60 Hodgkin’s Disease
Epidemiology and Risk Factors1
Hodgkin’s disease accounts for less than 1% of cancers diagnosed in the United States each year.2,3 There is a slight male predominance. The median age at the time of diagnosis is 26 to 30 years. The disease is uncommon in children younger than 10 years and the age-adjusted incidence is bimodal.4 Both peaks, at ages 20 to 24 and 75 to 84, show an annual incidence of approximately 5.5 cases per 100,000. A recent decline in the height of the incidence peak for older adults may be attributed to improved diagnostic accuracy and the ability to differentiate mixed cellularity or lymphocyte depletion Hodgkin’s disease from diffuse large-cell or anaplastic large-cell lymphomas.
There are no well-defined risk factors related to the development of Hodgkin’s disease.5 Older reports associating an increased risk with tonsillectomy have been refuted. There are no definite associations between Hodgkin’s disease and human leukocyte antigen (HLA) loci. The slight increased risk associated with small sibship size and higher degree of education has not been explained, but contributes to the hypothesis of an infectious cause. A relationship between Hodgkin’s disease and prior infection with Epstein-Barr virus (EBV) has been proposed. Components of the EBV genome have been detected in the cellular deoxyribonucleic acid (DNA) of Reed-Sternberg cells,6 and elevated levels of immunoglobulin G and immunoglobulin A against the EBV capsid antigen and elevated levels of antibody against the EBV nuclear antigen and early antigen D have been detected in the serum of patients who later develop Hodgkin’s disease.7 The risk for developing Hodgkin’s disease appears to be higher in the presence of a prior history of infectious mononucleosis.8 Evidence of prior EBV infection is most common in mixed cellularity Hodgkin’s disease and in pediatric Hodgkin’s disease in underdeveloped countries.9
Pathologic Conditions10,11
The cell of origin of the Reed-Sternberg cell is likely a precursor B cell. In most instances, the Reed-Sternberg cells stain positively for CD30 and CD15. They also express the interleukin 2 receptor (TAC) and HLA-DR antigens. They stain negatively with the leukocyte common antigen; occasionally, they are positive for pan–B cell markers such as CD20.12,13 However, the lymphocytic (L) and histiocytic (H) cells (Reed-Sternberg equivalents) in lymphocyte-predominant Hodgkin’s disease are strongly positive for CD20 and CD45, but negative for CD15.14 There are five histologic subtypes of Hodgkin’s disease, according to the World Health Organization modification of the Lukes and Butler system. These include nodular lymphocyte predominant Hodgkin’s disease and four subtypes of classical Hodgkin’s disease: nodular sclerosis, lymphocyte-rich, mixed cellularity, and lymphocyte depletion.15
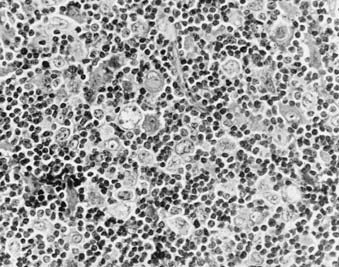
Nodular lymphocyte predominance (Fig. 60-1) is characterized by an abundance of normal-appearing lymphocytes and a paucity of abnormal cells. Patients frequently present with limited disease (stage I-II), and systemic symptoms are uncommon (<10%). The natural history of lymphocyte predominance is the most favorable among the histologic subtypes. In some reports, it demonstrates a pattern of late relapse similar to that of the low-grade lymphomas.16 However, the degree to which management programs should be influenced by this observation is not clear. In addition, these patients are at increased risk to develop transformation to other B-cell lymphomas, such as diffuse large-cell lymphoma. These observations, coupled with the different staining characteristics of the L and H cells of nodular lymphocyte-predominant Hodgkin’s disease, suggest that this entity may actually be a form of B-cell non-Hodgkin’s lymphoma.14,16
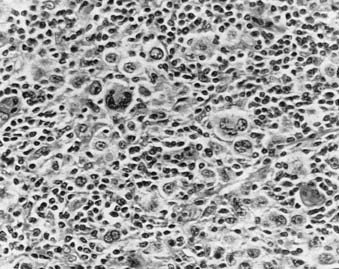
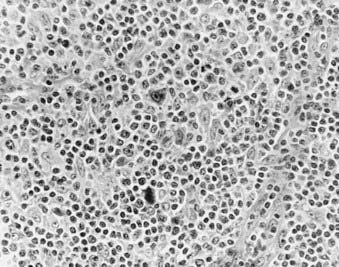
Nodular sclerosis Hodgkin’s disease (Fig. 60-2 and Fig. 60-3) is the most common histologic subtype in the United States (>70%). Involved nodes are traversed by broad bands of birefringent collagen surrounding nodules of cells that include lymphocytes, eosinophils, plasma cells, tissue histiocytes, and a variable proportion of atypical mononuclear cells and Reed-Sternberg cells. Women are more commonly affected than men, and the median age is younger than for other histologic subtypes. Clinically, the mediastinum and neck are often involved. One-third of patients have B symptoms. The natural history is somewhat less favorable than that of lymphocyte-predominant Hodgkin’s disease.
Mixed-cellularity Hodgkin’s disease (Fig. 60-4) is characterized by a diffuse effacement of lymph nodes by lymphocytes, eosinophils, and plasma cells, and there are relatively abundant atypical mononuclear and Reed-Sternberg cells. Patients with mixed-cellularity Hodgkin’s disease tend to present with more advanced disease and tend to be slightly older than those with nodular sclerosing or lymphocyte-predominant disease. The natural history of mixed-cellularity Hodgkin’s disease is less favorable than that of nodular sclerosing Hodgkin’s disease.
Interfollicular Hodgkin’s disease refers to a pattern of focal involvement of a lymph node in which there is a small focus of Hodgkin’s disease in the interfollicular zone. It is easy to confuse these cases with reactive lymphoid hyperplasia.17
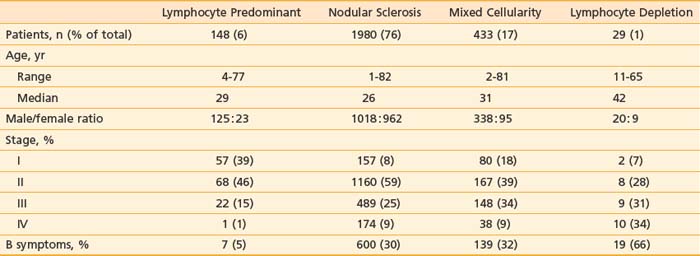
Table 60-1 summarizes the characteristics of 2590 patients treated at Stanford University from 1968 to 2001, according to histologic subtype. Other large centers in the United States and western Europe report a similar distribution of cases. However, the distribution of histologic subtypes from South America, Asia, Africa, Eastern Europe, and even some parts of the United States includes a greater proportion of unfavorable histologic types with a more aggressive clinical behavior.18
Clinical Presentation and Routes of Spread
Assuming contiguity between the supraclavicular lymph nodes and upper paraortic nodes–celiac axis–spleen (via the thoracic duct), 90% of patients with Hodgkin’s disease present with contiguous sites of involvement.19 The pattern of relapse among patients treated with irradiation alone also supports the concept of contiguity.20 Visceral involvement may be secondary to direct extension (especially lung, bone, or soft tissue) or hematogenous spread (such as to liver or multifocal bone).
