to the increased Hodgkin’s lymphoma risk as well as increased susceptibility to autoimmune conditions. In fact, an elevated risk of Hodgkin’s lymphoma has been found in patients with personal histories of several autoimmune conditions.25
Biologic Characteristics and Molecular Biology
The malignant cells in classical Hodgkin’s lymphoma, the Hodgkin/Reed-Sternberg (HRS) cells, are large, uninucleated or multinucleated cells that usually make up only 1% of the cells present in the tissue sample, with most of the tumor consisting of a variety of non-neoplastic inflammatory cells and fibrosis. Results of molecular single-cell studies have shown that in over 90% of cases, HRS cells have monoclonal immunoglobulin gene rearrangements that are characteristic of mature B lymphocytes, and somatically mutated VH genes that are specific markers for germinal center B cells and their descendants, supporting a germinal center or postgerminal center B-cell origin.25,26 Unlike normal B cells that have undergone successful maturation through the germinal cells, however, HRS cells characteristically show an absence of immunoglobulin gene expression. This has been attributed to mutations in the coding or regulatory regions,27 lack of expression of transcription factors that are responsible for activation of the promoters and enhancers,28 epigenetic silencing of the immunoglobulin heavy-chain transcription,29 and constitutive expression of Notch1 and STAT5.30,31 Despite their inability to express immunoglobulin receptors, HRS cells are resistant to apoptosis, which normally removes immunoglobulin-negative B cells that have traversed the germinal center.32
There is increasing evidence connecting the prevention of apoptosis and survival of HRS cells to the activation of the nuclear factor kappa B (NFκB) transcription factor-signaling pathway. Constitutive NFκB is required for proliferation and survival of Hodgkin’s lymphoma tumor cells.28 The cause of the constitutive activation of NFκB is probably multifactorial and may include amplification of the REL gene,33 mutations in NFκB inhibitors,34 and somatic mutations in the novel tumor suppressor gene TNFAIP3.35 There is an inverse relationship between EBV infection and inactivation of A20, the protein encoded by the TNFAIP3 gene, suggesting that they may represent alternative pathways of pathogenesis.35
Molecular profiling experiments using Hodgkin’s lymphoma-derived cell lines with suppressed and unsuppressed NFκB activity have been performed in order to better understand the NFκB signaling pathway and to identify its target genes. One of the regulators of apoptosis that is expressed in dependence of NFκB is cIAP2, a direct inhibitor of caspase 3, suggesting that HRS cells are protected from caspase 3-induced apoptosis by cIAP2.29 Another NFκB-dependent regulator of apoptosis is CD95, which has been shown to be up-regulated in HRS cells. Unlike the other NFκB-dependent regulators, however, CD95 is known to trigger apoptosis rather than prevent it. The resistance of HRS cells to CD95-mediated apoptotic cell death may be due to functional inhibition of death receptor pathways by cellular FADD-like IL1B-converting enzyme inhibitory proteins (c-FLIP), which is one of the most strongly NFκB-regulated genes.30 The contribution of NFκB signaling to the development of Hodgkin’s lymphoma is further supported by epidemiologic data showing that regular aspirin use is associated with a reduced risk of developing Hodgkin’s lymphoma, presumably through inhibition of NFκB transcription.31 Continued efforts in the elucidation of the NFκB pathway in the pathogenesis of Hodgkin’s lymphoma may have important therapeutic implications through pharmacologic down-regulation of NFκB activity and its target genes, and increasing the susceptibility of HRS cells to apoptosis. However, bortezomib, a proteasome inhibitor that inhibits NFκB activity, does not appear to be clinically active against Hodgkin’s lymphoma.
Pathology
Since the 1930s, a number of pathologic classification systems for Hodgkin’s lymphoma have been developed. The Rye classification system, introduced at a conference in Rye, New York, in 1966, divided cases of Hodgkin’s lymphoma into lymphocyte predominance (LP), nodular sclerosis (NS), mixed cellularity (MC), and lymphocyte depletion (LD) disease.32 This system was widely adopted in the next 25 years and was subsequently modified in the revised European-American classification of malignant lymphomas (REAL) and later in the World Health Organization (WHO) classification33,34 (Table 74-1). In the current classification system, HL is specifically recognized as a lymphoma. Based on morphologic, immunophenotypical, and clinical characteristics, it is divided into two distinct entities: classical Hodgkin’s lymphoma and nodular lymphocyte predominant Hodgkin’s lymphoma (NLPHD). Table 74-2 compares the morphologic and immunophenotypical features of the malignant cells of these two entities. The diagnosis of HL is based on morphologic assessment with identification of HRS cells or their variants, along with immunohistochemical studies.
TABLE 74-1 The WHO Histologic Classification of Hodgkin’s Lymphoma34
TABLE 74-2 Comparison of Morphologic and Immunophenotypical Characteristics of Malignant Cells of Classical Hodgkin’s Disease (HD) and Nodular Lymphocyte Predominance Hodgkin’s Disease (NLPHD)
| Classical HD (HRS Cells) | NLPHD (L/H Cells) | |
|---|---|---|
| Nuclei | Mononucleated and multinucleated, monolobulated and multilobulated | Mononucleated, multilobulated |
| Nucleoli | Large | Multiple, small |
| CD30 | Positive | Negative |
| CD15 | Positive in majority of cases | Negative |
| CD45 | Negative | Positive |
| CD20 | Negative in majority of cases | Positive |
| CD79a | Negative in majority of cases | Positive |
Classical Hodgkin’s Lymphoma
The NS subtype accounts for approximately 70% of classical Hodgkin’s lymphomas, affecting predominantly young adults. Morphologically, it is characterized by the presence of one or more sclerotic bands radiating from a thickened lymph node capsule. The British National Lymphomas Investigation subclassified NS Hodgkin’s lymphoma into two grades based on the percentage of nodules showing lymphocyte depletion or increased number of anaplastic-appearing HRS cells.35,36 The prognostic value of this grading system with modern therapy is unclear, however.
The MC subtype is more frequently seen in developing countries, accounting for over half of the cases, whereas in the more developed parts of the world, it makes up approximately 25% of classical Hodgkin’s lymphoma cases. Morphologically, HRS cells are seen scattered in a diffuse inflammatory background with the absence of nodular sclerosing fibrosis. In contrast to the NS and lymphocyte-rich classic Hodgkin’s disease (LRCHD), EBV positivity is much more frequent in the MC subtype.17
LRCHD accounts for approximately 5% of classic Hodgkin’s lymphoma cases and has a male predominance and older median age at presentation.33 It is characterized by a background infiltrate of small, mature, predominantly B lymphocytes with rare HRS cells and variants. It can resemble NLPHD morphologically, and immunohistochemical studies of the malignant cells are essential to make the distinction. The prognosis may be slightly better than for other subtypes of classic Hodgkin’s disease.33
The lymphocyte depletion subtype has an increased number of HRS cells and is depleted in lymphocytes. Many cases that were previously classified as lymphocyte depletion Hodgkin’s lymphoma are now determined to be anaplastic or large-cell non-Hodgkin’s lymphoma based on immunohistochemical studies.37 Reliable clinical data on this subtype are limited given its rarity and the uncertainty concerning its diagnosis.
Nodular Lymphocyte Predominance Hodgkin’s Lymphoma
NLPHD makes up 5% of Hodgkin’s lymphoma cases. The malignant cells of NLPHD are the lymphocytic-predominant (LP) cells, which are also known as popcorn cells due to their characteristic appearance. The pathogenesis of LP cells is probably distinct from that of HRS cells but probably shares constitutive NFκB activity.38 Unlike classic Hodgkin’s lymphoma, the neoplastic cells of NLPHD typically lack the expression of CD15 and CD30 markers but are consistently positive for CD20 and CD45.38 A nodular pattern is seen, usually completely or partially replacing the lymph node. The nodules tend to be large and closely packed, and L&H cells are typically seen within or around the nodules. There are usually large numbers of CD57-positive small lymphocytes in the nodules, often with ringing around the L&H cells. In about 3% to 5% of cases, transformation to diffuse large B-cell lymphoma may occur.
Clinical Manifestations, Patient Evaluation, and Staging
Clinical Presentation
Patients may also present exclusively with constitutional symptoms in the absence of any physical findings. These symptoms, also known as B symptoms, include fever, unexplained weight loss, and drenching night sweats. Severe, generalized pruritus, not classified as a B symptom, is noted in about 10% to 15% of patients and has been associated with a poorer prognosis.39 Alcohol-induced pain, typically at the site of lymphadenopathy or bony involvement, can be a presenting symptom in some patients.40
Staging System
The Ann Arbor staging classification (Table 74-3), developed in 1971, is a four-stage system formulated to provide prognostic information and to guide therapeutic decisions. It does not reflect other important prognostic factors such as bulky disease or multiple sites of involvement, however.41 The availability of improved imaging techniques has also changed its applicability. In 1988, a meeting was held in the Cotswolds, England, where revisions to the Ann Arbor staging system were made42 (Table 74-4). The following main changes were made: (1) The use of computed tomography (CT) scanning is allowed to assess disease involvement below the diaphragm. (2) For stage II disease, the number of anatomic nodal sites is indicated by a subscript (e.g., stage II3). (3) For stage III disease, upper and lower abdominal involvement was subdivided as III1 and III2, respectively. (4) Bulky disease is denoted by X, defined as more than one-third widening of the mediastinum at the T5 to T6 level or more than 10 cm maximum dimension of the nodal mass. (5) Unconfirmed/uncertain complete remission (CRu) was introduced to denote the presence of a residual imaging abnormality but the absence of pathologically confirmed residual disease.
TABLE 74-3 Ann Arbor Staging Classification for Hodgkin’s Lymphoma
| Stage | Definitions |
|---|---|
| Stage I | Involvement of single lymph node region (I) or of single extralymphatic organ or site (IE) |
| Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm alone (II) or with involvement of limited, contiguous extralymphatic organ or tissue (IIE) |
| Stage III | Involvement of lymph node regions on both sides of the diaphragm (III), which may include the spleen (IIIS) or limited, contiguous extralymphatic organ or site (IIIE), or both (IIISE) |
| Stage IV | Diffuse or disseminated foci of involvement of one or more extralymphatic organs or tissues, with or without associated lymphatic involvement |
The absence or presence of fever, night sweats, and or unexplained weight loss of 10% or more of body weight in the 6 months preceding admission are to be denoted in all cases by the suffix letters A or B, respectively. The clinical stage (CS) denotes the stage as determined by all diagnostic examinations and a single biopsy only. If a second biopsy of any kind has been obtained, whether negative or positive, the term pathologic stage (PS) is used.
TABLE 74-4 The Cotswolds Staging Classification for Hodgkin’s Lymphoma
| Stage | Definitions |
|---|---|
| Stage I | Involvement of a single lymph node region or lymphoid structure (e.g., spleen, thymus, Waldeyer’s ring) or involvement of a single extralymphatic site (IE) |
| Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm (hilar nodes, when involved on both sides, constitute stage II disease); localized contiguous involvement of only one extranodal organ or site and lymph node region(s) on the same side of the diaphragm (IIE) The number of anatomic regions involved should be indicated by a suffix (e.g., II3) |
| Stage III | Involvement of lymph node regions on both sides of the diaphragm (III), which may also be accompanied by involvement of the spleen (IIIS) or by localized contiguous involvement of only one extranodal organ site (IIIE) or both (IIISE) III1: With or without involvement of splenic, hilar, celiac, or portal nodes III2: With involvement of para-aortic, iliac, and mesenteric nodes |
| Stage IV | Diffuse or disseminated involvement of one or more extranodal organs or tissues, with or without associated lymph node involvement |
| Designations Applicable to Any Disease Stage | |
| A | No symptoms |
| B | Fever (temperature >38° C), drenching night sweats, unexplained weight loss >10% of body weight within the prior 6 months |
| X | Bulky disease (a widening of the mediastinum by more than one-third of the presence of a nodal mass with a maximal dimension >10 cm) |
| E | Involvement of a single extranodal site that is contiguous or proximal to the known nodal site |
| CS | Clinical stage |
| PS | Pathologic stage (as determined by staging laparotomy) |
Patient Evaluation and Staging Workup
An adequate surgical biopsy for pathologic assessment by an experienced hematopathologist is essential in the initial diagnosis of Hodgkin’s lymphoma. Careful history taking and physical examination are needed for all patients. Particular attention should be placed on the presence and duration of constitutional symptoms, as well as other symptoms that may be indicative of the extent and bulkiness of local disease. On physical examination, all nodal groups should be thoroughly palpated, with clear documentation of the extent of disease involvement. Baseline blood work, some of which has been shown to be of prognostic value in patients with Hodgkin’s lymphoma, should be obtained. Studies should include a complete blood count with differential, sedimentation rate, and serum albumin levels.43 A fluorine-18-fluorodeoxyglucose positron emission tomography (FDG-PET) scan, which has been shown to be more sensitive than CT scanning and results in up-staging of 15% to 25% of patients, is now considered part of the standard staging workup for Hodgkin’s lymphoma.44–50 A separate diagnostic CT scan is not necessary if it was done as part of an integrated PET/CT scan. The performance of bone marrow biopsy should be limited to patients with advanced-stage disease or those with constitutional symptoms given the low yield in patients with early-stage, favorable-prognosis disease of less than 1%.51,52 The recommended patient evaluation and staging studies for newly diagnosed Hodgkin’s lymphoma are listed in Table 74-5.
