HIV Infection and AIDS
Perspective
The acquired immunodeficiency syndrome (AIDS) is a pandemic caused by the human immunodeficiency virus (HIV). This disease has caused a tremendous degree of human suffering and has had an immeasurable impact on demographics, cultures, economics, and politics in most societies around the globe. There are an estimated 33.3 million people living with HIV infection worldwide, and 35 million people have died of it.1,2 Significant strides have been made in areas of prevention and treatment, resulting in stabilization of the epidemic, and the annual number of new infections has steadily decreased since the 1990s. Despite this decline in incidence of HIV infection, new infections and the widespread use of highly active antiretroviral therapy (HAART), thus decreasing AIDS-related deaths, have resulted in an increase of the global prevalence of HIV/AIDS (Fig. 132-1).
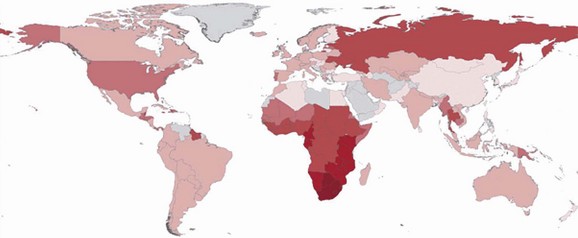
Figure 132-1 The distribution of people living with HIV infection globally. The darker shades represent higher prevalence of HIV infection. (From Joint United Nations Programme on HIV/AIDS: UNAIDS Report on the Global AIDS Epidemic 2010. Available at www.unaids.org/documents/20101123_2010_HIV_Prevalence_Map_em.pdf. Accessed November 25, 2011.)
HIV infection was first recognized in 1981 by clinicians in New York and Los Angeles in previously healthy homosexual men when they noted unusual clusters of Pneumocystis jiroveci (formerly Pneumocystis carinii) pneumonia (PCP) and Kaposi’s sarcoma. Early on, researchers were aware that these patients had a profound defect in their cell-mediated immune system. They discovered that this disease was spread through sexual activity or exposure to blood. Subsequent concerted scientific effort resulted in rapid identification of the infecting organism, prompt understanding of the natural history of the disease, and swift strides in providing therapeutic interventions.2
Means of transmission of HIV and the demographic distribution of the virus vary around the globe. Sub-Saharan Africa is the epicenter of the pandemic, with 22.5 million adults and children living with HIV infection (68% percent of the global HIV population). South Africa has the most people living with HIV infection (5.6 million), and Swaziland has the highest prevalence of HIV infection in the world (estimated to be 25.9%).1 Unprotected heterosexual intercourse with subsequent transmission of HIV to newborns and breast-fed babies (mother-to-child transmission) is the dominant mode of transmission worldwide, accounting for about 85% of all HIV infections.3 The HIV epidemic in Asia and eastern Europe is largely in people who inject drugs, sex workers and their clients, and men who have sex with men. In the Caribbean, the primary means of transmission is thought to be through unprotected sex between men and women, especially through paid sex. A large portion of the burden of disease in Central and South America is in men who have sex with men. In the Middle East and North Africa, the prevalence of HIV infection is relatively low.1 The main pattern of transmission in the higher income countries of North America and western and central Europe is in men who have sex with men. Other significant modes of transmission in these regions include injection drug use and paid unprotected sex.1