The mechanism of spread of disease to the spleen is unclear. However, there is a correlation between the burden of disease identified in the spleen and the likelihood of hematogenous dissemination. Nearly all patients with hepatic or bone-marrow involvement by Hodgkin’s disease have (or had) extensive involvement of the spleen.21
Hodgkin’s disease only rarely involves the lymphoid tissues of the Waldeyer ring and Peyer patches. In addition, although visceral involvement by Hodgkin’s disease may be identified in the lungs, liver, bone marrow, and skeletal system, it uncommonly involves other organ systems such as the upper aerodigestive tract, central nervous system, skin, and gastrointestinal tract.22
One-third of patients present with fever, night sweats, or weight loss (B symptoms). Fevers may have a classic intermittent Pel-Ebstein pattern. The night sweats may be drenching. The presence of B symptoms often signals more extensive disease; however, even patients with only one or two involved lymph node regions may have significant B symptoms. Patients who have both weight loss and fevers may have a particularly poor prognosis.23
Patient age may have an effect on the natural history of the disease. In the elderly, the presence of intercurrent illness often affects the selection of staging and treatment procedures.24
Hodgkin’s disease may be diagnosed during pregnancy, and it is common for pregnancy to follow successful treatment. However, there is no evidence that pregnancy has any effect on the natural history of Hodgkin’s disease in these women.25,26
Although patients infected with human immunodeficiency virus (HIV-1) do not appear to be at increased risk for the development of Hodgkin’s disease, patients infected with HIV-1 who develop Hodgkin’s disease display an unusual natural history. The disease tends to present in a more advanced stage, is more likely to be associated with systemic symptoms, and often has unusual patterns of involvement.27 Treatment is challenging because these patients have a poor tolerance for chemotherapy and opportunistic infections are common.
Diagnostic and Staging Studies28,29
Staging procedures commonly employed for Hodgkin’s disease are listed in Table 60-2. Routine hematologic evaluation may reveal a mild anemia, lymphopenia, leukopenia, leukocytosis, or thrombocytosis. These may reflect paraneoplastic effects, but in some cases may be indicative of bone marrow involvement. The erythrocyte sedimentation rate and alkaline phosphatase and lactate dehydrogenase levels may be elevated and serve as useful markers to assess response to treatment and subsequent disease activity.30 Serum albumin levels, white blood cell counts, and lymphocyte counts are all important prognostic factors, especially in advanced disease.31
CBC, Complete blood cell count; CT, computed tomography; ESR, erythrocyte sedimentation rate; 18FDG-PET, 2-fluoro-2-deoxy-d-glucose positron emission tomography; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging.
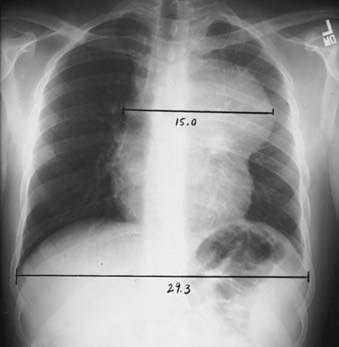
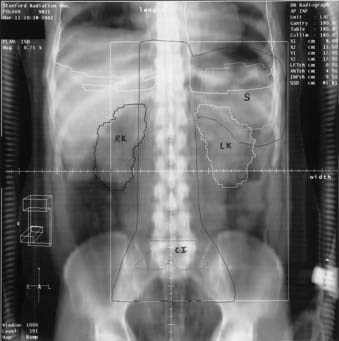
Radiographic evaluation should include posterior-anterior and lateral chest radiographs. A convenient means for measuring the extent of mediastinal adenopathy is to divide the maximum single width of the mediastinal mass by the maximum intrathoracic diameter (near the level of the diaphragm), as shown in Fig. 60-5. When this ratio exceeds 1 : 3, the disease is defined as bulky.32 Other measurements have also been used to define bulky mediastinal disease, including mass diameters that exceed 10 cm. A computed tomography (CT) scan of the chest should also be obtained if there is mediastinal disease, because it provides ancillary information regarding the extent of intrathoracic disease and assists in treatment planning and follow-up assessment.33
Abdominal and pelvic CT scans should be obtained with special attention paid to the retroperitoneal area, spleen, and liver. Lymph nodes are generally considered to be enlarged on a CT scan if their short axis measurement exceeds 1 to 1.5 cm. Splenomegaly or hepatomegaly alone does not equate with involvement by Hodgkin’s disease, because enlarged spleens are often uninvolved at the time of splenectomy; however, the presence of nodules visible on CT scan is considered indicative of involvement. The overall accuracy rate for detection of Hodgkin’s disease in the spleen by CT was reported to be only 58% in a laparotomy series.34 However, these data, obtained at a time when staging laparotomy was routine, may underestimate the detection rate with current CT scanning technology.
In the last several years,35 positron emission tomography (PET) using 2-fluoro-2-deoxy-d-glucose (18FDG) has been acknowledged as an important initial staging study in Hodgkin’s disease. FDG-PET is more sensitive than CT scanning36 or gallium imaging37 and is more convenient than gallium as a staging procedure because of the shorter interval between injection and scanning (1 hour versus 48 to 72 hours). PET scanning will identify additional sites of disease, beyond those identified by other clinical evaluation and imaging, in 10% to 15% of patients. PET scanning has proved to be highly useful as an early response indicator. When repeated early in the course of therapy, its prognostic value exceeds that of other well-established prognostic factors.38 PET is also useful as a follow-up study to evaluate residual masses present on CT scan.39
Magnetic resonance imaging (MRI) may be an alternative to chest or abdominopelvic CT scanning for initial staging, but has not been employed widely. Its main value may be in the staging evaluation of pregnant women with Hodgkin’s disease.40
Staging System41–44
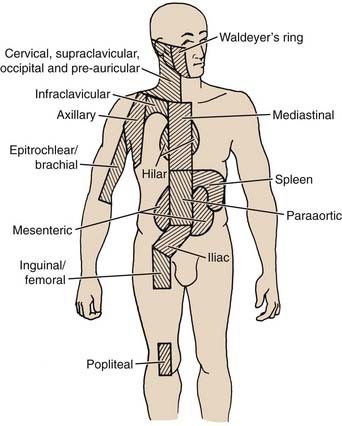
The Ann Arbor staging system for Hodgkin’s disease is outlined in Table 60-3.45 The lymphoid regions used in this system are displayed in Fig. 60-6. The designation of clinical stage (CS) is based on results of the initial biopsy and clinical staging studies, whereas the pathologic stage (PS) is based on the results of any subsequent biopsies, including bone marrow biopsy and laparotomy and splenectomy. In the postlaparotomy era, the distinction between CS and PS is rarely noted. Significant systemic (B) symptoms include fevers, night sweats, and weight loss. Other systemic symptoms of Hodgkin’s disease, including pruritus, alcohol-induced pain, and fatigue, should be noted, but are not considered B symptoms. The E designation indicates extralymphatic disease, although the concept is very poorly defined. In practical clinical terms, an E lesion is often considered to be a direct extension from involved lymph nodes and encompassable by a curative radiation field; however, the original description includes as an example “multiple nodules in the lung limited to one lobe.” In addition, the Ann Arbor system fails to deal with the possibility of multiple E lesions, which can be identified quite clearly in the chest with the careful use of contemporary imaging studies such as CT and MRI. Some authors’ inclusion of these patients in stage IV has led to a great deal of confusion in the literature. Another deficiency of the Ann Arbor system is its failure to consider bulk of disease. This has been shown to be important, especially in the mediastinum. The presence of bulky disease in the mediastinum should be noted, together with the stage.