TABLE 74-5 Patient Evaluation and Staging in Hodgkin’s Lymphoma
| History |
CT, computed tomography; MUGA, multiple gated acquisition (scanning); PET, positron emission tomography.
Prognostic Factors
For patients with early-stage Hodgkin’s lymphoma, several prognostic factors, largely based on patients treated with radiation therapy alone, have been identified. These include B symptoms, sedimentation rate, disease bulk, number of sites of disease, patient age, and histologic subtype. Cooperative groups have used varying combinations of these factors to stratify patients into favorable versus unfavorable disease in clinical trials. Examples of various prognostic classification systems used by cooperative groups are shown in Table 74-6.
TABLE 74-6 Prognostic Classification Systems for Clinical Stage I to II Hodgkin’s Lymphoma
| Institution | Prognostic Classification |
|---|---|
| European Organization for Research and Treatment of Cancer |
High Risk:
ESR, erythrocyte sedimentation rate; LP, lymphocyte predominance subtype; NS, nodular sclerosis subtype.
For patients with advanced-stage disease, Hasenclever and colleagues43 developed the International Prognostic Score (IPS), using data from over 5000 patients with advanced-stage Hodgkin’s lymphoma, mostly treated with doxorubicin-based combination chemotherapy. Seven factors were found to have similar independent prognostic values in predicting freedom from progression (FFP) and overall survival (OS). These included hypoalbuminemia (<4 g/dL), anemia (<10.5 g/dL), male sex, age 45 years or older, stage IV disease, leukocytosis (>15,000/µL), and lymphocytopenia (<600/µL or <8% of the white cell count, or both). The 5-year FFP ranged from 42% in patients with an IPS score of 5 or higher to 84% in patients with a score of 0 (Table 74-7). Patients with five or more of the adverse factors account for only 7% of the study population, however. The IPS has been used in trials for patient selection and patient stratification, and it may also have a role in guiding tailored therapy based on relapse risk in advanced-stage patients.
TABLE 74-7 International Prognostic Scoring System for Advanced-Stage Hodgkin’s Lymphoma*
| Prognostic Score | 5-yr Freedom from Progression | 5-yr Overall Survival |
|---|---|---|
| 0 | 84% | 89% |
| 1 | 77% | 90% |
| 2 | 67% | 81% |
| 3 | 60% | 78% |
| 4 | 51% | 61% |
| >5 | 42% | 56% |
* Each of the following factors carries a score of 1: hypoalbuminemia, anemia, male sex, age ≥45 years, stage IV disease, leukocytosis, and lymphocytopenia.
More recently, an early response to PET has been identified as a powerful prognostic tool in Hodgkin’s lymphoma. In a study by Gallamini and associates53 that included 260 patients with stage II to IVB Hodgkin’s lymphoma, the 2-year progression-free survival (PFS) for patients with positive versus negative PET results after two cycles of chemotherapy (PET2) were 12.8% and 95%, respectively (p <.0001). On multivariate regression analysis that included PET2 status and IPS as a continuous variable, IPS lost its prognostic value and only PET2 status had significant independent prognostic value for PFS rates (hazard ratio [HR], 38.3; p <.0001). Trials have been designed investigating the role of response-adapted therapy based on PET findings.
Primary Therapy for Early-Stage Hodgkin’s Lymphoma
Early-stage disease is found in about 60% of all cases of Hodgkin’s lymphoma. Historically, the primary therapy for early-stage Hodgkin’s lymphoma had been extended-field radiation therapy (EFRT) alone, with the addition of chemotherapy in the presence of unfavorable prognostic factors such as large mediastinal adenopathy, constitutional symptoms, high number of involved sites, and/or elevated sedimentation rate. Since the introduction of Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine (ABVD), a more effective and less toxic combination chemotherapy regimen than mechlorethamine, vincristine, procarbazine, and prednisone (MOPP), there has been a shift to the use of combined-modality therapy in early-stage patients, which yields long-term cure rates of 80% to 90%. Randomized studies have shown a significantly higher freedom from treatment failure (FFTF) rate with combined-modality therapy than with radiation therapy alone.54–56 Notably, in the European Organization for Research and Treatment of Cancer (EORTC) H8F trial comparing three cycles of MOPP/ABV plus IFRT versus subtotal nodal irradiation alone, at a median follow-up of 92 months, the significant difference in event-free survival (EFS) translated into a significant overall survival difference between the two arms at 10 years (97% vs. 92%; p = .001).55
Because of the excellent survival response in this relatively young group of patients, late effects of therapy have been increasingly recognized.* Most of the current trials in early-stage Hodgkin’s lymphoma focus on treatment reduction and modification. The key questions addressed by the trials on the use of combined-modality therapy for early-stage Hodgkin’s lymphoma include the following: (1) What is the optimal combination chemotherapy regimen? (2) How many cycles of chemotherapy are needed? (3) What is the appropriate radiation field size and dose? (4) Can radiation therapy be eliminated?
Optimal Combination Chemotherapy Regimen
Investigators have explored alternatives or modifications of the ABVD regimen to limit toxicity in favorable patients or to improve efficacy in unfavorable patients. Examples include vinblastine, bleomycin, and methotrexate (VBM),65–68 methotrexate, vinblastine, and prednisone (MVP),66 Adriamycin (doxorubicin), cyclophosphamide, etoposide, vincristine, bleomycin, and prednisolone (VAPEC-B),69 Adriamycin (doxorubicin) and vinblastine (AV),54 Novantrone (mitoxantrone), Oncovin (vincristine), vinblastine, and prednisone (NOVP),70 and epirubicin, bleomycin, vinblastine, and prednisone (EBVP II, adminstered monthly).71 Most of these regimens showed promising results, at least in patients with favorable-prognosis disease, with relapse-free survival (RFS) of about 90%. The Stanford V regimen (nitrogen mustard, Adriamycin (doxorubicin), vincristine, vinblastine, etoposide, bleomycin, and prednisone, followed by radiation therapy to initial nodal involvement in selected cases), a short but intensive 12-week regimen, was originally developed for patients with advanced-stage disease or bulky, early-stage disease.72,73 The Stanford group recently reported the results on 87 patients with nonbulky, stage I to IIA disease treated with an abbreviated 8-week course of Stanford V therapy followed by IFRT to 30 Gy.74 At a median follow-up of 9 years, the FFP and OS were 94% and 96%, respectively. In the most recent version (Stanford V-C), the nitrogen mustard is replaced by cyclophosphamide and the radiation dose is reduced from 30 Gy to 20 Gy. The German Hodgkin’s Study Group (GHSG) HD13 trial compared two cycles of ABVD, AVD, ABV, and AV, all followed by 30 Gy of IFRT in clinical stage I to II patients without risk factors. The two arms without dacarbazine (AV and ABV) were closed early due to higher than expected relapse rates, but the final results of this trial are pending at this time.
Attempts to use less intensive chemotherapy in patients with unfavorable-prognosis disease have been disappointing. In the EORTC H7U study, comparing unfavorable-prognosis clinical stage I to II patients treated with six cycles of EBVP II and IFRT versus six cycles of MOPP/ABV and IFRT, the 10-year EFS (68% vs. 88%; p <.0001) and 10-year OS (79% vs. 87%; p = .0175) were significantly lower in the EBVP II arm.75 In order to improve the treatment outcome in patients with unfavorable features, trials had been conducted to determine whether these patients may benefit from the regimen containing bleomycin, etoposide, Adriamycin (doxorubicin), cyclophosphamide, Oncovin (vincristine), procarbazine, and prednisone (BEACOPP), originally developed for patients with advanced-stage disease. Both the EORTC H9U and the GHSG HD11 studies compared four to six cycles of ABVD with four cycles of baseline BEACOPP, followed by IFRT to 20 to 30 Gy. No significant differences in 4-year EFS or OS rates were observed between BEACOPP and ABVD in the EORTC H9U trial.76 In the GHSG HD11 trial, at a median follow-up of 82 months, a significantly higher 5-year FFTF in the four cycles of baseline BEACOPP arm over the four cycles of ABVD arm, if followed by 20 Gy of IFRT (86.8% vs. 81.1%; 95% CI of difference, 0.1% to 11.3%). There was no significant difference between BEACOPP and ABVD if followed by 30 Gy of IFRT, however.77 Patients treated with baseline BEACOPP had a higher rate of severe toxicity than patients treated with ABVD (73.8% vs. 51.5%; p <.001). The GHSG HD14 trial tested increasing dose intensity using dose-escalated BEACOPP in this population. This trial randomized patients with unfavorable clinical stage I to II disease to four cycles of ABVD versus two cycles of dose-escalated BEACOPP and two cycles of ABVD, followed by IFRT to 30 Gy. Preliminary results showed a significantly superior 3-year FFTF in the dose-escalated BEACOPP-containing arm (96% vs. 90%) but no differences in OS.78
Optimal Duration of Chemotherapy
Whether the number of cycles of chemotherapy can be shortened in patients with favorable-prognosis disease was addressed by the GHSG HD10 trial, in which clinical stage I to II patients without risk factors were randomized to four cycles or two cycles of ABVD, followed by 30-Gy or 20-Gy IFRT. At a median follow-up of 7.5 years, there were no significant differences between four or two cycles of chemotherapy in 8-year FFTF (88.4% vs. 85.7%) or 8-year OS (94.6% vs. 94.4%).79 In patients with unfavorable-prognosis disease, the optimal number of cycles of chemotherapy was one of the study questions in two successive EORTC trials using different chemotherapy regimens. In the EORTC-H8U trial, patients were randomized to six cycles of MOPP/ABV followed by IFRT, four cycles of MOPP/ABV followed by IFRT, or four cycles of MOPP/ABV followed by EFRT.80 At a median follow-up of 92 months, there was no significant difference in 5-year EFS among the three treatment groups (84%, 88%, and 87%). The OS at 10 years was also not significantly different (88%, 85%, and 84%).55 In the previously described EORTC-H9U trial, randomizing patients to six cycles of ABVD, four cycles of ABVD, or four cycles of BEACOPP, all followed by IFRT, at a median follow up of 57 months, there was no difference in 4-year EFS among the three arms (91%, 87%, and 90%; p = .38).76
Optimal Radiation Field Size
Several randomized trials have compared EFRT and IFRT after chemotherapy for early-stage Hodgkin’s lymphoma. In the EORTC H8U trial, two of the three arms compared four cycles of MOPP/ABV followed by either IFRT or EFRT, and no significant differences in failure-free or OS rates were detected at a median follow-up of 92 months.55 Similarly, the GHSG HD8 trial for unfavorable early-stage patients found no differences in 5-year FFTF and OS between EFRT or IFRT after two cycles of cyclophosphamide, Oncovin (vincristine), procarbazine, and prednisone (COPP) and ABVD at a median follow-up of 54 months.81 An Italian trial randomized 136 patients with clinical stage I unfavorable Hodgkin’s lymphoma and clinical stage IIA favorable and unfavorable Hodgkin’s lymphoma to either EFRT or IFRT after four cycles of ABVD.82 At a median follow-up of 116 months, no differences in 12-year FFP or OS were observed. Three cases of secondary malignant disease were reported, all of which were in the EFRT arm.