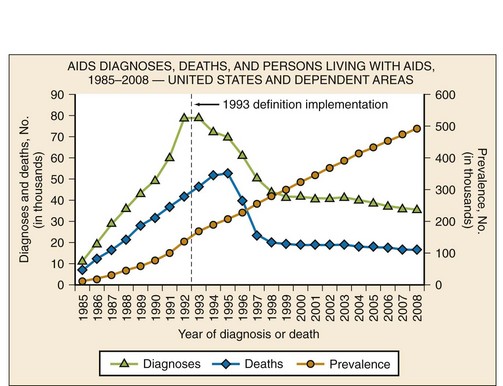
The prevalence of HIV infection in the United States, estimated to be 1,106,400 adults and adolescents in 2009, is currently the highest it has been.4 The increase in prevalence is likely to be due to a combination of factors, including more people knowing their HIV status and receiving medical therapy, identification of new infections, and fewer people dying of HIV/AIDS. Although the prevalence of HIV/AIDS in the United States continues to rise, the incidence has remained relatively stable since the late 1990s5 (Fig. 132-2). In the United States, HIV disproportionately affects men who have sex with men, African Americans, Hispanics, and people living in urban areas.4 Of the estimated 56,300 new HIV infections in the United States each year, 53% are in men who have sex with men, 31% are through high-risk heterosexual contact, 12% are in injection drug users, and 27% are in women.3,4
Principles of Disease
The mature HIV virion is a spherical structure with an outer envelope and an inner core (Fig. 132-3). The core contains two copies of the RNA genome, enzymes (reverse transcriptase and integrase), and regulatory proteins.6 Surrounding the core is the viral membrane, containing the glycoproteins gp41 and gp120, which are responsible for the attachment and entry of the virus into a CD4+ cell.7
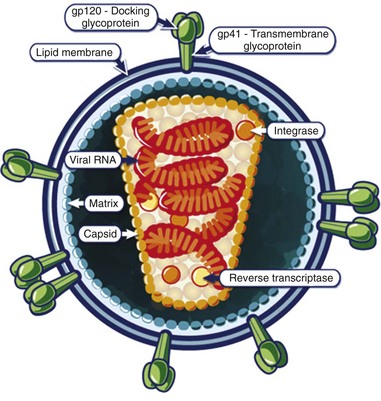
Figure 132-3 The structure of the HIV virion. (From the National Institute of Allergy and Infectious Diseases. Available at http://www.niaid.nih.gov/topics/HIVAIDS/Understanding/Biology/Pages/structure.asPx.)
In a multistep process, the HIV virion invades the host cell and integrates its genetic material into the host’s chromosome (Fig. 132-4). The infection begins with binding of the virus to the CD4+ host cell; the interaction is mediated by a high-affinity interaction between the surface proteins gp41 and gp120 with the CD4+ antigen and coreceptors. Different coreceptors, CCR5 and CXCR4, have been identified. The virus enters the cell by fusing its envelope with the target cell membrane. After internalization, reverse transcriptase forms viral DNA from the original RNA. The viral enzyme integrase then transports the newly formed viral DNA into the nucleus, where it integrates with human chromosomal DNA. Viral polyproteins and RNA are formed and subsequently modified and spliced by the viral enzyme protease, and new infectious viral particles are created.3,8 This cycle continues with HIV infecting more CD4+ cells. Major targets of antiretroviral therapy (ART) include reverse transcriptase, protease, integrase, and the CCR5 coreceptor.
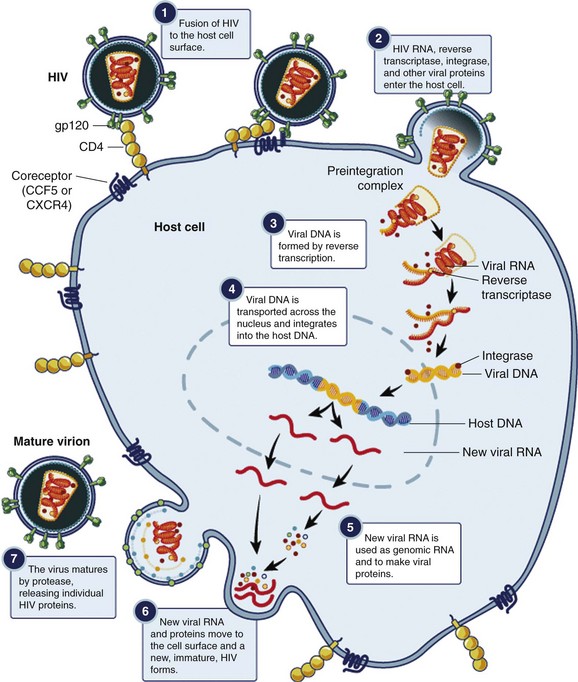
Figure 132-4 Replicative cycle of the HIV virion. (From the National Institute of Allergy and Infectious Diseases. Available at www.niaid.nih.gov/TOPICS/HIVAIDS/UNDERSTANDING/BIOLOGY/Pages/hivReplicationCycle.aspx.)