| Stage I | Involvement of a single lymph node region |
| Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm (II) or localized involvement of an extralymphatic organ or site and of one or more lymph node regions on the same side of the diaphragm (IIE) |
| Stage III | Involvement of lymph node regions on both sides of the diaphragm (III), which may also be accompanied by involvement of the spleen (IIIS) or by localized involvement of an extralymphatic organ or site (IIIE) or both (IIISE) |
| Stage IV | Diffuse or disseminated involvement of one or more extralymphatic organs or tissues, with or without associated lymph node involvement |
The absence or presence of fever, night sweats, or unexplained loss of 10% or more of body weight in the 6 months before diagnosis is denoted by the suffix letter A or B, respectively.
Patients are assigned a clinical stage based on the initial biopsy and all subsequent nonsurgical staging studies. A pathologic stage is assigned based on all clinical studies as well as subsequent surgical staging procedures such as bone marrow biopsy, staging laparotomy, and splenectomy.
From Carbone PP, Kaplan HS, Mushoff K, et al: Report of the committee on Hodgkin’s disease staging classification, Cancer Res 31:1860, 1971.
In addition to Ann Arbor stage, other indicators may be helpful in predicting prognosis or selecting therapy. An international study evaluated prognosis in advanced Hodgkin’s disease by analyzing prognostic features among 5141 patients.31 Seven factors were identified, each of which had a similar prognostic affect. These are summarized in Table 60-4.
Table 60-4 A Prognostic Score for Advanced Hodgkin’s Disease
| Factor | Unfavorable Covariate |
|---|---|
| Serum albumin | <4 g/dl |
| Hemoglobin | <10.5 g/dl |
| Gender | Male |
| Age | 45 years and older |
| Stage | IV (Ann Arbor) |
| Leukocyte count | >15,000/mm3 |
| Lymphocyte count | <600/mm3 or <8% of white count |
Standard Therapeutic Approaches
Chemotherapy
Combination chemotherapy has excellent curative potential for patients with Hodgkin’s disease.47 The initial drug combination that achieved substantial success in treating Hodgkin’s disease was nitrogen mustard (mechlorethamine), vincristine (Oncovin), procarbazine, and prednisone (MOPP), reported by De Vita and associates48 from the National Cancer Institute. The acute toxicities of MOPP included nausea, vomiting, peripheral neuropathy, constipation, leukopenia, and thrombocytopenia. The major potential long-term toxicities were sterility (80% of men; in women it was directly linked to age) and secondary acute nonlymphocytic leukemia (usual latent period, 3 to 7 years; actuarial risk approximately 3% to 5% at 7 to 10 years).
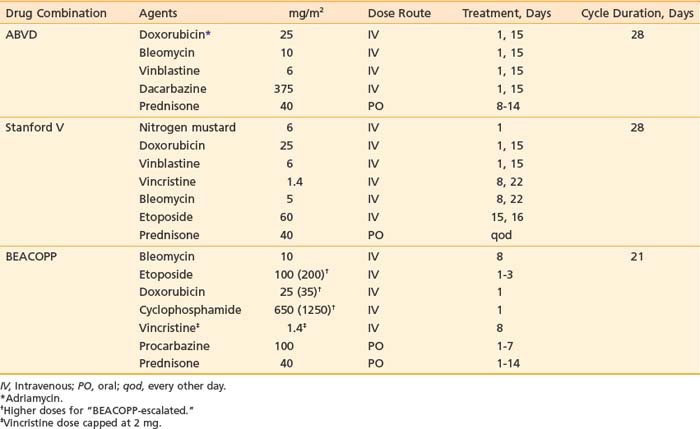
With the introduction of doxorubicin (Adriamycin), completely novel drug combinations were developed. The most successful of these has been the combination of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD; Table 60-5).49 Acute toxicities include nausea and vomiting, hair loss, and blood count depression. The hematologic effects are less severe than with MOPP. Sterility is not usually a complication, and secondary leukemias have been reported only rarely. Important potential long-term risks include possible cardiac and pulmonary toxicity from the doxorubicin and bleomycin, respectively.
Different combinations of drugs have been combined in an alternating fashion to address the problem of drug resistance. Most notable among “alternating non–cross-resistant” regimens is MOPP/ABVD, in which monthly cycles of MOPP are alternated with monthly cycles of ABVD.50 A subsequent innovation was the MOPP-ABV hybrid program.51,52 A superiority of outcome assoiciated with ABVD regimens and relatively greater toxicity associated with MOPP-containing regimens has led to the recommendation for ABVD alone in most clinical guidelines, such as those of the National Cancer Center Network (http://www.nccn.org).
In an effort to develop more effective combination chemotherapy, especially for patients with advanced, unfavorable disease, the German Hodgkin’s Study Group (GHSG) initiated a series of clinical trials testing a more intense drug combination—bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP)—with variations in scheduling referred to as BEACOPP-baseline, BEACOPP-escalated, and BEACOPP-14.53 The BEACOPP schedule is shown in Table 60-5. In general, the BEACOPP regimens have greater risk for secondary leukemia and sterility than are associated with ABVD.
Another approach in the modification of chemotherapy programs has been to develop schedules of brief, more intensive chemotherapy, with lower total doses of drugs, but with inclusion of limited radiotherapy. An example of this is the Stanford V program (see Table 60-5).54
Combined-Modality Therapy54,55
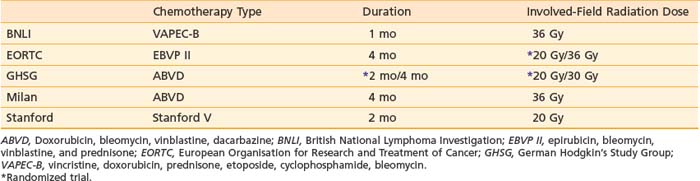
Combined-modality therapy plays an essential role in the management of many patients with Hodgkin’s disease.56 In the past 10 years, there has been a rapid move to the adoption of combined-modality therapy programs for early stage Hodgkin’s disease. This has largely been due to the identification of significant late effects among patients who are long-term (>15 years) survivors after treatment with radiation therapy alone. These newer programs generally include modified chemotherapy regimens (different combinations of drugs or fewer cycles of established regimens) followed by limited radiation treatment. Clinical trials indicate that in this setting, involved field (IF) irradiation is sufficient. For example, in Milan, a randomized trial evaluated outcome for patients treated with four cycles of ABVD plus subtotal lymphoid irradiation versus four cycles of ABVD plus IF. With a median follow-up in excess of 7 years, there was no significant difference in outcome for the two treatment approaches and patients treated with ABVD plus IF (36 Gy) had survival and freedom-from-relapse rates of 94%.57 At Stanford, 65 patients were treated with 8 weeks of Stanford V chemotherapy followed by IF (30 Gy). The 3-year freedom-from-progression rate was 96%.58 Other similar programs are summarized in Table 60-6. Current clinical trials are studying variations in radiation dose and number of cycles of chemotherapy (see the following text).