An involved field encompasses not only the involved nodes but also the other lymph nodes within the same lymph node region, as denoted by the Rye classification for staging (Fig. 74-1). Guidelines for IFRT to specific sites have been detailed by Yahalom and Mauch.83 For example, in a patient presenting with an enlarged cervical lymph node, an involved field would include the entire ipsilateral cervical chain and the supraclavicular region because these nodes are considered to be within one region. Similarly, an involved field for a patient with groin disease would encompass the inguinal and femoral nodes. In a patient with mediastinal involvement, the field would cover the mediastinal, hilar, subcarinal, and medial supraclavicular nodes. Although the hilar nodes are scored separately traditionally, the hilar and subcarinal nodes are included in the mediastinal field. In addition, the medial supraclavicular nodes are included in order to cover the upper mediastinum (top of T1). Of note, IFRT was developed in the two-dimensional treatment planning era, in which the field design was based largely on bony landmarks, with inclusion of a considerable volume of normal tissue in the irradiated field.
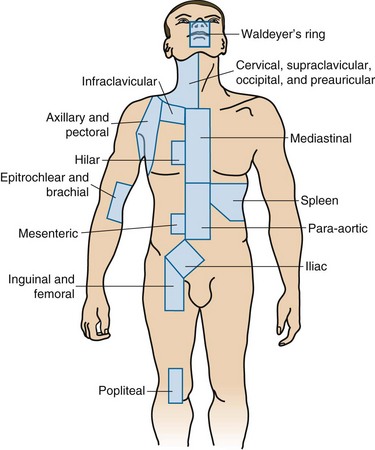
Figure 74-1 The anatomic definitions of separate lymph node regions based on the Rye classification for staging.
Although IFRT is considered the standard treatment field at this time as part of combined-modality therapy, there are data suggesting that relapse sites in Hodgkin’s lymphoma after chemotherapy alone are largely limited to the initially involved node.84 This has led to the concept of involved-node radiotherapy (INRT). The definition of INRT is in evolution and varies somewhat among groups. In general, the clinical target volume (CTV) is based on the prechemotherapy and postchemotherapy nodal volumes, with exclusion of normal displaced structures based on the postchemotherapy scan. The use of PET scanning in the treatment position is encouraged but is not mandated. In the European Organization for Research and Treatment of Cancer/Groupe d’Etudes des Lymphomes de l’Adulte (EORTC-GELA) H10 and H11 trials for early-stage favorable and unfavorable Hodgkin’s lymphoma, INRT was adopted in both the standard and experimental arms. The EORTC-GELA group defines the planning target volume (PTV) as a 1-cm isotropic expansion of the CTV to allow for organ motion and setup variations.85 In addition, four-dimensional planning and intensity-modulated radiation therapy (IMRT) were allowed.
Investigators from British Columbia reported results of their experience with INRT for early-stage Hodgkin’s lymphoma patients. At a median follow-up of 50 months, no local recurrences were observed. However, the INRT employed in this study encompassed the initially involved lymph nodes with a margin ranging from 1.5 cm to 5 cm to the field edge and therefore included a larger volume than that of the INRT as defined by the European groups.86 In addition, two-dimensional planning was performed in some of the patients.
The GHSG is planning a randomized trial for patients with risk factors (HD17) comparing IFRT and INRT. The GHSG defines the PTV as a 2-cm axial and 3-cm cranial caudal expansion of the CTV (if necessary, it can be reduced to 1 to 1.5 cm if there is close proximity to critical structures) and for mediastinal disease, a 1-cm axial and 2-cm cranial caudal expansion.78 This is the only randomized trial directly comparing IFRT with INRT, the results of which may provide information on the effectiveness of INRT after chemotherapy in preserving local control while limiting acute and long-term side effects. In the meantime, efforts to standardize the definition of INRT using modern image guidance and conformal radiotherapy technique are essential.
Optimal Radiation Dose
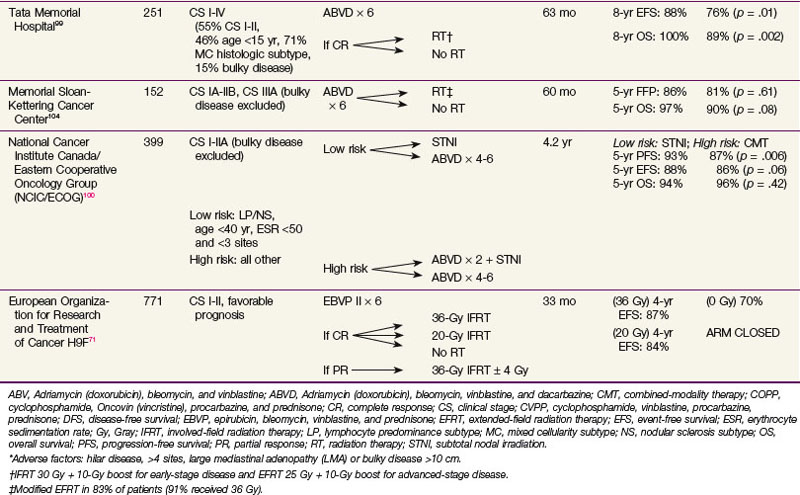
The appropriate radiation dose after chemotherapy in early-stage Hodgkin’s lymphoma has been explored by three trials.71,87,88 The EORTC H9F trial is a three-arm trial in which all patients receive six cycles of EBVP II.71 After a complete response, patients were randomized to receive no further treatment, 36-Gy IFRT, or 20-Gy IFRT. Patients with a partial response all received 36 Gy of IFRT with or without a 4-Gy boost. A complete response or complete response unconfirmed was achieved in 619 patients. At a median follow-up of 33 months, the 4-year EFS rates in the three arms of 36-Gy IFRT, 20-Gy IFRT, and no radiotherapy were 87%, 84%, and 70%, respectively. The difference in treatment results between the two doses of radiotherapy was not significant. However, the no-radiotherapy arm was closed early because stopping rules were met (>20% of events) (see later discussion).
The GHSG HD10 trial is a four-arm trial for low-risk disease (no bulky mediastinal mass or extranodal disease, fewer than three nodal sites, low sedimentation rate) in clinical stage I to II patients, in which comparisons were made between IFRT to 30 Gy versus 20 Gy after four or two cycles of ABVD.87 At a median follow-up of 7.5 years, there were no significant differences between 30 Gy and 20 Gy of IFRT in 8-year OS (94.9% vs. 95.6%), 8-year FFTF (87.8% vs. 88.6%), and 8-year PFS (88.1% vs. 88.9%). The question of optimal radiation dose in unfavorable patients was addressed by the GHSG HD11 trial, a four-arm trial comparing 20 Gy versus 30 Gy after four cycles of ABVD or baseline BEACOPP.77 At a median follow-up of 82 months, the 5-year FFTF rates were 81.1%, 85.3%, 86.8%, and 87%, respectively. Baseline BEACOPP was more effective than ABVD when followed by 20 Gy of IFRT, but the two regimens yielded similar outcomes when followed by 30 Gy of IFRT. There was no significant difference in 5-year FFTF rates between baseline BEACOPP and ABVD when followed by 30 Gy of IFRT; however, an inferiority of 20 Gy cannot be excluded after four cycles of ABVD, leading to the authors’ conclusion that four cycles of ABVD followed by 30 Gy of IFRT is the optimal therapy for early-stage, unfavorable Hodgkin’s lymphoma.
Chemotherapy Alone for Early-Stage Hodgkin’s Lymphoma
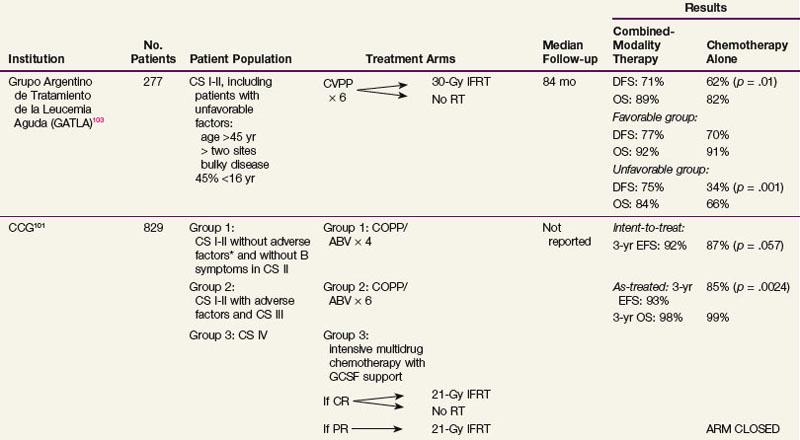
There are well-documented late effects of radiotherapy for Hodgkin’s lymphoma, based largely on patients treated with radiation therapy alone with larger treatment fields and higher doses of radiation than currently employed. These include risks of secondary malignant diseases,89–91,92 in particular, the risk of breast cancer in women irradiated at a young age,93,94 and lung cancer among smokers,95–97 and risks of cardiovascular disease.98 Because of these concerns, investigators have explored the option of eliminating radiation therapy and treating patients with early-stage disease with chemotherapy alone. Table 74-8 summarizes randomized trials that had compared combined-modality therapy with chemotherapy alone.* These trials varied in the study design, patient population, types of chemotherapy, and radiation fields employed. Three of the trials were limited to patients with early-stage disease only,100,102,103 and in the remaining three trials, advanced-stage patients were included.99,101,104 All but one trial showed a significant disease-free survival benefit with the addition of radiation therapy.99,100,101,102,103 One trial, which included stage I to IV patients, showed an overall survival benefit with radiation therapy.99 The Cochrane Haematological Malignancies Group recently conducted a meta-analysis105 in which five randomized controlled trials that compared chemotherapy alone with identical chemotherapy combined with radiotherapy for patients with stage I to II Hodgkin’s lymphoma were included. The results showed that, in addition to a significant disease-control benefit favoring the combined-modality therapy approach, there was a highly significant overall survival benefit with the addition of radiation therapy (HR, 0.4; p <.00001).
TABLE 74-8 Randomized Trials Comparing Combined-Modality Therapy and Chemotherapy Alone in Early-Stage Hodgkin’s Lymphoma
One of the key criticisms of trials that showed a significantly inferior outcome in the chemotherapy alone arm was the inadequate chemotherapy used in some of the trials, including the EBVP II used in the EORTC H9F study and the etoposide, epirubicin, bleomycin, cyclophosphamide, and prednisone (VEBEP) regimen used in an Italian trial discussed below.102,106 It therefore appears that less effective or abbreviated chemotherapy, or alternatives to ABVD designed to limit chemotherapy-related toxicity, is not acceptable when radiation therapy is omitted, and the addition of radiation therapy may allow the use of lower cumulative doses of doxorubicin or less toxic regimens. Toxicities associated with full-course ABVD may not be trivial and include myelosuppression, peripheral neuropathy, bleomycin lung toxicity, and cardiac toxicity.107,108,109 A significantly increased excess cardiac mortality rate has been demonstrated after ABVD without mediastinal irradiation.108,109
Convincing data on the high prognostic value of response to chemotherapy based on PET findings, especially for advanced-stage patients,53,110 have led to an increasing interest in the use of PET response (either early in the course of chemotherapy or at the end of chemotherapy) to identify patients in whom radiation therapy can be eliminated. Picardi and colleagues106 conducted a randomized trial designed to evaluate whether radiation therapy can be safely eliminated if a complete response by PET scanning is achieved after chemotherapy. A total of 260 patients with stage I to IV Hodgkin’s lymphoma with a tumor mass of more than 5 cm were included in the study. One hundred sixty patients became PET negative and had a 75% or more reduction in the tumor mass at the completion of six cycles of VEBEP. These patients were randomized to 32 Gy of IFRT versus no further treatment. At a median follow-up of 40 months, there was a significant disease-free survival benefit with the addition of consolidative radiation therapy (96% vs. 86%; p = .03), suggesting that even in carefully selected patients based on an optimal functional imaging response to chemotherapy, the omission of radiation therapy is associated with a higher relapse rate. Several randomized trials were designed to specifically address the use of PET response to identify patients with early-stage Hodgkin’s lymphoma who may be candidates for chemotherapy alone.69,111 In the EORTC-GELA H10 trial,111 favorable early-stage patients were randomized to the standard arm of three cycles of ABVD followed by INRT, versus the experimental arm of two cycles of ABVD followed by PET scanning. If the scan was negative, patients received two additional cycles of ABVD and then no further treatment. If the PET scan was positive, patients received two cycles of dose-escalated BEACOPP, followed by INRT. In the EORTC-GELA H11 trial for patients with unfavorable early-stage disease, the standard arm consisted of four cycles of ABVD followed by INRT, whereas patients on the experimental arm received two cycles of ABVD followed by PET scanning. If the scan was negative, patients received four additional cycles of ABVD and then no further treatment. If the PET scan was positive, patients received two cycles of dose-escalated BEACOPP, followed by INRT. The experimental arms in both trials were recently closed at the recommendation of an independent data monitoring committee, based on the interim analysis results demonstrating that it is likely that both studies will show that chemotherapy alone is inferior to combined-modality treatment in interim-PET negative patients. In a British trial,69 patients with clinical stage IA and IIA nonbulky Hodgkin’s lymphoma underwent PET scanning after three cycles of ABVD. If the scan was negative, patients were randomized to IFRT versus no further treatment. This trial has completed accrual, although results are currently pending. The GHSG recently initiated the HD16 trial for low-risk early-stage patients, comparing two cycles of ABVD followed by 30-Gy IFRT irrespective of PET results after chemotherapy, versus two cycles of ABVD followed by 30-Gy IFRT only for patients with positive PET scans after chemotherapy and no further therapy if PET scans are negative after chemotherapy. Therefore, at the current time, there are no available data to support the omission of radiation therapy based on PET response or early PET response in patients with early-stage Hodgkin’s lymphoma, an approach that should be reserved in the context of a clinical trial.