The hallmark of HIV infection is CD4+ T-cell destruction, leading to a deficient cell-mediated arm of the immune system. The opportunistic infections (PCP and infections due to Toxoplasma gondii, Mycobacterium avium complex [MAC], and cytomegalovirus [CMV]) and malignant neoplasms (including Kaposi’s sarcoma) of AIDS patients are primarily due to the lack of CD4+ activity. Although it is unclear exactly how HIV depletes CD4+ cells, increased peripheral destruction and decreased production of CD4+ cells occur with the infection. Humoral immunity is also impaired, through B-cell proliferation and production of abnormal antibodies, making HIV-infected individuals more vulnerable to infections by encapsulated bacteria.2,7
HIV infection also leads to a chronic immune activation. Ongoing viremia, along with proinflammatory cytokines, B-cell proliferation, and hypergammaglobulinemia, leads to a chronic inflammatory state that contributes to cardiovascular disease, cancer, and other chronic diseases in long-term HIV-infected individuals.7
Risk Factors for HIV Transmission
Activities that result in exposure to infectious body fluids increase the likelihood of HIV transmission, and the risk of transmission varies according to the nature of the exposure. The highest risk exposure is transfusion with HIV-positive blood. Other factors associated with increased risk of HIV transmission include exposure to serum with high viral load,9–12 lack of male circumcision,13–15 presence of ulcerative sexually transmitted disease,12 and similarity of HLA class I alleles among discordant couples.11 The risk of transmission of HIV varies by the type of sexual contact: it is 1 to 30% for receptive anal intercourse; 0.1 to 10% for receptive vaginal and insertive anal intercourse; and 0.1 to 1% for insertive vaginal intercourse.16,17
Clinical Features
Stages of HIV Infection
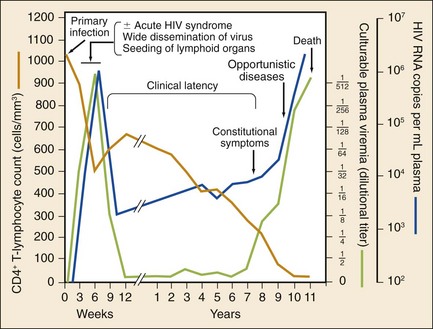
The stages of untreated HIV infection are described by the World Health Organization (Box 132-1) and the Centers for Disease Control and Prevention (CDC; Table 132-1). Figure 132-5 also illustrates the natural history of untreated HIV infection.
Table 132-1
CDC Surveillance Case Definitions for HIV Infection
| STAGE | LABORATORY EVIDENCE |
| Stage 1 | No AIDS-defining condition and either CD4+ T-lymphocyte count of ≥500 cells/µL or CD4+ T-lymphocyte percentage of total lymphocytes of ≥29 |
| Stage 2 | No AIDS-defining condition and either CD4+ T-lymphocyte count of 200-499 cells/µL or CD4+ T-lymphocyte percentage of total lymphocytes of 14-28 |
| Stage 3 (AIDS) | CD4+ T-lymphocyte count of <200 cells/µL or CD4+ T-lymphocyte percentage of total lymphocytes of <14 or documentation of an AIDS-defining condition |
| Stage unknown | No information available on CD4+ T-lymphocyte count or percentage and no information available on AIDS-defining conditions |
CDC, Centers for Disease Control and Prevention.
From Schneider E, et al: Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recomm Rep 57:1-12, 2008.