Table 60-6 Chemotherapy Plus Involved-Field Radiation Therapy for Stage I and II Favorable-Prognosis Hodgkin’s Disease
The treatment of choice for patients with bulky mediastinal Hodgkin’s disease (mediastinal mass greater than one-third of the maximum intrathoracic diameter) is combined-modality therapy. Several reports show that patients with bulky mediastinal disease are at greater risk for relapse after treatment with irradiation alone than after treatment with combined-modality therapy.59 Likewise, the risk of relapse is substantial in patients with large mediastinal masses treated with chemotherapy alone.60 The general recommendation has been that these patients be treated with a combination of chemotherapy and irradiation (http://www.nccn.org).
With respect to the choice of chemotherapy, the potential overlapping toxicities of doxorubicin and bleomycin with irradiation (cardiac and pulmonary effects) should be considered. The recommended radiation dose in this situation varies from 25 to 40 Gy, but most data cite doses of at least 30 to 36 Gy.53,56 Doses in the higher part of the range should be used when the response to chemotherapy is incomplete, or PET imaging remains positive after chemotherapy, or the chemotherapy course is abbreviated. The use of lower doses (as low as 20 Gy) should be restricted to clinical trials.61
Treatment may include all initially involved sites or simply to the area of initial bulk (mediastinum). When treatment is limited to the mediastinum, the bilateral supraclavicular areas are often included. The irradiation fields should conform to the area of residual mediastinal disease with adequate margins laterally, not to the initial width of disease before chemotherapy. However, the fields generally extend at least 2 cm proximal and distal beyond the original extent of disease. Fig. 60-7 and Fig. 60-8 display typical supradiaphragmatic and infradiaphragmatic “involved fields.”
The use of combined-modality therapy for patients with stage III and IV Hodgkin’s disease is rational, because most patients who relapse after treatment with chemotherapy alone do so in sites of initial disease; however, clinical trials have failed to support this rationale.62–64
The most important recent trial to bear on this issue is the European Organisation for Research and Treatment of Cancer–Groupe Pierre-et-Marie Curie (EORTC-GPMC) H34 (#20884) trial. All patients were treated with MOPP-ABV chemotherapy and then randomly assigned to receive either no further therapy or treatment with IF irradiation (25 Gy). The study accrued 736 patients and revealed no difference in survival, event-free survival, or relapse-free survival between the two groups.65
The combined-modality approach that has been adopted at Stanford for bulky stage II and stage III and IV disease is to employ intense but low-total-dosage chemotherapy (12 weeks of Stanford V) followed by radiation only to initially bulky sites (>5 cm), and at a higher dose (36 Gy). Irradiation is initiated 1 to 3 weeks after the completion of 12 weeks of Stanford V chemotherapy. With this approach, because chemotherapy is abbreviated, the radiation is considered to be an integral part of the treatment, not simply an adjuvant therapy.53 This approach has been tested in a phase 3 intergroup trial, the results of which have not yet been reported.
Many salvage treatment programs also incorporate radiation therapy in a combined-modality approach.66 There is a growing experience with IF irradiation as a component of high-dose chemotherapy programs accompanied by autologous bone marrow or peripheral stem cell transplantation.67–69
At the Memorial Hospital, investigators have reported the use of accelerated hyperfractionated total lymphoid irradiation in patients scheduled for high-dose therapy and bone marrow transplantation.70 After standard induction chemotherapy and before transplantation, patients receive IF irradiation to 15 Gy (1.5 Gy twice a day) followed by total lymphoid irradiation to 20.04 Gy (1.67 Gy three times a day). They report a 6.5-year disease-free survival rate of 50%.
Irradiation may also be restricted to actual sites of relapse in high-dose therapy programs. This approach is supported by the observation that subsequent relapse among patients treated with high-dose therapy is often within sites of prior involvement.71 Retrospective data from a number of institutions now point to better long-term disease control when these patients receive local irradiation.72–74 For example, at Stanford, 24 of 100 patients received IF irradiation (median 30 Gy) in addition to high-dose chemotherapy preparatory regimens.73 For patients with disease classified as stage I, II, or III at the time of relapse, the 3-year event-free survival rate was 84% when IF irradiation was included, compared with 55% for high-dose therapy and transplant alone (P = 0.13). In patients who had received no previous irradiation, the 3-year event-free survival rate after high-dose therapy that incorporated consolidative irradiation was 79%, compared with 52% without irradiation (P = 0.10). For a subgroup of patients with relapse stage I, II, or III and no history of irradiation, the event-free survival rate was 82% with consolidative irradiation and 43% without it (P = 0.07).