Recommendation on Treatment for Early-Stage Hodgkin’s Lymphoma
The optimal treatment for early-stage Hodgkin’s lymphoma is in evolution. At this time, in early-stage patients with favorable disease based on GHSH criteria (no bulky mediastinal mass or extranodal disease, fewer than three nodal sites, low sedimentation rate), two cycles of ABVD chemotherapy followed by IFRT to 20 Gy may be adequate.87 In patients with unfavorable early-stage disease, four cycles of ABVD may be adequate, but 30 Gy of IFRT is needed after ABVD.
Primary Treatment for Advanced-Stage Hodgkin’s Lymphoma
The introduction of the combination chemotherapy regimen MOPP in the mid-1960s substantially improved the curability of patients with advanced-stage Hodgkin’s lymphoma.4 The ABVD regimen was initially introduced as a form of second-line therapy in patients who had a poor response to, or relapse after, MOPP chemotherapy.112 Its role as primary treatment in newly diagnosed patients was subsequently substantiated by several randomized trials in advanced-stage patients, showing that ABVD-containing regimens are associated with a higher failure-free survival rate than MOPP regimens.113,114,115,116 Results of an intergroup randomized trial comparing MOPP/ABV hybrid versus ABVD in advanced-stage Hodgkin’s lymphoma showed that the addition of MOPP to ABVD did not confer any therapeutic benefit but did add to treatment-related toxicity.117 ABVD is significantly less myelosuppressive and it also does not carry the risk of gonadal dysfunction and leukemogenesis. Currently, in the United States, ABVD is accepted by most as the standard systemic therapy for advanced-stage Hodgkin’s lymphoma, which yields long-term failure-free survival rates of 60% to 65% and OS rates of 70% to 75%. To further improve treatment results, dose-escalated and/or dose-dense regimens for advanced-stage Hodgkin’s lymphoma have been developed. The two major regimens, BEACOPP and its variants, and Stanford V, are discussed below.
Alternatives to ABVD
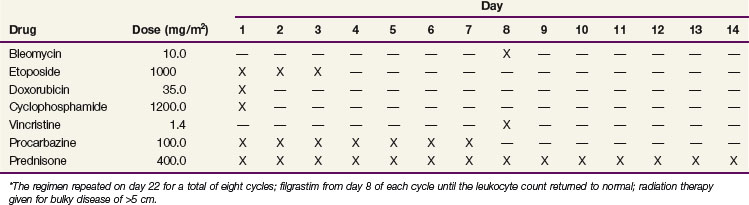
There have been promising data on more dose-dense and dose-intense regimens, including the dose-escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, Oncovin (vincristine), procarbazine, and prednisone (BEACOPP) (Table 74-9) and Stanford V regimens.118,119 In the most recent update of the GHSG HD9 study on 1196 evaluable patients, at a median follow-up of 111 months, the 10-year freedom from relapse was significantly higher in the dose-escalated BEACOPP arm than the baseline BEACOPP and COPP-ABVD arms (82%, 70%, and 64%; p <.0001).120 The corresponding 10-year OS were 86%, 80%, and 75% (p = .0005). In the standard arm of this trial, however, COPP-ABVD, rather than modern ABVD, was used. Also, it was noted that patients on the dose-escalated BEACOPP arm had a significantly higher 10-year cumulative incidence of acute myelogenous leukemia/myelodysplasia (3.2%, 2.2%, and 0.4%; p = .03).
The GHSG and others have explored modifications of the dose-escalated BEACOPP regimen, including dose-escalated BEACOPP followed by standard-dose BEACOPP,121 and the BEACOPP-14 regimen,78,122 a time-intensified variant of standard-dose BEACOPP. Comparisons of modified dose-escalated BEACOPP against ABVD have shown higher PFS rates but at the expense of increased toxicity and a lack of overall survival benefit.123,124 The EORTC protocol 20012 is comparing four cycles of escalated-dose BEACOPP followed by four cycles of standard-dose BEACOPP to eight cycles of ABVD in stage III to IV patients with an IPS of 3, and the results are pending.
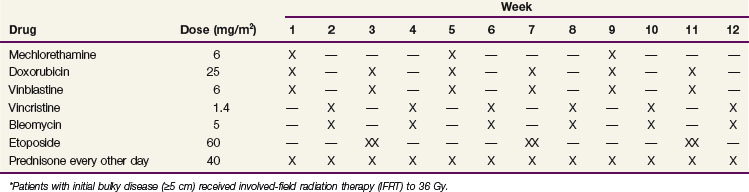
The Stanford V regimen is a 12-week, seven-drug regimen that is administered on a weekly basis118 (Table 74-10). It contains lower cumulative doses of mechlorethamine, Adriamycin (doxorubicin), and bleomycin than MOPP and ABVD, respectively, in order to limit leukemogenesis, sterility, and cardiac and pulmonary toxicity. Patients with initial disease of 5 cm or larger and/or macroscopic splenic disease (≈90% of patients) receive 36 Gy of IFRT 2 weeks after the chemotherapy. Phase II studies have shown promising results with the Stanford V regimen in patients with advanced-stage or locally extensive Hodgkin’s lymphoma.118,125 Stanford V, when given with radiotherapy as specified by the original protocol (IFRT to initial sites 5 cm or larger and/or macroscopic splenic disease), appears to yield results comparable to ABVD126 but has a poorer than expected treatment outcome when radiotherapy is not delivered according to the guidelines.127 Results are pending from the Eastern Cooperative Oncology Group (ECOG) E2496 Intergroup phase II trial comparing six to eight cycles of ABVD with Stanford V.
The Role of Radiation Therapy in Advanced-Stage Hodgkin’s Lymphoma
The rationale for the addition of radiation therapy to combination chemotherapy in advanced-stage Hodgkin’s lymphoma is based on the patterns of failure after chemotherapy, in which the majority of relapses are at the site of initial disease.128,129 A number of randomized trials have been performed addressing the role of consolidative radiation therapy after chemotherapy in advanced-stage Hodgkin’s lymphoma.80,130–133,134 Table 74-11 summarizes some of the more recent randomized studies comparing combined-modality therapy versus chemotherapy alone, using the same chemotherapy regimens in both arms, for advanced-stage Hodgkin’s lymphoma.121,130,133,134 The results of these trials were largely negative for significant benefit from the addition of radiation therapy. The previously described study from Tata Memorial Hospital, which included patients of all stages, is the only study that showed a significant survival benefit of combined-modality therapy over chemotherapy alone.99 On subgroup analysis, the survival benefit was limited to patients with advanced-stage disease. The 8-year OS for patients with stage III to IV disease was 80% in the chemotherapy-alone arm versus 100% in the combined-modality arm (p = .006). Other subgroups that appeared to especially benefit from radiation therapy in this trial included patients with B symptoms and younger-age patients.
TABLE 74-11 Randomized Trials Comparing Combined-Modality Therapy and Chemotherapy Alone in Advanced-Stage Hodgkin’s Lymphoma
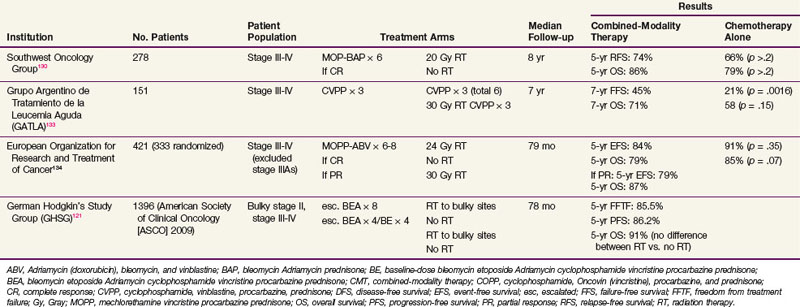
In the randomized trial conducted by the Southwest Oncology Group (SWOG),130 patients who achieved a complete remission were randomized to 20 Gy of radiation therapy to sites of initial disease versus no further treatment. Although no differences in relapse-free survival and OS were detected for the overall group, patients with the nodular sclerosis histologic subtype and/or bulky disease (defined as ≥6 cm) had a significant relapse-free survival benefit with the addition of radiation therapy. The 5-year relapse-free survival for patients with the nodular sclerosis histologic subtype randomized to radiation therapy was 77% compared with 56% for those randomized to no further treatment (p = .01). For patients with bulky disease and nodular sclerosis histologic findings, the 5-year remission duration estimates with and without radiation therapy were 76% and 46%, respectively (p = .006).
In the EORTC trial, stage III to IV patients with a complete response to MOPP-ABV were randomized to receive either radiation therapy or no further treatment.134 At a median follow-up of 79 months, there were no differences in the 5-year EFS or OS. The 5-year cumulative risk of secondary malignant disease was significantly higher in the 172 patients randomized to radiation therapy than the 161 patients randomized to no radiation therapy (7.8% and 4%; p = .05). However, among the patients with a partial response who received radiation therapy to a higher dose, the 5-year cumulative risk of secondary malignant disease was only 3.2%; the reason for the discrepancy is not clear. The authors also reported results of the 227 patients with a partial response who went on to receive 30 Gy of consolidative radiation therapy with or without an additional boost.98 The 8-year EFS and OS were 76% and 84%, respectively, which were comparable to the treatment results of patients who had a complete response, leading to the conclusion that patients with a partial response after chemotherapy may benefit from radiation therapy.
One study analyzed the outcomes of nonrandomized consolidative IFRT given after an objective response to chemotherapy in 702 patients with advanced-stage or early-stage unfavorable Hodgkin’s lymphoma.69 Consolidative radiation therapy of at least 30 Gy was recommended for patients with an incomplete response to chemotherapy or bulky disease at presentation, and was received by 300 of the 702 patients. With a median follow-up of 6.9 years, a significantly higher PFS (71% vs. 86%; p <.0001) and OS (87% vs. 93%; p = .014) were found in patients who received radiation therapy, despite the fact that there were significantly more patients with bulky disease and patients with a partial response in the radiation therapy cohort.
As previously described, one of the study questions of the GHSG HD12 study is the role of radiation therapy in advanced-stage Hodgkin’s lymphoma.121 At a median follow-up of 78 months, there was no significant difference between the radiotherapy or no-radiotherapy arms after either eight cycles of dose-escalated BEACOPP or four cycles of escalated and four cycles of baseline BEACOPP. In the GHSH HD15 trial comparing eight cycles of dose-escalated BEACOPP, six cycles of dose-escalated BEACOPP, versus eight cycles of BEACOPP-14, radiation therapy was limited to patients with residual disease of 2.5 cm and with residual PET avidity. Kobe and colleagues78 reported results of 311 patients from this trial who had residual disease of 2.5 cm and underwent postchemotherapy PET scanning. Sixty-six patients (21%) were found to have residual PET avidity, 63 of whom received 30 Gy of radiation therapy to the residual mass as per the protocol. Among patients with at least 12 months of follow-up, the 1-year PFS rates for PET-negative patients and PET-positive patients were 96% and 85%, respectively (p = .011). Longer follow-up is needed to confirm the excellent negative predictive value of PET in this study. Although the treatment outcome of patients with residual PET avidity who received radiation therapy was inferior to those with negative PET in this study, the results are far superior to those reported in other series for patients with PET residual disease after chemotherapy, with PFS ranging from 0% to 33%.110,135
Optimal Radiation Dose and Field Size
There are limited data on the optimal dose and appropriate radiation treatment field after chemotherapy in advanced-stage Hodgkin’s lymphoma. Randomized data are not available comparing radiation doses or fields as part of combined-modality therapy in advanced-stage Hodgkin’s lymphoma. An earlier GHSG study combined data from two of the randomized trials on patients with stage I to III disease, with or without bulk, and found no differences in FFTF and OS rates between 20 Gy, 30 Gy, and 40 Gy of consolidative radiation therapy.132 The radiation dose employed in several more recent trials of advanced-stage Hodgkin’s lymphoma varied. In general, initially nonbulky sites, if treated, received doses of up to 20 to 30 Gy, initially bulky sites received 30 to 36 Gy, and sites with partial response were treated to 30 to 40 Gy.*
The treatment fields that have been used in the trials are also variable. For instance, in the EORTC trial, the protocol mandated irradiating all initially involved areas (except for bone marrow) and that both the spleen and the para-aortic nodes be included in the treatment field even if only one of the sites was involved.134 Patients also received low-dose whole-lung or whole-liver irradiation if disease was initially present at these sites. Although referred to as “involved-field” radiation therapy, patients can be treated to a large volume due to their disease extent. Other groups have restricted the radiation treatment field to initially bulky sites. In both the Stanford V and the original BEACOPP regimens in the GHSG HD9 trial, only sites that were initially 5 cm or greater were included in the radiation field.118,119 Now that more effective systemic therapy has been developed and functional imaging has become available to assess the response to systemic therapy, and because concerns have been raised about the toxicity associated with large-field radiation therapy, further efforts have been made to limit the field size if radiation therapy is to be given. In the HD15 trial, for patients receiving radiation therapy, the treatment field was limited to postchemotherapy residual masses. Analysis of patterns of failure of patients in these trials will provide useful information on the optimal radiation treatment field in advanced-stage patients.