Primary HIV Infection
Primary infection with HIV often causes an acute, self-limited viral infection. The most common findings are mononucleosis-like symptoms consisting of fever, pharyngitis, and lymphadenopathy. This usually occurs 2 to 6 weeks after transmission. During this time, the virus is actively replicating and antibodies to HIV have not been produced. The virus has many potential targets, and viral loads are often enormous.18 The diagnosis of acute HIV infection has significant public health benefits. Patients with acute HIV infection transmit the infection disproportionately; these patients often do not know that they are infected, and their viral load may be in the range of millions of RNA copies per milliliter.19
Diagnosis of acute HIV infection requires keeping the constellation of symptoms in mind and understanding the pitfalls of laboratory testing for early infection. The results of routine HIV antibody testing may be negative for several weeks or even months after exposure. The diagnosis of acute HIV infection is confirmed with the presence of high titers of viral RNA and a negative antibody screen. Assay reactivity is dynamic; a plasma RNA test will detect HIV infection approximately 1 week before the ability to detect the p24 antigen and 12 days before antibodies to HIV develop20 (Table 132-2). A viral load test in the absence of symptoms of acute HIV infection is not recommended; false-positive results occur, and the test is costly.21 Confirmed cases of acute HIV infection should be referred immediately to a specialist, preferably one who actively enrolls patients in clinical trials.
Table 132-2
HIV Testing by Laboratory Stage
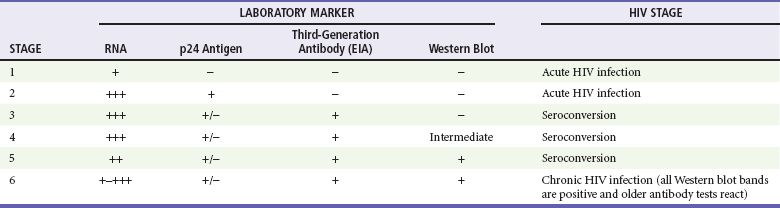
Modified from Fiebig EW, et al: Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS 17:1871-1879, 2003.
AIDS
The CDC definition of AIDS is a CD4+ cell count below 200 cells/µL or the presence of an AIDS-defining condition (Box 132-2). At this level, immune dysfunction is severe, and without ART, survival is short. Those with a CD4+ cell count below 50 cells/µL have advanced AIDS and are at much higher risk for death and for development of opportunistic infections. Some infections are so common in patients with AIDS that primary prophylaxis is indicated and cost effective. Prophylaxis is started for PCP when CD4+ counts are less than 200 cells/µL; for toxoplasmosis, when CD4+ counts are less than 100 cells/µL; and for MAC disease, when CD4+ counts are less than 50 cells/µL (Table 132-3). Isoniazid is given to patients with a positive response to purified protein derivative. The risk of opportunistic infection in relation to the CD4+ cell count is progressive rather than an all-or-none phenomenon.
Table 132-3
Prophylaxis to Prevent First Episode of Selected Opportunistic Infections
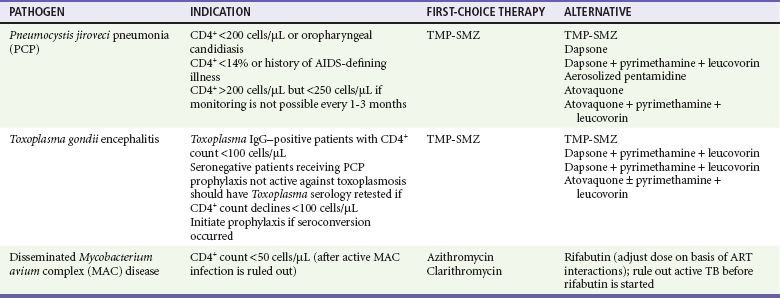
ART, antiretroviral therapy; TB, tuberculosis; TMP-SMZ, trimethoprim-sulfamethoxazole.
Modified from Kaplan JE, et al: Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58:1-207, 2009. Available at www.cdc.gov/mmwr/preview/mmwrhtml/rr5804a1.htm.