Role of Radiation Therapy
Radiation therapy has a potential role in any presentation of Hodgkin’s disease. It has a long history in the treatment of Hodgkin’s disease, including the curative treatment of patients with stage I and II and certain subsets of stage IIIA disease. However, the prolonged survival of patients who have been treated with irradiation has permitted the identification of significant late effects associated with that treatment. Largely because of the risk for these effects, as well as the identification of safer chemotherapy regimens such as ABVD and Stanford V, radiation theray is now used most commonly in conjunction with chemotherapy, in which setting the fields and doses employed can be more limited and safer.75
Techniques of Radiotherapy
Radiation Treatment Plan76
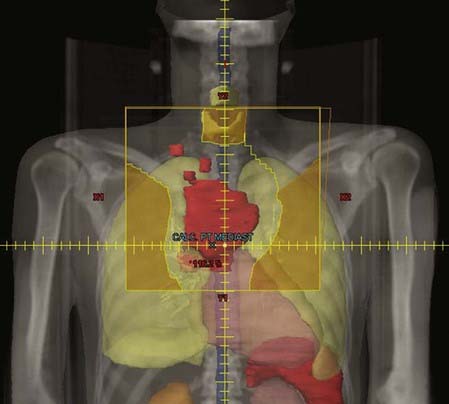
In the most common clinical situation, patients undergo initial imaging with diagnostic CT and PET scanning. Treatment is often initiated with chemotherapy, after which the patient receives consolidative irradiation. The most precise way to accomplish this is to transfer information from the pretreatment imaging studies to the CT simulation scan. Ideally, a fused PET/CT scan is obtained in the treatment position prior to initiation of therapy (on a PET/CT simulator, if possible). The initially involved nodes will constitute the gross tumor volume (GTV); however, the post-chemotherapy GTV may need to be adjusted for shrinkage of lymph node masses. This is especially important in the mediastinum, where mediastinal masses that were pushing on the lungs have regressed—only the residual width of the disease need be treated. The clinical tumor volume in this situation has not been strictly defined, but most commonly includes 3 to 5 cm of the lymph node region proximal and distal to the sites of initial involvement. The planning tumor volume (PTV) adds an additional 0.5 to 1 cm. Isodose curves should confirm at least 95% of the prescribed dose delivered to the PTV. The prescribed dose varies depending on the clinical situation, as noted in the following text. But fraction size is generally 1.5 to 1.8 Gy per day.77,78
In the mediastinum, tumor shrinkage needs to be taken into account, as noted previously. Many protocols call for inclusion of the pulmonary hilar nodes and low supraclavicular nodes whenever the mediastinum is treated. AP-PA-opposed technique generally provides the best tumor coverage for this region. Intensity-modulated radiation therapy or 3-D conformal techniques in settings in which the mediastinal disease is very anterior may be advantageous with respect to achieving a conformal dose, but this will be at the expense of a higher integral dose to the lungs and delivery of low-dose irradiation to the breasts. Lung dose-volume histograms should be generated, especially for patients with large mediastinal masses. Because many patients will have been treated with ABVD chemotherapy, which contains bleomycin, the mean lung dose should be limited to 15 Gy. Limiting daily fractions to just 1.5 Gy may further decrease risk. The use of techniques such as respiratory gating or active breathing control may provide aditional benefit in reducing the lung dose.79
Recently, the concept of involved node irradiation has been defined by European clinical trials groups.80 Involved node irradiation incorporates findings on pretreatment PET and diagnostic CT scans, and post-treatment diagnostic CT and CT-simulation studies to define fields that are even more restricted than “involved fields,” and therefore less likely to be associated with long-term morbidity. This concept has been incorporated into the clincial trials of both the EORTC and the GHSG.81–84
Critical Normal Tissues85
The number of organs and the volume of normal tissue within the treatment fields for Hodgkin’s may be substantial, and careful consideration of both short-term toxicity and long-term morbidity is essential in treatment planning. In the supradiaphragmatic region, there is potential toxicity involving the salivary glands, thyroid, soft tissues of the neck and shoulder girdle (especially in younger people), brachial plexus, spinal cord, heart, and lungs. For subdiaphragmatic fields, dose-limiting organs include the kidneys, spinal cord, liver, bone marrow, gastrointestinal tract, and gonads. Specific discussion of radiation tolerance of these organs is included in the individual organ site chapters (see Chapters 17–21 and 26–43). Fortunately, the doses employed in Hodgkin’s disease are usually well below the threshold of toxicity for most organs; however, given the long duration of follow-up for most patients with Hodgkin’s disease, no dose can be ignored.
Symptomatic pericarditis may develop after doses of 30 Gy when the majority of the cardiac silhouette is treated. Depending on the location of disease, the use of partial-field blocking can reduce that risk. The myocardium is slightly more resistant than the pericardium, but doses of 35 Gy or more may be associated with long-term risk of myocardial dysfunction. The coronary arteries are moderately radiation-resistant; however, doses in excess of 30 Gy are associated with an increased risk of coronary artery disease and myocardial infarction.86
There are generally few long-term effects on salivary gland function after standard treatment for Hodgkin’s disease. However, xerostomia may develop in rare patients who require treatment to the Waldeyer region. Thyroid gland function is often diminished, despite partial thyroid shielding.87 This is often manifest initially as an elevation of the sensitive thyroid-stimulating hormone (S-TSH), and exogenous replacement therapy is appropriate at that time.
In addition to organ-specific complications, a significant risk for patients is the development of treatment-related malignancies. Long-term follow-up is essential to define this risk. Leukemia may develop in approximately 5% of patients with a latent period of 3 to 7 years after treatment with chemotherapy that includes a significant dose of alkylating agents (e.g., MOPP). Secondary solid tumors are related primarily to irradiation, but may also appear after treatment with chemotherapy alone. Secondary solid tumors have a longer latent period (at least 7 to 10 years). Patients who smoke should be counseled to stop because of a high risk of lung cancer when smoking is combined with irradiation. Women should undergo mammography according to the usual American Cancer Society guidelines to detect breast cancer early, which may or may not be treatment-related. In addition, even younger women should begin mammogram screening 10 years after they have completed mantle irradiation. Recently, the American Cancer Society has recommended that women who received chest irradiation before age 30 undergo both routine and magnetic resonance mammography. The relative risk that women will develop breast cancer after treatment for Hodgkin’s disease is 4.1. This is manifest primarily in women who are younger than 30 years when they are treated.88
Outcome
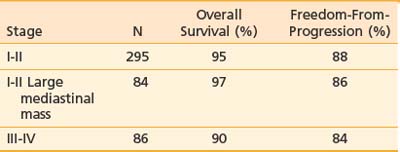
Clinical trial groups generally define three distinct groups of patients with Hodgkin’s disease: a favorable group that is usually restricted to those who have stage I or IIA disease, without any bulky adenopathy; an unfavorable group who have stage III or IV disease; and an intermediate group with a various mix of factors, always including those who have stage I or II disease with large mediastinal adenopathy. The Stanford results for patients categorized in this fashion are displayed in Table 60-7.
Table 60-7 Ten-Year Outcome for 565 Patients With Classic Hodgkin’s Disease Treated at Stanford, 1989-2001
Favorable Presentations of Stage I and IIA
The standard treatment for these patients is with combined-modality therapy. Generally, 2 to 4 months of chemotherapy is followed by IF irradiation. The rate of freedom from progression is generally reported to be at least 90% and survival 95% at 8 years. Since 1995 at Stanford, we have treated these patients with the Stanford V program. After 8 weeks of chemotherapy, patients receive irradiation to the involved regions to a dose of 30 Gy. The 3-year freedom-from-progression rate is 95% and 3-year survival is 97%.89
Unfavorable Presentations of Stage I and II (Bulky Mediastinal Involvement)90,91
Approximately 20% of patients with stage I or II Hodgkin’s disease present with a large mediastinal mass. Combined-modality therapy is the treatment of choice for these patients. The expected 5-year freedom-from-progression rate is approximately 85% and the 10-year survival rate is approximately 90%.92 Since the 1990s, we have used the Stanford V program, in which 12 weeks of chemotherapy is followed by irradiation to 36 Gy to the mediastinum and bilateral supraclavicular areas. The 5-year freedom-from-progression rate is 97% and the 5-year survival rate is 100% for this cohort.53
Stage III and IV41
Systemic chemotherapy is the mainstay of treatment for patients with advanced-stage Hodgkin’s disease. Although early clinical trials of systemic therapy for Hodgkin’s disease often excluded patients with stage IIIA, these patients are now included as patients with “advanced Hodgkin’s disease” in most clinical trials. An important clinical trial of the Cancer and Leukemia Group B compared the efficacy of MOPP, ABVD, and MOPP-ABVD in a prospective randomized study of patients with advanced-stage Hodgkin’s disease.52 The complete response rates and actuarial failure-free survival rates were statistically superior for the two doxorubicin-containing arms of the trial (ABVD or MOPP-ABVD). Among the patients treated with ABVD chemotherapy, the complete response rate was 82%, the 5-year failure-free-survival rate was 61%, and the 5-year survival rate was 73%.