Primary Treatment For Nodular Lymphocyte Predominance Hodgkin’s Lymphoma
In the Revised European-American Lymphoma (REAL) and World Health Organization (WHO) classification systems, nodular lymphocyte predominance Hodgkin’s lymphoma (NLPHD) is classified as a distinct entity based on morphologic and immunophenotypical features.33,34 Clinically, it is characterized by a male predominance, older age at diagnosis of 30 to 50 years, peripheral nodal presentation with seldom-seen mediastinal, liver, spleen, or bone marrow involvement, predominantly early-stage disease, an indolent clinical course, and late multiple relapses.38,136–138,139,140 Because of its rarity (≈5% of all cases of Hodgkin’s lymphoma), there is a lack of randomized data about the guidance of its management, which ranges from watching and waiting,137 surgery alone,141 radiation therapy with or without chemotherapy,* or immunotherapy.145 Observations have been made that ABVD may not be as effective in patients with NLPHD and that regimens that containing alkylating agents may be a better choice when chemotherapy is indicated.136,146 Because NLPHD is rarely fatal, and the main causes of death in these patients are treatment related rather than disease related,136,137,140 it is sensible to choose a modality with well-established effectiveness while limiting the treatment exposure of these patients. Several series have reported on results of radiation therapy for early-stage NLPHD, with relapses occurring in 20% to 25% of patients, typically at sites outside of the irradiated field.136,139,143,144,147 Patients tend to remain responsive to further therapy despite multiple relapses, however.137 The use of more limited treatment fields such as IFRT or regional-field radiation therapy to 30 to 36 Gy did not appear to compromise the treatment outcome compared with the use of more extensive fields.139,144 In patients with neck or axillary involvement, the mediastinum, which is rarely involved, can be blocked, thereby avoiding exposure of the lungs and heart to radiation. Because of the small number of available cases, collaborative efforts among multiple large institutions are essential to provide answers on the optimal treatment for this disease entity.
Refractory and Recurrent Disease
The standard salvage therapy for patients with refractory disease or relapsed disease after a short initial remission following chemotherapy is high-dose therapy with autologous bone marrow or stem cell transplantation. Two randomized trials compared high-dose therapy with bone marrow or hematopoietic stem cell rescue versus conventional-dose chemotherapy as salvage for patients with refractory or relapsed Hodgkin’s lymphoma after chemotherapy.148,149 The British National Lymphoma Investigation group prospectively randomized 40 patients to high-dose BCNU, etoposide, cytarabine, melphalan (BEAM therapy) followed by autologous bone marrow transplantation or to mini-BEAM therapy.149 The inclusion criteria were a lack of complete response after MOPP or a similar regimen or relapsed disease within 1 year, or disease failure after two or more chemotherapy regimens. At a median follow-up of 34 months, the 3-year actuarial EFS rates of the two arms were 53% and 10%, respectively (p = .025) and the PFS rates were 88% and 35%, respectively (p = .005), favoring the high-dose therapy arm. The GHSG conducted a similar study comparing high-dose BEAM followed by autologous stem cell transplantation and dexamethasone with conventional-dose BEAM.148 Only patients with chemosensitive disease were included in the trial. At a median follow-up of 39 months, the FFTF rates of the two arms were 55% and 34%, respectively (p = .019). Significant survival differences were not detected in either of the two trials, however.
One of the key factors that influences the outcome is chemosensitivity to second-line cytoreductive chemotherapy prior to the high-dose therapy and transplantation.65,150–156 Other factors that have been shown to be of prognostic significance include duration of complete response to initial treatment,151 extranodal disease,151,152 and constitutional symptoms65,151,155 or bulky disease150 at the time of relapse. The Hasenclever index score can also be used as a prognostic indicator.156
Role of Radiation Therapy Before or After High-Dose Therapy
Further relapses after high-dose therapy tend to occur at sites of initial relapsed disease.157 The role of IFRT given either before or after high-dose therapy has not been addressed prospectively by randomized trials, but retrospective studies have indicated that the addition of radiation therapy may contribute to improved outcome. In one study from Stanford, subgroups of patients who significantly benefited from IFRT given either before or after the transplantation included patients with relapsed stage I to III disease and patients who did not receive prior radiation therapy.72 In a study from the University of Chicago, the use of IFRT in conjunction with high-dose therapy significantly improved local control of all sites of disease.158 Patients with persistent disease following high-dose therapy had significantly improved PFS (40% vs. 12.1%; p = .04). Wendland and associates159 reviewed 65 patients with refractory or relapsed Hodgkin’s lymphoma who received high-dose therapy and transplantation. Twenty-one patients received IFRT, and 44 patients did not receive radiation therapy. Half of the patients in the no IFRT group (22 of 44) died compared with 23.8% (5 of 21) of patients in the IFRT group, a finding that was of borderline significance (p = .06). Similarly, Kahn and colleagues,159a in a study examining the role of IFRT in Hodgkin’s lymphoma and non-Hodgkin’s lymphoma patients receiving transplant, found that patients who did not receive IFRT had a 2.09 relative risk of death, which was of borderline statistical significance on multivariable analysis (p = .066). Results of these retrospective series need to be interpreted with caution because of differences in characteristics of patients who did and did not receive radiation therapy. For example, patients who were offered radiation therapy tended to have bulkier disease or chemotherapy-refractory disease, whereas patients who did not receive radiation therapy may have had more disseminated disease.
Limited Relapse After Chemotherapy
A small proportion of patients who relapsed after chemotherapy can be successfully salvaged with conventional-dose salvage chemotherapy and/or radiation therapy. A number of series showed that in selected patients with favorable features, a salvage rate as high as 80% can be achieved without exposing patients to high-dose therapy.160–165 Potential candidates for conventional dose-salvage therapy include patients with an initial remission duration of 1 year or longer, limited nodal relapse without extranodal disease, and absence of constitutional symptoms at relapse. In one series of 28 patients with limited nodal relapse after chemotherapy, the combination of salvage chemotherapy and radiation therapy yielded outcomes significantly superior to those of radiation therapy alone.162 In patients who received combined-modality salvage therapy, the 7-year freedom from further relapse rate was 93% and the OS rate was 85%, whereas the corresponding actuarial estimates in patients treated with radiation therapy alone were significantly lower at 36% (p = .002) and 36% (p = .03), respectively. These data suggest that in carefully selected patients with favorable criteria at relapse, salvage with conventional-dose therapy can be considered, thereby sparing patients the toxicity of high-dose therapy.
Techniques of Radiation Therapy
Modern Conformal Therapy
IMRT uses multiple beams over the targeted volume to provide a highly conformal radiation dose distribution. Girinsky and colleagues166 compared five different IMRT treatment plans against three-dimensional conformal radiation therapy and conventional treatment in patients with Hodgkin’s lymphoma with mediastinal involvement. IMRT setups were associated with significantly better PTV coverage and lower doses to the heart and to the origin of the coronaries. However, compared with conventional treatment and three-dimensional conformal therapy, IMRT resulted in significantly higher median doses to the lungs and to the breasts. The spreading out of low doses to large volumes and the associated potential increased secondary malignant disease risks with IMRT techniques have precluded its routine use in the treatment of Hodgkin’s lymphoma patients. It continues to have a role in selected cases, however, including extensive chest wall involvement where conventional technique would result in exposing large volumes of the lungs in the irradiated field, or in the retreatment setting where there is significant dose limitation to vital structures such as the spinal cord. Goodman and associates167 reported on their experience of using IMRT for extremely large mediastinal masses or in patients with prior radiation therapy to the mediastinum. In these special case scenarios, the use of IMRT resulted not only in improved PTV coverage but also in lower mean doses to the lungs.
Another modern conformal technique is proton therapy, in which charged particles deposit most of their energy at a proportional depth, resulting in a characteristic dose distribution known as the Bragg peak. With the addition of multiple energies, a spread-out Bragg peak can be created such that the target can be covered by only a limited number of fields. Investigators from the University of Florida compared the hypothetical plans of conventional radiotherapy, IMRT, and three-dimensional proton INRT among nine patients with stage II Hodgkin’s lymphoma.168 In this study, the CTV was based on a 5-mm expansion of the postchemotherapy volume, and the PTV was defined as a uniform 3-mm expansion of the CTV. Proton beam therapy was found to result in significantly lower mean doses to the whole body, lungs, and breasts compared with the other two techniques, and PTV coverage was comparable among the three techniques. Although the results suggested that there are dosimetric advantages with protons, the adoption of proton beam therapy for Hodgkin’s lymphoma is limited by the lack of clinical data and the limited availability of proton facilities.
The Role of PET in Guiding Radiation Planning
PET scanning, which has been shown to be more accurate than CT in the staging of Hodgkin’s lymphoma, can allow more precise definition of the initially involved nodes. Hutchings and colleagues169 identified 30 patients with Hodgkin’s lymphoma treated with IFRT. All patients had prechemotherapy PET/CT scans, although only the CT component was used for planning purposes. When the PET data were incorporated retrospectively in the planning, the IFRT was increased in seven patients (23%) and decreased in two patients (7%). Girinsky and colleagues166 fused the prechemotherapy PET scan with the postchemotherapy CT planning scan in 30 Hodgkin’s lymphoma patients treated with INRT. In 11 of the 30 patients (36%), the PET scan identified involved nodal disease that was missed by the CT scan, resulting in changes of the radiation treatment field. It was also noted that on average only 25% of the lymphoma volume on CT showed FDG avidity on PET, leading to the conclusion that the target must include both CT-positive and PET-positive disease. In the current guidelines for INRT by both the EORTC/GELA and GHSG, the use of prechemotherapy PET in the treatment position fused with the planning CT is encouraged, but not mandatory, in determining the CTV.
Respiratory Motion Adaptation Techniques
As radiation treatment fields become more conformal, it is important to take into account respiratory motion, especially when treating sites that are subject to the effect of diaphragmatic motion, such as the mediastinum, lungs, pericardial nodes, or spleen. Breathing adaptation techniques, in the simplest form, involve covering the entire span of respiratory motion of the target on a four-dimensional CT scan. With respiratory gating, the radiation beam is turned on only during a prespecified period of the respiratory cycle, such that organ motion within the radiation field is limited to the motion taking place during the prespecified beam-on time. Four-dimensional planning may be of particular relevance with INRT in which the expansion from CTV to PTV can be as limited as 1 cm. Other breathing adaptation techniques include breath-holding techniques, which have been studied in Hodgkin’s lymphoma patients.170,171 Radiation delivery at deep inspiration was shown to result in reduced doses to the heart and lungs in Hodgkin’s lymphoma patients receiving mediastinal irradiation. In the EORTC/GELA and GHSG guidelines for INRT, respiratory-gated radiotherapy is considered an option.