Initial Evaluation
HIV-infected patients are at risk for some of the same infections and medical problems that noninfected patients have, but they are more vulnerable to opportunistic and unusual infections. Knowledge of the CD4+ count, along with the patient’s clinical presentation, is critical in the ED management of the HIV-infected patient. Before HAART, HIV-positive individuals were primarily hospitalized for opportunistic infections. Patients with HIV infection are now dying of diseases that were not traditionally considered AIDS diseases, such as heart disease, liver failure, and non–AIDS-related cancers.22
The current CD4+ count is a marker of the degree of immunosuppression and is critical background information for the interpretation of signs and symptoms. However, many patients have undiagnosed HIV infection, and they often present in later stages of disease.23 Others may not be taking ART because of issues involving access to care, difficulties with medication adherence, or viral resistance to ART. Opportunistic infections such as PCP, disseminated mycobacterial infections, cryptococcal meningitis, and CMV disease do not occur until CD4+ counts are dramatically reduced. A total lymphocyte count may provide a rough surrogate for the absolute CD4+ count; a count between 1000 and 2000 cells/µL appears to be a reasonable surrogate of significant immunosuppression. A cell count of more than 2000 cells/µL makes it less likely that a patient is profoundly immunosuppressed. Acute illness will drop peripheral lymphocyte counts and thus limits the utility of the peripheral lymphocyte count as a diagnostic aid in an acute setting. However, an ED study showed that patients with a peripheral lymphocyte count below 950 cells/µL were highly likely to have AIDS.24
Certain clinical features suggest the possibility of a new diagnosis of HIV infection. A careful look at the skin and in the mouth may be revealing; certain skin findings, such as oral hairy leukoplakia and Kaposi’s sarcoma, are found almost exclusively in HIV-infected individuals.25 Unexplained oral candidiasis is often the initial presenting symptom in someone with undiagnosed HIV infection.
Clinical Manifestations by Organ System
HIV-infected patients have an increased risk of acute coronary syndrome, although it is not fully understood whether this is the result of the HIV infection itself, a complication of ART, or a combination of both. Patients receiving ART suffer from a myriad metabolic abnormalities (hyperglycemia, hyperlipidemia, lipodystrophy) and accelerated atherosclerosis, which may increase their risk for cardiovascular disease and acute coronary syndrome.26–32 Studies have shown that the virus alone is associated with dyslipidemia,26 endothelial damage,33–35 inflammation,36,37 and hypercoagulability.38–42 Stopping of ART has been shown to result in systemic inflammation, coagulation cascade activation and an increase in biomarkers associated with endothelial activation,36,43 and increased risk of major cardiovascular events.44
Antiretroviral agents cause varying degrees of dyslipidemia. Protease inhibitors, particularly ritonavir in higher doses, can especially be a problem.30 Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are associated with increases in low-density lipoprotein cholesterol and total cholesterol but also a significant increase in high-density lipoprotein cholesterol.45 The Data Collection on Adverse Events of Anti-HIV Drugs (D : A : D) study found that the nucleoside reverse transcriptase inhibitor abacavir was associated with a fourfold increased risk of myocardial infarction and a significant increase in stroke, peripheral vascular disease, and congestive heart failure.46 Despite the reality that some ART is likely to contribute to the development of coronary artery disease, the benefits of therapy outweigh the risks of accelerated atherosclerosis.
Pulmonary
Pulmonary infections are the leading cause of morbidity and mortality for people living with HIV infection.47 Noninfectious pulmonary complications are also common. The patient’s immune status (as suggested by the last CD4+ count) and the current treatment regimen determine the severity of the presenting illness. The tempo of the illness and geographic risk factors also help guide the diagnostic evaluation and initial therapy.