In an effort to improve these results, the GHSG developed a series of protocols to test the BEACOPP regimen. In an initial trial (HD9) comparing COPP/ABVD, BEACOPP baseline and BEACOPP escalated, the BEACOPP-escalated arm was superior to the other two arms of the trial. In common clincial practice, because the BEACOPP regimen is associated with significantly greater toxicity than ABVD, it is often reserved for patients with stage III or IV Hodgkin’s disease who have three or more adverse factors on the international prognostic score (see Table 60-4).
Since 1990, we have used the Stanford V program of combined-modality therapy for patients with stage III and IV disease. After 12 weeks of chemotherapy, they receive 36 Gy to initial sites of disease greater than 5 cm. With a median follow-up of more than 5 years, the 5-year freedom from progression is 85%, and survival is 96%.53
Lymphocyte Predominance
Patients with stage III and IV disease are treated in a fashion analogous to that for patients with classic Hodgkin’s disease. However, patients with stage I and II disease may be treated with IF irradiation alone. Retrospective trials report 10-year freedom-from-progression and overall survival rates of approximately 90% for patients treated with this limited therapy.93,94
Current Clinical Trials
For patients with favorable presentations of stage I and II disease, current clinical trials attempt to define the minimum treatment necessary to achieve a cure. The GHSG HD10 trial tested two versus four cycles of ABVD and found them to be equivalent; in addition, 20 Gy and 30 Gy provide equivalent outcomes.95 A subsequent GHSG trial tested variants of ABVD and found AVD to be equivalent to ABVD, whereas ABV and AV were inferior.
The possibility of using chemotherapy alone for patients with favorable presentations is also being tested. The National Cancer Institute of Canada HD6 trial included arms of treatment with chemotherapy alone.96 Although the freedom-from-progression rate was significantly better in the radiation-containing arms of the trial (5-year freedom-from-progression was 93% versus 87%, P = 0.008), the survival was identical. In addition, a very favorable cohort was identified that included patients who achieved a CT-based complete response after just two cycles of ABVD. After completing two additional cycles of ABVD, they enjoyed a freedom-from-progression rate of 95%. The current GHSG HD16 and EORTC H9 trials both include possibilities for ABVD treatment alone (two cycles or four cycles, respectively) for patients in whom FDG-PET examinations are negative after two cycles of ABVD.
1 DeVita VT, Bonadonna G. The history of the chemotherapy of Hodgkin’s disease. In: Mauch PM, Diehl V, Hoppe R, et al, editors. Hodgkin’s disease. Philadelphia: Lippincott Williams and Wilkins; 1999:9-22.
2 Stat bite. J Natl Cancer Inst. 1998;90(5):352.
3 Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin. 2001;51(1):15-36.
4 Medeiros LJ, Greiner TC. Hodgkin’s disease. Cancer. 1995;75(1 Suppl):357-369.
5 Grufferman S, Delzell E. Epidemiology of Hodgkin’s disease. Epidemiol Rev. 1984;6:76-106.
6 Weiss LM, Movahed LA, Warnke RA, et al. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med. 1989;320(8):502-506.
7 Mueller N, Evans A, Harris NL, et al. Hodgkin’s disease and Epstein-Barr virus. Altered antibody pattern before diagnosis. N Engl J Med. 1989;320(11):689-695.
8 Hjalgrim H, Askling J, Sorensen P, et al. Risk of Hodgkin’s disease and other cancers after infectious mononucleosis. J Natl Cancer Inst. 2000;92(18):1522-1528.
9 Ambinder RF, Browning PJ, Lorenzana I, et al. Epstein-Barr virus and childhood Hodgkin’s disease in Honduras and the United States. Blood. 1993;81(2):462-467.
10 Rosenberg S. Development of the concept of Hodgkin’s disease as a curable illness: the American experience. In: Mauch P AJ, Diehl V, Hoppe R, et al, editors. Hodgkin’s disease. Philadelphia: Lippincott Williams and Wilkins; 1999:47-57.
11 Weiss LM, Warnke RA, Hansmann M-L, et al. Pathology of Hodgkin lymphoma. In: Hoppe RT, Mauch PM, Armitage JO, et al, editors. Hodgkin lymphoma. Philadelphia: Lippincott Williams & Wilkins; 2007:43-72.
12 Chittal SM, Caveriviere P, Schwarting R, et al. Monoclonal antibodies in the diagnosis of Hodgkin’s disease. The search for a rational panel. Am J Surg Pathol. 1988;12(1):9-21.
13 Rassidakis GZ, Medeiros LJ, Viviani S, et al. CD20 expression in Hodgkin and Reed-Sternberg cells of classical Hodgkin’s disease: associations with presenting features and clinical outcome. J Clin Oncol. 2002;20(5):1278-1287.
14 Pinkus GS, Said JW. Hodgkin’s disease, lymphocyte predominance type, nodular—a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985;118(1):1-6.
15 Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835-3849.
16 Regula DPJr, Hoppe RT, Weiss LM. Nodular and diffuse types of lymphocyte predominance Hodgkin’s disease. N Engl J Med. 1988;318(4):214-219.
17 Doggett RS, Colby TV, Dorfman RF. Interfollicular Hodgkin’s disease. Am J Surg Pathol. 1983;7(2):145-149.
18 Hu E, Hufford S, Lukes R, et al. Third-world Hodgkin’s disease at Los Angeles County–University of Southern California Medical Center. J Clin Oncol. 1988;6(8):1285-1292.
19 Rosenberg SA, Kaplan HS. Evidence for an orderly progression in the spread of Hodgkin’s disease. Cancer Res. 1966;26(6):1225-1231.
20 Kaplan HS. The radical radiotherapy of regionally localized Hodgkin’s disease. Radiology. 1962;78:553-561.
21 Hoppe RT, Cox RS, Rosenberg SA, et al. Prognostic factors in pathologic stage III Hodgkin’s disease. Cancer Treat Rep. 1982;66(4):743-749.
22 Kaplan H. Patterns of anatomic distribution, Hodgkin’s disease, vol 2. Cambridge: Harvard University Press; 1980.
23 Crnkovich MJ, Hoppe RT, Rosenberg SA. Stage IIB Hodgkin’s disease: the Stanford experience. J Clin Oncol. Apr 1986;4(4):472-479.
24 Austin-Seymour MM, Hoppe RT, Cox RS, et al. Hodgkin’s disease in patients over sixty years old. Ann Intern Med. 1984;100(1):13-18.
25 Jacobs C, Donaldson SS, Rosenberg SA, et al. Management of the pregnant patient with Hodgkin’s disease. Ann Intern Med. 1981;95(6):669-675.