Treatment Algorithm, Challenges, and Future Directions
The treatment algorithm for patients with early-stage and advanced-stage Hodgkin’s lymphoma is outlined in Tables 74-12 and 74-13, respectively. In patients with early-stage disease without unfavorable features, cure rates of over 90% have been achieved. The key challenge in the management of these patients is to identify ways to limit late effects of Hodgkin’s lymphoma therapy because multiple studies have demonstrated that over time, there is a significantly increased risk of mortality from causes other than Hodgkin’s lymphoma, with secondary malignant disease and cardiac disease being the two leading problems.64
TABLE 74-12 Treatment Algorithm for Patients with Early-Stage Hodgkin’s Lymphoma
| Classical Hodgkin’s Lymphoma, Favorable Prognosis |
| Classical Hodgkin’s Lymphoma, Unfavorable Prognosis |
ABVD, Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine; CT, computed tomography; GHSG, German Hodgkin’s Study Group; Gy, Gray; IFRT, involved-field radiation therapy; PET, positron emission tomography.
* The use of two cycles of ABVD and 20-Gy IFRT limited to early-stage patients without risk factors as per GHSG criteria.
TABLE 74-13 Treatment Algorithm for Patients with Advanced-Stage Hodgkin’s Lymphoma
| Classical Hodgkin’s Lymphoma |
|
Six to eight cycles of ABVD (restaging PET/CT after two or three cycles and at the end of chemotherapy)
|
| Nodular Lymphocyte Predominent Hodgkin’s Lymphoma |
ABVD, Adriamycin (doxorubicin), bleomycin, vinblastine; and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, Oncovin [vincristine], procarbazine, and prednisone; ChlVPP, chlorambucil, vinblastine, procarbazine, and prednisolone; CT, computed tomography; Gy, Gray; PET, positron emission tomography; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone.
There are increasing data available showing that these late effects are related to radiation dose and volume.* A significant radiation dose-response relationship has been shown for the development of breast cancer,93,94,173 lung cancer,95,96 and, more recently, gastric cancer174 in Hodgkin’s lymphoma survivors. Data are also available showing a significantly reduced risk of breast cancer with more restricted treatment fields.92,175 These data justify current efforts of reducing radiation dose and field sizes in the treatment of early-stage Hodgkin’s lymphoma. For patients with an unfavorable prognosis or advanced-stage disease, the challenge remains of further improving the cure of Hodgkin’s lymphoma. Potentially more effective multiagent chemotherapy regimens are being developed and tested in clinical trials. In addition to traditional chemotherapy regimens, other promising approaches include the use of targeted immunotherapy regimens that may serve as adjuncts to chemotherapy,145,176–179 and myeloablative or nonmyeloablative allogeneic stem cell transplantation, in which the graft-versus-Hodgkin’s lymphoma effect may provide therapeutic benefits in patients with chemotherapy-refractory disease.125,180–182
15 Weiss LM, Movahed LA, Warnke RA, et al. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med. 1989;320:502-506.
33 Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms. A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392.
34 Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835-3849.
43 Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506-1514.
53 Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma. A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746-3752.
54 Press OW, LeBlanc M, Lichter AS, et al. Phase III randomized intergroup trial of subtotal lymphoid irradiation versus doxorubicin, vinblastine, and subtotal lymphoid irradiation for stage IA to IIA Hodgkin’s disease. J Clin Oncol. 2001;19:4238-4244.
55 Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med. 2007;357:1916-1927.
56 Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin’s lymphoma. Final results of the GHSG HD7 trial. J Clin Oncol. 2007;25:3495-3502.
60 Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101-2108.
61 Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21:3431-3439.
69 Johnson PW, Sydes MR, Hancock BW, et al. Consolidation radiotherapy in patients with advanced Hodgkin’s lymphoma. Survival data from the UKLG LY09 randomized controlled trial (ISRC TN97144519). J Clin Oncol. 2010;28:3352-3359.
73 Horning SJ, Williams J, Bartlett NL, et al. Assessment of the stanford V regimen and consolidative radiotherapy for bulky and advanced Hodgkin’s disease. Eastern Cooperative Oncology Group pilot study E1492. J Clin Oncol. 2000;18:972-980.
75 Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin’s lymphoma. Long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol. 2006;24:3128-3135.
77 Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma. Final analysis of the German Hodgkin Study Group HD11 Trial. J Clin Oncol. 2010;28:4199-4206.
78 Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112:3989-3994.
79 Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640-652.
81 Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma. Results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601-3608.
82 Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease. Long-term results. J Clin Oncol. 2004;22:2835-2841.
83 Yahalom J, Mauch P. The involved field is back. Issues in delineating the radiation field in Hodgkin’s disease. Ann Oncol. 2002;13(Suppl 1):79-83.
85 Girinsky T, van der Maazen R, Specht L, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma Concepts and guidelines. Radiother Oncol. 2006;79:270-277.
86 Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma. A question of field size. J Clin Oncol. 2008;26:5170-5174.
92 Franklin J, Pluetschow A, Paus M, et al. Second malignancy risk associated with treatment of Hodgkin’s lymphoma. Meta-analysis of the randomised trials. Ann Oncol. 2006;17:1749-1760.
93 van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971-980.
94 Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465-475.
95 van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiotherapy and smoking in lung cancer following Hodgkin’s disease. J Natl Cancer Inst. 1995;87:1530-1537.
96 Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182-192.
97 Swerdlow AJ, Schoemaker MJ, Allerton R, et al. Lung cancer after Hodgkin’s disease. A nested case-control study of the relation to treatment. J Clin Oncol. 2001;19:1610-1618.
98 Aleman BM, Raemaekers JM, Tomisic R, et al. Involved-field radiotherapy for patients in partial remission after chemotherapy for advanced Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2007;67:19-30.
99 Laskar S, Gupta T, Vimal S, et al. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy. Is there a need? J Clin Oncol. 2004;22:62-68.
101 Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765-3771.
104 Straus DJ, Portlock CS, Qin J, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 2004;104:3483-3489.
105 Herbst C, Rehan F, Brillant C, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin’s lymphoma. A systematic review. Hematologica. 2010;95:494-500.
106 Picardi M, De Renzo A, Pane F, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48:1721-1727.
108 Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease. A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206-214.
110 Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52-59.
115 Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478-1484.
118 Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease. Mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630-637.
119 Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:2386-2395.
120 Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma. 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548-4554.
126 Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s lymphoma. United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390-5396.
127 Gobbi PG, Levis A, Chisesi T, et al. ABVD versus modified stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin’s lymphoma. Final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol. 2005;23:9198-9207.
134 Aleman BM, Raemaekers JM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin’s lymphoma. N Engl J Med. 2003;348:2396-2406.
135 Advani R, Maeda L, Lavori P, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin’s disease. J Clin Oncol. 2007;25:3902-3907.
139 Nogova L, Reineke T, Eich HT, et al. Extended field radiotherapy, combined modality treatment or involved field radiotherapy for patients with stage IA lymphocyte-predominant Hodgkin’s lymphoma. A retrospective analysis from the German Hodgkin Study Group (GHSG). Ann Oncol. 2005;16:1683-1687.
144 Chen RC, Chin MS, Ng AK, et al. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma. Patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol. 2010;28:136-141.
158 Mundt AJ, Sibley G, Williams S, et al. Patterns of failure following high-dose chemotherapy and autologous bone marrow transplantation with involved field radiotherapy for relapsed/refractory Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1995;33:261-270.
169 Hutchings M, Loft A, Hansen M, et al. Clinical impact of FDG-PET/CT in the planning of radiotherapy for early-stage Hodgkin lymphoma. Eur J Haematol. 2007;78:206-212.
172 De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928-937.
174 van den Belt-Dusebout AW, Aleman BM, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420-1429.
175 De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma. Lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239-4246.
1 Hodgkin T. On some morbid experiences of the absorbent glands and spleen. Med Chir Trans. 1832;17:69-97.
2 Gilbert R. Radiotherapy in Hodgkin’s disease (malignant granulomatosis); anatomic and clinical foundations; governing principles, results. Am J Roentgenol. 1939;41:198-241.
3 Peters M. A study in survivals in Hodgkin’s disease treated radiologically. Am J Roentgenol. 1950;63:299-311.
4 DeVita V, Serpick A, Carbone P. Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann Intern Med. 1970;73:881-895.
5 Gloeckler Ries LA, Reichman ME, Lewis DR, et al. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541-552.
6 Macmahon B. Epidemiological evidence of the nature of Hodgkin’s disease. Cancer. 1957;10:1045-1054.
7 Grufferman S, Delzell E. Epidemiology of Hodgkin’s disease. Epidemiol Rev. 1984;6:76-106.
8 Serraino D, Franceschi S, Talamini R, et al. Socio-economic indicators, infectious diseases and Hodgkin’s disease. Int J Cancer. 1991;47:352-357.
9 Westergaard T, Melbye M, Pedersen JB, et al. Birth order, sibship size and risk of Hodgkin’s disease in children and young adults. A population-based study of 31 million person-years. Int J Cancer. 1997;72:977-981.
10 Chang ET, Zheng T, Weir EG, et al. Childhood social environment and Hodgkin’s lymphoma. New findings from a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1361-1370.
11 Miller RW, Beebe GW. Infectious mononucleosis and the empirical risk of cancer. J Natl Cancer Inst. 1973;50:315-321.
12 Kvale G, Hoiby EA, Pedersen E. Hodgkin’s disease in patients with previous infectious mononucleosis. Int J Cancer. 1979;23:593-597.
13 Mueller N, Evans A, Harris NL, et al. Hodgkin’s disease and Epstein-Barr virus. Altered antibody pattern before diagnosis. N Engl J Med. 1989;320:689-695.
14 Mueller N, Mohar A, Evans A, et al. Epstein-Barr virus antibody patterns preceding the diagnosis of non-Hodgkin’s lymphoma. Int J Cancer. 1991;49:387-393.
15 Weiss LM, Movahed LA, Warnke RA, et al. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med. 1989;320:502-506.
16 Wu TC, Mann RB, Charache P, et al. Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin’s disease. Int J Cancer. 1990;46:801-804.
17 Glaser SL, Lin RJ, Stewart SL, et al. Epstein-Barr virus-associated Hodgkin’s disease. Epidemiologic characteristics in international data. Int J Cancer. 1997;70:375-382.
18 Grufferman S, Cole P, Smith PG, et al. Hodgkin’s disease in siblings. N Engl J Med. 1977;296:248-250.
19 Grufferman S, Barton JW3rd, Eby NL. Increased sex concordance of sibling pairs with Behcet’s disease, Hodgkin’s disease, multiple sclerosis, and sarcoidosis. Am J Epidemiol. 1987;126:365-369.
20 Mack TM, Cozen W, Shibata DK, et al. Concordance for Hodgkin’s disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N Engl J Med. 1995;332:413-418.
21 Cozen W, Gill PS, Ingles SA, et al. IL-6 levels and genotype are associated with risk of young adult Hodgkin lymphoma. Blood. 2004;103:3216-3221.
22 Hors J, Dausset J. HLA and susceptibility to Hodgkin’s disease. Immunol Rev. 1983;70:167-192.
23 Oza AM, Tonks S, Lim J, et al. A clinical and epidemiological study of human leukocyte antigen-DPB alleles in Hodgkin’s disease. Cancer Res. 1994;54:5101-5105.
24 Staratschek-Jox A, Shugart YY, Strom SS, et al. Genetic susceptibility to Hodgki’s lymphoma and to secondary cancer. Workshop report. Ann Oncol. 2002;13(Suppl 1):30-33.
25 Kanzler H, Kuppers R, Hansmann ML, et al. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184:1495-1505.
26 Marafioti T, Hummel M, Foss HD, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95:1443-1450.
27 Rajewsky K, Kanzler H, Hansmann ML, et al. Normal and malignant B-cell development with special reference to Hodgkin’s disease. Ann Oncol. 1997;8(Suppl 2):79-81.
28 Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest. 1997;100:2961-2969.
29 Diehl V, Stein H, Hummel M, et al. Hodgkin’s lymphoma. Biology and treatment strategies for primary, refractory, and relapsed disease. Hematology Am Soc Hematol Educ Program. 2003:225-247.
30 Mathas S, Lietz A, Anagnostopoulos I, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med. 2004;199:1041-1052.
31 Chang ET, Zheng T, Weir EG, et al. Aspirin and the risk of Hodgkin’s lymphoma in a population-based case-control study. J Natl Cancer Inst. 2004;96:305-315.
32 Lukes RJ, Craver LF, Hall TC, et al. Report of the nomenclature committee. Cancer Res. 1966;26:1311.
33 Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms. A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392.
34 Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835-3849.