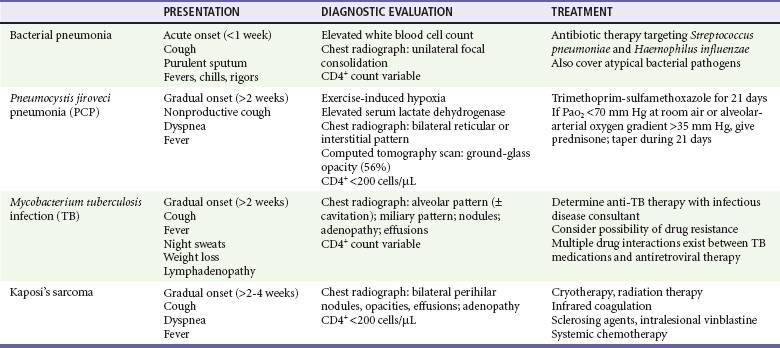
The most frequent respiratory infections in people with HIV infection are upper respiratory tract infections and acute bronchitis. The incidence of lower respiratory tract infections increases as CD4+ counts decline. Potential causes of lower respiratory tract infections include viruses (influenza, respiratory syncytial, parainfluenza), bacteria, and fungi (P. jiroveci) (Table 132-4). Bacterial pneumonia is more frequent in people infected with HIV than in noninfected persons, and the incidence increases as the CD4+ count drops; the most common organism is Streptococcus pneumoniae. The incidence of PCP also increases as the CD4+ count drops below 200 cells/µL; the risk of infection progressively increases as immune function worsens.
Table 132-4
Differential Diagnosis of Respiratory Infections in HIV-Infected Patients by CD4+ Count
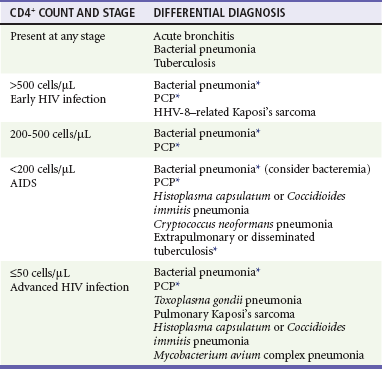
HHV-8, human herpesvirus 8; PCP, Pneumocystis jiroveci pneumonia.
Diagnostic evaluation of patients with HIV infection who present with respiratory complaints should be based on their HIV disease stage as well as their clinical presentation (Table 132-5). Evaluation includes pulse oximetry, chest radiography, and complete blood count. Additional tests may include arterial blood gas analysis (to determine the need for corticosteroids in patients with PCP), serum lactate dehydrogenase (elevated in PCP), serum cryptococcal antigen, urine Histoplasma capsulatum antigen, and sputum studies (including Gram’s stain, acid-fast bacillus, and staining for P. jiroveci). Blood cultures should be considered before antibiotic therapy initiation; an aggressive search for the pathogen is generally recommended in patients with HIV infection.
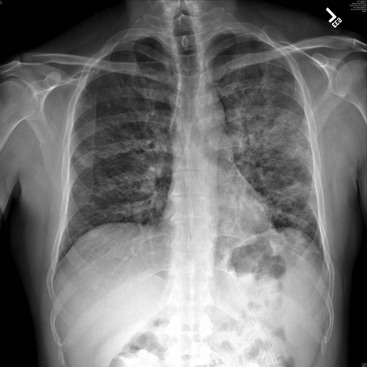
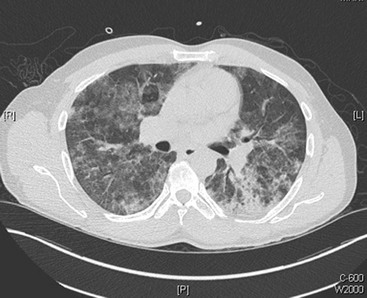
PCP is one of the most common AIDS-defining opportunistic infections. With the widespread use of HAART as well as chemoprophylaxis against PCP in patients with CD4+ counts below 200 cells/µL, the incidence of PCP in developed countries has greatly decreased during the last several years.48 The clinical presentation of PCP is characterized by the gradual onset of fever (79-100%), cough (95%), and progressive dyspnea (95%).49 The cough is nonproductive, although sputum production does not exclude this diagnosis, and patients can be coinfected with a bacterial pneumonia. Some patients, especially those taking nonsystemic prophylaxis (e.g., aerosolized pentamidine), may have extrapulmonary manifestations of PCP, such as hepatosplenomegaly, skin lesions, and ocular lesions. The most common associated laboratory abnormalities are a CD4+ count below 200 cells/µL and an elevated lactate dehydrogenase level. Whereas chest radiographs can be normal, most commonly they show diffuse, bilateral, interstitial, or alveolar infiltrates (Fig. 132-6). High-resolution computed tomography (CT), which has a high sensitivity for PCP, often reveals ground-glass or cystic lesions; a normal scan makes the diagnosis of PCP very unlikely50 (Fig. 132-7). The definitive diagnosis is made by isolation of the organism, commonly from respiratory specimens obtained by sputum induction, bronchoalveolar lavage, or endotracheal aspiration.