26 Le Floch O, Donaldson SS, Kaplan HS. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin’s disease. Cancer. 1976;38(6):2263-2268.
27 Schoeppel SL, Hoppe RT, Dorfman RF, et al. Hodgkin’s disease in homosexual men with generalized lymphadenopathy. Ann Intern Med. 1985;102(1):68-70.
28 Kadin M, Liebowitz D. Cytokines and cytokine receptors in Hodgkin’s disease. In: Mauch PAJ, Diehl V, Hoppe R, Weiss LM, editors. Hodgkin’s disease. Philadelphia: Lippincott Williams and Wilkins; 1999:139-157.
29 Grossmann A. Anatomic imaging. In: Hoppe RT, Mauch PM, Armitage JO, et al, editors. Hodgkin lymphoma. Philadelphia: Lippincott Williams & Wilkins; 2007:133-142.
30 Friedman S, Henry-Amar M, Cosset JM, et al. Evolution of erythrocyte sedimentation rate as predictor of early relapse in posttherapy early-stage Hodgkin’s disease. J Clin Oncol. 1988;6(4):596-602.
31 Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506-1514.
32 Piro AJ, Weiss DR, Hellman S. Mediastinal Hodgkin’s disease: a possible danger for intubation anesthesia. Intubation danger in Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1976;1(5–6):415-419.
33 Castellino RA, Blank N, Hoppe RT, et al. Hodgkin disease: contributions of chest CT in the initial staging evaluation. Radiology. 1986;160(3):603-605.
34 Castellino RA, Hoppe RT, Blank N, et al. Computed tomography, lymphography, and staging laparotomy: correlations in initial staging of Hodgkin disease. AJR Am J Roentgenol. 1984;143(1):37-41.
35 Front D, Israel O. The role of Ga-67 scintigraphy in evaluating the results of therapy of lymphoma patients. Semin Nucl Med. 1995;25(1):60-71.
36 Partridge S, Timothy A, O’Doherty MJ, et al. 2-Fluorine-18-fluoro-2-deoxy-D glucose positron emission tomography in the pretreatment staging of Hodgkin’s disease: influence on patient management in a single institution. Ann Oncol. 2000;11(10):1273-1279.
37 Wirth A, Seymour JF, Hicks RJ, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography, gallium-67 scintigraphy, and conventional staging for Hodgkin’s disease and non-Hodgkin’s lymphoma. Am J Med. 2002;112(4):262-268.
38 Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746-3752.
39 De Wit M, Bohuslavizki KH, Buchert R, et al. 18FDG-PET following treatment as valid predictor for disease-free survival in Hodgkin’s lymphoma. Ann Oncol. 2001;12(1):29-37.
40 Armitage JO, Carella AM, Schmitz N, et al. Role of hematopoietic stem-cell transplantation in Hodgkin lymphoma. In: Hoppe RT, Mauch PM, Armitage JO, et al, editors. Hodgkin lymphoma. Philadelphia: Lippincott Williams & Wilkins; 2007:281-292.
41 Hodgson DC, Gospodarowicz MK. Clinical evaluation and staging of Hodgkin lymphoma. In: Hoppe RT, Mauch PM, Armitage JO, et al, editors. Hodgkin lymphoma. Philadelphia: Lippincott Williams & Wilkins; 2007:123-132.
42 Carde P, Hagenbeek A, Hayat M, et al. Clinical staging versus laparotomy and combined modality with MOPP versus ABVD in early-stage Hodgkin’s disease: the H6 twin randomized trials from the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Group. J Clin Oncol. 1993;11(11):2258-2272.
43 Donaldson SS, Moore MR, Rosenberg SA, et al. Characterization of postsplenectomy bacteremia among patients with and without lymphoma. N Engl J Med. 1972;287(2):69-71.
44 Leibenhaut MH, Hoppe RT, Efron B, et al. Prognostic indicators of laparotomy findings in clinical stage I-II supradiaphragmatic Hodgkin’s disease. J Clin Oncol. 1989;7(1):81-91.
45 Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31(11):1860-1861.
46 Sarris A, Jhanwar S, Cabanillas F. Cytogenetics of Hodgkin’s disease. In: Mauch PAJ, Diehl V, Hoppe R, Weiss LM, editors. Hodgkin’s disease. Philadelphia: Lippincott Williams and Wilkins; 1999:195-212.
47 Fonatsch C, Jox A, Streubel B, et al. Cytogenetic findings in Hodgkin’s disease cell lines. In: Mauch PAJ, Diehl V, Hoppe R, et al, editors. Hodgkin’s disease. Philadelphia: Lippincott Williams and Wilkins; 1999:213-219.
48 De Vita VTJr, Hubbard SM, Longo DL. The chemotherapy of lymphomas: looking back, moving forward—the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1987;47(22):5810-5824.
49 Bonadonna G. Chemotherapy strategies to improve the control of Hodgkin’s disease: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1982;42(11):4309-4320.
50 Bonadonna G, Valagussa P, Santoro A. Alternating non-cross-resistant combination chemotherapy or MOPP in stage IV Hodgkin’s disease. A report of 8-year results. Ann Intern Med. 1986;104(6):739-746.
51 Klimo P, Connors JM. MOPP/ABV hybrid program: combination chemotherapy based on early introduction of seven effective drugs for advanced Hodgkin’s disease. J Clin Oncol. 1985;3(9):1174-1182.
52 Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478-1484.
53 Diehl V, Engert A, Re D. New strategies for the treatment of advanced-stage Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2007;21(5):897-914.
54 Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease: mature results of a prospective clinical trial. J Clin Oncol. 2002;20(3):630-637.
55 Engel C, Loeffler M, Schmitz S, et al. Acute hematologic toxicity and practicability of dose-intensified BEACOPP chemotherapy for advanced stage Hodgkin’s disease. German Hodgkin’s Lymphoma Study Group (GHSG). Ann Oncol. 2000;11(9):1105-1114.
56 Connors J, Yahalom J, Noordijk E. Principles of combined-modality therapy in Hodgkin’s disease. In: Mauch PM, Diehl V, Hoppe R, et al, editors. Hodgkin’s disease. Philadelphia: Lippincott Williams and Wilkins; 1999:395-407.
57 Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease: long term results. J Clin Oncol. 2004;22(14):2836-2841.
58 Horning S, Hoppe R, Breslin S, et al. Very brief (8 week) chemotherapy (CT) and low dose (30 Gy) radiotherapy (RT) for limited stage Hodgkin’s disease (HD): preliminary results of the Stanford-Kaiser G4 Study of Stanford V and radiotherapy [Abstract]. Blood. 1999;94(1):387a.
59 Hoppe RT, Coleman CN, Cox RS, et al. The management of stage I–II Hodgkin’s disease with irradiation alone or combined modality therapy: the Stanford experience. Blood. 1982;59(3):455-465.
60 Longo DL, Russo A, Duffey PL, et al. Treatment of advanced-stage massive mediastinal Hodgkin’s disease: the case for combined modality treatment. J Clin Oncol. 1991;9(2):227-235.