35 Bennett MH, MacLennan KA, Easterling MJ, et al. The prognostic significance of cellular subtypes in nodular sclerosing Hodgkin’s disease. An analysis of 271 non-laparotomised cases (BNLI report no. 22). Clin Radiol. 1983;34:497-501.
36 MacLennan KA, Bennett MH, Vaughan Hudson B, et al. Diagnosis and grading of nodular sclerosing Hodgkin’s disease. A study of 2190 patients. Int Rev Exp Pathol. 1992;33:27-51.
37 Kant JA, Hubbard SM, Longo DL, et al. The pathologic and clinical heterogeneity of lymphocyte-depleted Hodgkin’s disease. J Clin Oncol. 1986;4:284-294.
38 Anagnostopoulos I, Hansmann ML, Franssila K, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease. Histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96:1889-1899.
39 Gobbi PG, Cavalli C, Gendarini A, et al. Reevaluation of prognostic significance of symptoms in Hodgkin’s disease. Cancer. 1985;56:2874-2880.
40 Atkinson K, Austin DE, McElwain TJ, et al. Alcohol pain in Hodgkin’s disease. Cancer. 1976;37:895-899.
41 Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860-1861.
42 Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease. Cotswolds meeting. J Clin Oncol. 1989;7:1630-1636.
43 Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506-1514.
44 Rigacci L, Vitolo U, Nassi L, et al. Positron emission tomography in the staging of patients with Hodgkin’s lymphoma. A prospective multicentric study by the Intergruppo Italiano Linfomi. Ann Hematol. 2007;86:897-903.
45 Pelosi E, Pregno P, Penna D, et al. Role of whole-body [18F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) and conventional techniques in the staging of patients with Hodgkin and aggressive non Hodgkin lymphoma. Radiol Med. 2008;113:578-590.
46 Naumann R, Beuthien-Baumann B, Reiss A, et al. Substantial impact of FDG PET imaging on the therapy decision in patients with early-stage Hodgkin’s lymphoma. Br J Cancer. 2004;90:620-625.
47 Friedberg JW, Fischman A, Neuberg D, et al. FDG-PET is superior to gallium scintigraphy in staging and more sensitive in the follow-up of patients with de novo Hodgkin lymphoma. A blinded comparison. Leuk Lymphoma. 2004;45:85-92.
48 Erturk SM, Van den Abbeele AD. Role of PET/CT scanning in initial and post-treatment assessment of Hodgkin disease. J Natl Compr Canc Netw. 2008;6:623-632.
49 Elstrom R, Guan L, Baker G, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875-3876.
50 Hutchings M, Loft A, Hansen M, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica. 2006;91:482-489.
51 Macintyre EA, Vaughan Hudson B, Linch DC, et al. The value of staging bone marrow trephine biopsy in Hodgkin’s disease. Eur J Haematol. 1987;39:66-70.
52 Gomez-Almaguer D, Ruiz-Arguelles GJ, Lopez-MartInez B, et al. Role of bone marrow examination in staging Hodgkin’s disease. Experience in Mexico. Clin Lab Haematol. 2002;24:221-223.
53 Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma. A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746-3752.
54 Press OW, LeBlanc M, Lichter AS, et al. Phase III randomized intergroup trial of subtotal lymphoid irradiation versus doxorubicin, vinblastine, and subtotal lymphoid irradiation for stage IA to IIA Hodgkin’s disease. J Clin Oncol. 2001;19:4238-4244.
55 Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med. 2007;357:1916-1927.
56 Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin’s lymphoma. Final results of the GHSG HD7 trial. J Clin Oncol. 2007;25:3495-3502.
57 van Rijswijk RE, Verbeek J, Haanen C, et al. Major complications and causes of death in patients treated for Hodgkin’s disease. J Clin Oncol. 1987;5:1624-1633.
58 Cosset JM, Henry-Amar M, Meerwaldt JH. Long-term toxicity of early stages of Hodgkin’s disease therapy. The EORTC experience. EORTC Lymphoma Cooperative Group. Ann Oncol. 1991;2(Suppl 2):77-82.
59 Hoppe RT. Hodgkin’s disease. Complications of therapy and excess mortality. Ann Oncol. 1997;8:115-118.
60 Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101-2108.
61 Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21:3431-3439.
62 Mauch P, Ng A, Aleman B, et al. Report from the Rockefellar Foundation Sponsored International Workshop on reducing mortality and improving quality of life in long-term survivors of Hodgkin’s disease. July 9-16, 2003, Bellagio, Italy. Eur J Haematol Suppl. 2005:68-76.
63 Hodgson DC. Hodgkin lymphoma. The follow-up of long-term survivors. Hematol Oncol Clin North Am. 2008;22:233-244. vi
64 Ng AK, Mauch PM. Late effects of Hodgkin’s disease and its treatment. Cancer J. 2009;15:164-168.
65 Horning SJ, Chao NJ, Negrin RS, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease. Analysis of the Stanford University results and prognostic indices. Blood. 1997;89:801-813.
66 Moody AM, Pratt J, Hudson GV, et al. British National Lymphoma Investigation. Pilot studies of neoadjuvant chemotherapy in clinical stage Ia and IIa Hodgkin’s disease. Clin Oncol (R Coll Radiol). 2001;13:262-268.
67 Martinelli G, Cocorocchio E, Saletti PC, et al. Efficacy of vinblastine, bleomycin, methotrexate (VBM) combination chemotherapy with involved field radiotherapy in early stage (I-IIA) Hodgkin disease patients. Leuk Lymphoma. 2003;44:1919-1923.
68 Gobbi PG, Broglia C, Merli F, et al. Vinblastine, bleomycin, and methotrexate chemotherapy plus irradiation for patients with early-stage, favorable Hodgkin lymphoma. The experience of the Gruppo Italiano Studio Linfomi. Cancer. 2003;98:2393-2401.
69 Johnson PW, Sydes MR, Hancock BW, et al. Consolidation radiotherapy in patients with advanced Hodgkin’s lymphoma. Survival data from the UKLG LY09 randomized controlled trial (ISRC TN97144519). J Clin Oncol. 2010;28:3352-3359.
70 Tormo M, Terol MJ, Marugan I, et al. Treatment of stage I and II Hodgkin’s disease with NOVP (mitoxantrone, vincristine, vinblastine, prednisone) and radiotherapy. Leuk Lymphoma. 1999;34:137-142.
71 Thomas J, Fermé C, Noordijk E, et al. Results of the EORTC-GELA H9 randomized trials. The H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin’s lymphoma (HL). Haematologica. 2007;92:27.
72 Poen JC, Hoppe RT, Horning SJ. High-dose therapy and autologous bone marrow transplantation for relapsed/refractory Hodgkin’s disease. The impact of involved field radiotherapy on patterns of failure and survival. Int J Radiat Oncol Biol Phys. 1996;36:3-12.
73 Horning SJ, Williams J, Bartlett NL, et al. Assessment of the stanford V regimen and consolidative radiotherapy for bulky and advanced Hodgkin’s disease. Eastern Cooperative Oncology Group pilot study E1492. J Clin Oncol. 2000;18:972-980.
74 Advani R, Hoppe R, Baer D, et al. Efficacy of abbreviated Stanford v chemotherapy and involved field radiotherapy in early stage Hodgkin’s disease. Mature results of the G4 trial. Blood. 2009. Abstract 1670
75 Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin’s lymphoma. Long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol. 2006;24:3128-3135.
76 Ferme C, Diviné M, Vranovsky A, et al. Four ABVD and involved-field radiotherapy in unfavorable supradiaphragmatic clinical stages (CS) I-II Hodgkin’s lymphoma (HL). Preliminary results of the EORTC-GELA H9-U trial. Blood. 106, 2005. Abstract 813
77 Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma. Final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199-4206.
78 Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112:3989-3994.
79 Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640-652.
80 Ferme C, Sebban C, Hennequin C, et al. Comparison of chemotherapy to radiotherapy as consolidation of complete or good partial response after six cycles of chemotherapy for patients with advanced Hodgkin’s disease. Results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 trial. Blood. 2000;95:2246-2252.
81 Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma. Results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601-3608.
82 Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease. Long-term results. J Clin Oncol. 2004;22:2835-2841.
83 Yahalom J, Mauch P. The involved field is back: issues in delineating the radiation field in Hodgkin’s disease. Ann Oncol. 2002;13(Suppl 1):79-83.
84 Shahidi M, Kamangari N, Ashley S, et al. Site of relapse after chemotherapy alone for stage I and II Hodgkin’s disease. Radiother Oncol. 2006;78:1-5.
85 Girinsky T, van der Maazen R, Specht L, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma. Concepts and guidelines. Radiother Oncol. 2006;79:270-277.
86 Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma. A question of field size. J Clin Oncol. 2008;26:5170-5174.
87 Engert A, Diehl V, Pluetschow A, et al. Two cycles of ABVD followed by involved field radiotherapy with 20 Gray (Gy) is the new standard of care in the treatment of patients with early-stage Hodgkin lymphoma. Final analysis of the randomized German Hodgkin Study Group (GHSG) HD10. Study Supported by the Deutsche Krebshilfe and in part by the Competence Network Malignant Lymphoma. Blood. 2009. Abstract 716
88 Borchmann P, Diehl V, Goergen H, et al. Combined modality treatment with intensified chemotherapy and dose-reduced involved field radiotherapy in patients with early unfavourable Hodgkin lymphoma (HL). Final analysis of the German Hodgkin Study Group (GHSG) HD11 trial. Blood. 2009. Abstract 717
89 Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort. The relation to age at treatment. J Clin Oncol. 2000;18:498-509.
90 van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin’s disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487-497.
91 Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy. Long-term risks and risk factors. Blood. 2002;100:1989-1996.
92 Franklin J, Pluetschow A, Paus M, et al. Second malignancy risk associated with treatment of Hodgkin’s lymphoma. Meta-analysis of the randomised trials. Ann Oncol. 2006;17:1749-1760.
93 van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971-980.
94 Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465-475.
95 van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiotherapy and smoking in lung cancer following Hodgkin’s disease. J Natl Cancer Inst. 1995;87:1530-1537.
96 Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182-192.
97 Swerdlow AJ, Schoemaker MJ, Allerton R, et al. Lung cancer after Hodgkin’s disease. A nested case-control study of the relation to treatment. J Clin Oncol. 2001;19:1610-1618.
98 Aleman BM, Raemaekers JM, Tomisic R, et al. Involved-field radiotherapy for patients in partial remission after chemotherapy for advanced Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2007;67:19-30.
99 Laskar S, Gupta T, Vimal S, et al. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy. Is there a need? J Clin Oncol. 2004;22:62-68.
100 Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin’s disease. A phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14:1762-1767.
101 Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765-3771.
102 Noordijk E, Thomas J, Fermé C, et al. First results of the EORTC-GELA H9 randomized trials. The H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin’s lymphoma (HL). ASCO Annual Meeting Proceedings. 2005;23:6505.
103 Pavlovsky S, Maschio M, Santarelli MT, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage I-II Hodgkin’s disease. J Natl Cancer Inst. 1988;80:1466-1473.
104 Straus DJ, Portlock CS, Qin J, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 2004;104:3483-3489.
105 Herbst C, Rehan F, Brillant C, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin lymphoma. A systematic review. Haematologica. 2010;95:494-500.
106 Picardi M, De Renzo A, Pane F, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48:1721-1727.
107 Martin WG, Ristow KM, Habermann TM, et al. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23:7614-7620.
108 Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease. A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206-214.
109 Aviles A, Neri N, Nambo JM, et al. Late cardiac toxicity secondary to treatment in Hodgkin’s disease. A study comparing doxorubicin, epirubicin and mitoxantrone in combined therapy. Leuk Lymphoma. 2005;46:1023-1028.
110 Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52-59.
111 André M, Reman O, Fédérico M, et al. First Report On the H10 EORTC/GELA/IIL randomized intergroup trial on early fdg-pet scan guided treatment adaptation versus standard combined modality treatment in patients with supra-diaphragmatic stage I/II Hodgkin’s lymphoma, for the Groupe d’Etude des Lymphomes de l’Adulte (GELA), European Organisation for the Research and Treatment of Cancer (EORTC) Lymphoma Group and the Intergruppo Italiano Linfomi (IIL). Blood. 2009. Abstract 97
112 Santoro A, Bonadonna G, Bonfante V, et al. Alternating drug combinations in the treatment of advanced Hodgkin’s disease. N Engl J Med. 1982;306:770-775.
113 Santoro A, Bonadonna G, Valagussa P, et al. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin’s disease. Superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5:27-37.