61 Hoppe RT, Engert A, Noordijk EM. Treatment of unfavorable prognosis, stage I-II Hodgkin lymphoma. In: Hoppe RT, Mauch PM, Armitage JO, et al, editors. Hodgkin lymphoma. Philadelphia: Lippincott Williams & Wilkins; 2007:241-252.
62 Young RC, Canellos GP, Chabner BA, et al. Patterns of relapse in advanced Hodgkin’s disease treated with combination chemotherapy. Cancer. 1978;42(2 Suppl):1001-1007.
63 Fabian CJ, Mansfield CM, Dahlberg S, et al. Low-dose involved field radiation after chemotherapy in advanced Hodgkin disease. A Southwest Oncology Group randomized study. Ann Intern Med. 1994;120(11):903-912.
64 Diehl V, Loeffler M, Pfreundschuh M, et al. Further chemotherapy versus low-dose involved-field radiotherapy as consolidation of complete remission after six cycles of alternating chemotherapy in patients with advance Hodgkin’s disease. German Hodgkin’s Study Group (GHSG). Ann Oncol. 1995;6(9):901-910.
65 Aleman BMP, Raemaekers JMM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin’s lymphoma. N Engl J Med. 2003;348(24):2396-2406.
66 Hoppe RT. The management of Hodgkin’s disease in relapse after primary radiation therapy. Eur J Cancer. 1992;28A(11):1920-1922.
67 Hodgkin lymphoma. In: Hoppe RT, Mauch PM, Armitage JO, et al, editors. Hodgkin lymphoma. Philadelphia: Lippincott Williams & Wilkins; 2007:411-418.
68 Nademanee A, O’Donnell MR, Snyder DS, et al. High-dose chemotherapy with or without total body irradiation followed by autologous bone marrow and/or peripheral blood stem cell transplantation for patients with relapsed and refractory Hodgkin’s disease: results in 85 patients with analysis of prognostic factors. Blood. 1995;85(5):1381-1390.
69 Kaplan HS. Evidence for a tumoricidal dose level in the radiotherapy of Hodgkin’s disease. Cancer Res. 1966;26(6):1221-1224.
70 Yahalom J, Gulati SC, Toia M, et al. Accelerated hyperfractionated total-lymphoid irradiation, high-dose chemotherapy, and autologous bone marrow transplantation for refractory and relapsing patients with Hodgkin’s disease. J Clin Oncol. 1993;11(6):1062-1070.
71 Shamash J, Lee SM, Radford JA, et al. Patterns of relapse and subsequent management following high-dose chemotherapy with autologous haematopoietic support in relapsed or refractory Hodgkin’s lymphoma: a two centre study. Ann Oncol. 2000;11(6):715-719.
72 Mundt AJ, Sibley G, Williams S, et al. Patterns of failure following high-dose chemotherapy and autologous bone marrow transplantation with involved field radiotherapy for relapsed/refractory Hodgkin’s disease,. Int J Radiat Oncol Biol Phys. 1995;33(2):261-270.
73 Pezner RD, Nademanee A, Niland JC, et al. Involved field radiation therapy for Hodgkin’s disease autologous bone marrow transplantation regimens. Radiother Oncol. 1995;34(1):23-29.
74 Poen JC, Hoppe RT, Horning SJ. High-dose therapy and autologous bone marrow transplantation for relapsed/refractory Hodgkin’s disease: the impact of involved field radiotherapy on patterns of failure and survival. Int J Radiat Oncol Biol Phys. 1996;36(1):3-12.
75 Noordijk EM, Carde P, Mandard AM, et al. Preliminary results of the EORTC-GPMC controlled clinical trial H7 in early-stage Hodgkin’s disease. EORTC Lymphoma Cooperative Group. Groupe Pierre-et-Marie-Curie. Ann Oncol. 1994;5(Suppl 2):107-112.
76 Lutz WR, Larsen RD. Technique to match mantle and para-aortic fields. Int J Radiat Oncol Biol Phys. 1983;9(11):1753-1756.
77 Smith A. Treatment planning: patterns of care study newsletter. Philadelphia: American College of Radiology; 1993.
78 Carlsen SE, Bergin CJ, Hoppe RT. MR imaging to detect chest wall and pleural involvement in patients with lymphoma: effect on radiation therapy planning. AJR Am J Roentgenol. 1993;160(6):1191-1195.
79 Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44(4):911-919.
80 Girinsky T, Specht L, Ghalibafian M, et al. The conundrum of Hodgkin lymphoma nodes: to be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol. 2008;88(2):202-210.
81 Hoppe R. Hodgkin’s disease: patterns of care study newsletter 3. Philadelphia: American College of Radiology; 1990–1991. 8(3):1–8
82 Hoppe RT. Radiation therapy in the management of Hodgkin’s disease. Semin Oncol. 1990;17(6):704-715.
83 Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin’s disease. An analysis of technique, tumor eradication, and complications. Cancer. 1976;37(6):2813-2825.
84 Palos B, Kaplan HS, Karzmark CJ. The use of thin lung shields to deliver limited whole-lung irradiation during mantle-field treatment of Hodgkin’s disease. Radiology. 1971;101(2):441-442.
85 Hoppe RT, Burke JS, Glatstein E, et al. Non-Hodgkin’s lymphoma: involvement of Waldeyer’s ring. Cancer. 1978;42(3):1096-1104.
86 Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270(16):1949-1955.
87 Hancock SL, Cox RS, McDougall IR. Thyroid diseases after treatment of Hodgkin’s disease. N Engl J Med. 1991;325(9):599-605.
88 Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin’s disease. J Natl Cancer Inst. 1993;85(1):25-31.
89 Horning S, Hoppe RT, Advani R, et al. Efficacy and late effects of Stanford V chemotherapy and radiotherapy in untreated Hodgkin’s disease: mature data in early and advanced stage patients. Blood. 2004;104:308.
90 Leibenhaut MH, Hoppe RT, Varghese A, et al. Subdiaphragmatic Hodgkin’s disease: laparotomy and treatment results in 49 patients. J Clin Oncol. 1987;5(7):1050-1055.
91 Liao Z, Ha CS, Fuller LM, et al. Subdiaphragmatic stage I & II Hodgkin’s disease: long-term follow-up and prognostic factors. Int J Radiat Oncol Biol Phys. 1998;41(5):1047-1056.
92 Behar RA, Horning SJ, Hoppe RT. Hodgkin’s disease with bulky mediastinal involvement: effective management with combined modality therapy. Int J Radiat Oncol Biol Phys. 1993;25(5):771-776.
93 Nogova L, Reineke T, Eich HT, et al. Extended field radiotherapy, combined modality treatment or involved field radiotherapy for patients with stage IA lymphocyte-predominant Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin Study Group (GHSG). Ann Oncol. 2005;16(10):1683-1687.
94 Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma: a retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer. 2005;104(6):1221-1229.
95 Engert A, Diehl V, Pluetschow A, et al. Two cycles of ABVD followed by involved field radiotherapy with 20 Gray is the new standard of care in the treatment of patients with early-stage Hodgkin lymphoma. Blood. 2009;114:716.
96 Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(21):4634-4642.