114 Somers R, Carde P, Henry-Amar M, et al. A randomized study in stage IIIB and IV Hodgkin’s disease comparing eight courses of MOPP versus an alteration of MOPP with ABVD. A European Organization for Research and Treatment of Cancer Lymphoma Cooperative Group and Groupe Pierre-et-Marie-Curie controlled clinical trial. J Clin Oncol. 1994;12:279-287.
115 Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478-1484.
116 Canellos G, Niedzwiecki D, Johnson J. Long-term follow-up of survival in Hodgkin’s lymphoma. N Engl J Med. 2009;361:2390-2391.
117 Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease. Report of an intergroup trial. J Clin Oncol. 2003;21:607-614.
118 Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease. Mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630-637.
119 Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:2386-2395.
120 Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma. 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548-4554.
121 Diehl V, Haverkamp H, Mueller R, et al. Eight cycles of BEACOPP escalated compared with 4 cycles of BEACOPP escalated followed by 4 cycles of BEACOPP baseline with or without radiotherapy in patients in advanced stage Hodgkin lymphoma (HL). Final analysis of the HD12 trial of the German Hodgkin Study Group (GHSG). J Clin Oncol. 27, 2009. Abstract 8544
122 Sieber M, Bredenfeld H, Josting A, et al. 14-day variant of the bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone regimen in advanced-stage Hodgkin’s lymphoma. Results of a pilot study of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:1734-1739.
123 Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma. Results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27:805-811.
124 Gianni A, Rambaldi A, Zinzani P, et al. Comparable 3-year outcome following ABVD or BEACOPP first-line chemotherapy, plus pre-planned high-dose salvage, in advanced Hodgkin lymphoma (HL). A randomized trial of the Michelangelo, GITIL and IIL cooperative groups. J Clin Oncol. 26, 2008. Abstract 8506
125 Edwards-Bennett SM, Jacks LM, Moskowitz CH, et al. Stanford V program for locally extensive and advanced Hodgkin lymphoma. The Memorial Sloan-Kettering Cancer Center experience. Ann Oncol. 2010;21:574-581.
126 Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s lymphoma. United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390-5396.
127 Gobbi PG, Levis A, Chisesi T, et al. ABVD versus modified stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin’s lymphoma. Final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol. 2005;23:9198-9207.
128 Brizel DM, Winer EP, Prosnitz LR, et al. Improved survival in advanced Hodgkin’s disease with the use of combined modality therapy. Int J Radiat Oncol Biol Phys. 1990;19:535-542.
129 Yahalom J, Ryu J, Straus DJ, et al. Impact of adjuvant radiation on the patterns and rate of relapse in advanced-stage Hodgkin’s disease treated with alternating chemotherapy combinations. J Clin Oncol. 1991;9:2193-2201.
130 Fabian CJ, Mansfield CM, Dahlberg S, et al. Low-dose involved field radiation after chemotherapy in advanced Hodgkin disease. A Southwest Oncology Group randomized study. Ann Intern Med. 1994;120:903-912.
131 Diehl V, Loeffler M, Pfreundschuh M, et al. Further chemotherapy versus low-dose involved-field radiotherapy as consolidation of complete remission after six cycles of alternating chemotherapy in patients with advance Hodgkin’s disease. German Hodgkin’s Study Group (GHSG). Ann Oncol. 1995;6:901-910.
132 Loeffler M, Diehl V, Pfreundschuh M, et al. Dose-response relationship of complementary radiotherapy following four cycles of combination chemotherapy in intermediate-stage Hodgkin’s disease. J Clin Oncol. 1997;15:2275-2287.
133 Pavlovsky S, Santarelli MT, Muriel FS, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage III-IV A & B Hodgkin’s disease. Ann Oncol. 1992;3:533-537.
134 Aleman BM, Raemaekers JM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin’s lymphoma. N Engl J Med. 2003;348:2396-2406.
135 Advani R, Maeda L, Lavori P, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin’s disease. J Clin Oncol. 2007;25:3902-3907.
136 Bodis S, Kraus MD, Pinkus G, et al. Clinical presentation and outcome in lymphocyte-predominant Hodgkin’s disease. J Clin Oncol. 1997;15:3060-3066.
137 Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease. Report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol. 1999;17:776-783.
138 Feugier P, Labouyrie E, Djeridane M, et al. Comparison of initial characteristics and long-term outcome of patients with lymphocyte-predominant Hodgkin lymphoma and classical Hodgkin lymphoma at clinical stages IA and IIA prospectively treated by brief anthracycline-based chemotherapies plus extended high-dose irradiation. Blood. 2004;104:2675-2681.
139 Nogova L, Reineke T, Eich HT, et al. Extended field radiotherapy, combined modality treatment or involved field radiotherapy for patients with stage IA lymphocyte-predominant Hodgkin’s lymphoma. A retrospective analysis from the German Hodgkin Study Group (GHSG). Ann Oncol. 2005;16:1683-1687.
140 Nogova L, Reineke T, Brillant C, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma. A comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol. 2008;26:434-439.
141 Pellegrino B, Terrier-Lacombe MJ, Oberlin O, et al. Lymphocyte-predominant Hodgkin’s lymphoma in children. Therapeutic abstention after initial lymph node resection—a study of the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:2948-2952.
142 Wilder RB, Schlembach PJ, Jones D, et al. European Organization for Research and Treatment of Cancer and Groupe d’Etude des Lymphomes de l’Adulte very favorable and favorable, lymphocyte-predominant Hodgkin disease. Cancer. 2002;94:1731-1738.
143 Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma. A retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer. 2005;104:1221-1229.
144 Chen RC, Chin MS, Ng AK, et al. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma. Patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol. 2010;28:136-141.
145 Ekstrand BC, Lucas JB, Horwitz SM, et al. Rituximab in lymphocyte-predominant Hodgkin disease. Results of a phase 2 trial. Blood. 2003;101:4285-4289.
146 Canellos GP, Mauch P. What is the appropriate systemic chemotherapy for lymphocyte-predominant Hodgkin’s lymphoma? J Clin Oncol. 2010;28:e8.
147 Chera BS, Olivier K, Morris CG, et al. Clinical presentation and outcomes of lymphocyte-predominant Hodgkin disease at the University of Florida. Am J Clin Oncol. 2007;30:601-606.
148 Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease. A randomised trial. Lancet. 2002;359:2065-2071.
149 Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease. Results of a BNLI randomised trial. Lancet. 1993;341:1051-1054.
150 Crump M, Smith AM, Brandwein J, et al. High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin’s disease. Importance of disease status at transplant. J Clin Oncol. 1993;11:704-711.
151 Reece DE, Connors JM, Spinelli JJ, et al. Intensive therapy with cyclophosphamide, carmustine, etoposide +/− cisplatin, and autologous bone marrow transplantation for Hodgkin’s disease in first relapse after combination chemotherapy. Blood. 1994;83:1193-1199.
152 Wheeler C, Eickhoff C, Elias A, et al. High-dose cyclophosphamide, carmustine, and etoposide with autologous transplantation in Hodgkin’s disease. A prognostic model for treatment outcomes. Biol Blood Marrow Transplant. 1997;3:98-106.
153 Sureda A, Arranz R, Iriondo A, et al. Autologous stem-cell transplantation for Hodgkin’s disease. Results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19:1395-1404.
154 Lazarus HM, Loberiza FRJr, Zhang MJ, et al. Autotransplants for Hodgkin’s disease in first relapse or second remission. A report from the autologous blood and marrow transplant registry (ABMTR). Bone Marrow Transplant. 2001;27:387-396.
155 Ferme C, Mounier N, Divine M, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy. Results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 Trial. J Clin Oncol. 2002;20:467-475.
156 Sirohi B, Cunningham D, Powles R, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma. Ann Oncol. 2008;19:1312-1319.
157 Dhakal S, Biswas T, Liesveld JL, et al. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2009;75:188-192.
158 Mundt AJ, Sibley G, Williams S, et al. Patterns of failure following high-dose chemotherapy and autologous bone marrow transplantation with involved field radiotherapy for relapsed/refractory Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1995;33:261-270.
159 Wendland MM, Smith DC, Boucher KM, et al. The impact of involved field radiation therapy in the treatment of relapsed or refractory non-Hodgkin lymphoma with high-dose chemotherapy followed by hematopoietic progenitor cell transplant. Am J Clin Oncol. 2007;30:156-162.
159a Kahn ST, Flowers CR, Lechowicz MJ, et al. Refractory or relapsed Hodgkin’s disease and non-Hodgkin’s lymphoma. Optimizing involved-field radiotherapy in transplant patients. Cancer J. 2005;11(5):425-431.
160 Roach M3rd, Kapp DS, Rosenberg SA, et al. Radiotherapy with curative intent. An option in selected patients relapsing after chemotherapy for advanced Hodgkin’s disease. J Clin Oncol. 1987;5:550-555.
161 Brada M, Eeles R, Ashley S, et al. Salvage radiotherapy in recurrent Hodgkin’s disease. Ann Oncol. 1992;3:131-135.
162 Uematsu M, Tarbell NJ, Silver B, et al. Wide-field radiation therapy with or without chemotherapy for patients with Hodgkin disease in relapse after initial combination chemotherapy. Cancer. 1993;72:207-212.
163 Wirth A, Corry J, Laidlaw C, et al. Salvage radiotherapy for Hodgkin’s disease following chemotherapy failure. Int J Radiat Oncol Biol Phys. 1997;39:599-607.
164 Campbell B, Wirth A, Milner A, et al. Long-term follow-up of salvage radiotherapy in Hodgkin’s lymphoma after chemotherapy failure. Int J Radiat Oncol Biol Phys. 2005;63:1538-1545.
165 Josting A, Nogova L, Franklin J, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin’s lymphoma. A retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005;23:1522-1529.
166 Girinsky T, Pichenot C, Beaudre A, et al. Is intensity-modulated radiotherapy better than conventional radiation treatment and three-dimensional conformal radiotherapy for mediastinal masses in patients with Hodgkin’s disease, and is there a role for beam orientation optimization and dose constraints assigned to virtual volumes? Int J Radiat Oncol Biol Phys. 2006;64:218-226.
167 Goodman KA, Toner S, Hunt M, et al. Intensity-modulated radiotherapy for lymphoma involving the mediastinum. Int J Radiat Oncol Biol Phys. 2005;62:198-206.
168 Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients. Conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173-1180.
169 Hutchings M, Loft A, Hansen M, et al. Clinical impact of FDG-PET/CT in the planning of radiotherapy for early-stage Hodgkin lymphoma. Eur J Haematol. 2007;78:206-212.
170 Stromberg JS, Sharpe MB, Kim LH, et al. Active breathing control (ABC) for Hodgkin’s disease. Reduction in normal tissue irradiation with deep inspiration and implications for treatment. Int J Radiat Oncol Biol Phys. 2000;48:797-806.
171 Claude L, Malet C, Pommier P, et al. Active breathing control for Hodgkin’s disease in childhood and adolescence. Feasibility, advantages, and limits. Int J Radiat Oncol Biol Phys. 2007;67:1470-1475.
172 De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928-937.
173 Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27:3901-3907.
174 van den Belt-Dusebout AW, Aleman BM, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420-1429.
175 De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma. Lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239-4246.
176 Borchmann P, Schnell R, Fuss I, et al. Phase 1 trial of the novel bispecific molecule H22xKi-4 in patients with refractory Hodgkin lymphoma. Blood. 2002;100:3101-3107.
177 Schnell R, Borchmann P, Staak JO, et al. Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin’s lymphoma. Ann Oncol. 2003;14:729-736.
178 Yazbeck V, Georgakis GV, Wedgwood A, et al. Hodgkin’s lymphoma. Molecular targets and novel treatment strategies. Future Oncol. 2006;2:533-551.
179 Kasamon YL, Ambinder RF. Immunotherapies for Hodgkin’s lymphoma. Crit Rev Oncol Hematol. 2008;66:135-144.
180 Cooney JP, Stiff PJ, Toor AA, et al. BEAM allogeneic transplantation for patients with Hodgkin’s disease who relapse after autologous transplantation is safe and effective. Biol Blood Marrow Transplant. 2003;9:177-182.
181 Ruiz-Arguelles GJ, Lopez-Martinez B, Lopez-Ariza B. Successful allogeneic stem cell transplantation with nonmyeloablative conditioning in patients with relapsed Hodgkin’s disease following autologous stem cell transplantation. Arch Med Res. 2003;34:242-245.
182 Byrne BJ, Gockerman JP. Salvage therapy in Hodgkin’s lymphoma. Oncologist. 2007;12:156-167.