The use of primary and secondary prophylaxis greatly diminishes the risk of acquiring PCP. Risk factors for development of PCP include a history of PCP; a CD4+ count below 200 cells/µL (about 95% of the cases occur in people with CD4+ counts below 200); and recurrent fever, night sweats, oropharyngeal thrush, and unintentional weight loss among patients with CD4+ counts above 200 cells/µL. The average CD4+ count at the time of the first episode of PCP for patients not receiving prophylaxis is about 100 cells/µL; and for those receiving prophylaxis, about 20 cells/µL. Double-strength trimethoprim-sulfamethoxazole (TMP-SMZ) administered orally daily is the preferred PCP prophylaxis. TMP-SMZ is also the preferred treatment of PCP; the route is dependent on the severity of disease. There are a number of other possible regimens for those who are intolerant (Table 132-6).
Table 132-6
Treatment of Pneumocystis jiroveci Pneumonia in Patients with HIV Infection
| PREFERRED THERAPY | ALTERNATIVE THERAPY | |
| Moderate to severe illness | TMP-SMZ intravenously; switch to oral administration after clinical improvement 21-day therapy |
Pentamidine Primaquine + clindamycin |
| Mild to moderate illness | TMP-SMZ | Dapsone + trimethoprim Primaquine + clindamycin Atovaquone |
TMP-SMZ, trimethoprim-sulfamethoxazole.
Modified from Kaplan JE, et al: Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58:1-207, 2009. Available at www.cdc.gov/mmwr/preview/mmwrhtml/rr5804a1.htm.
Before initiation of antibiotics, the presence of resting hypoxemia or tachypnea should be assessed to determine whether an arterial blood gas analysis is indicated. Hypoxic patients (partial pressure of oxygen ≤70 mm Hg or an alveolar-arterial oxygen gradient ≥35 mm Hg) receive significant benefit from concurrent corticosteroid treatment. The clinical manifestations of PCP infection are caused by the replication of the organism and the host inflammatory response. Fungal death exacerbates the inflammatory response, and hypoxic patients should receive a 21-day prednisone taper in addition to antibiotic therapy.49
Concurrent epidemics of HIV infection and tuberculosis (TB) have resulted in significant morbidity and mortality for affected populations, particularly in Africa, where the burden of both diseases is immense. The risk of TB increases with declining immune function51,52; it doubles within the first year after HIV seroconverion.53 Patients with HIV infection are more likely to develop active TB from reactivated latent infection, and HIV infection is a risk factor for accelerated progression of TB.54 For patients with HIV infection, TB coinfection increases the risk of progression to AIDS or death.55
Clinical manifestations and radiographic findings of TB vary by the degree of underlying immunosuppression of the patient. Patients with early HIV infection and TB have presentations similar to those of individuals without HIV infection; they often have classic symptoms of pulmonary TB, such as fever, cough, weight loss, malaise, and night sweats. The typical radiographic findings of cavitation in the upper lung fields are usually present. With worsening immune function, there is less likelihood of pulmonary cavitation. In these cases, atypical radiographs are more common, such as pulmonary infiltrates without preference for the upper lung fields, and normal radiographs have been reported. With advanced HIV infection and severe immunosuppression, there is greater risk of extrapulmonary and disseminated TB.51,56
Oropharyngeal and Gastrointestinal
Oropharyngeal and gastrointestinal diseases are often the presenting symptoms of HIV infection. HIV-infected patients with gastrointestinal symptoms may present with common abdominal disease or may have HIV-related infections, malignant neoplasms, or medication side effects. Patients receiving ART may suffer treatment-related adverse gastrointestinal events, including pancreatitis, hepatic steatosis, lactic acidosis, and drug-induced hepatotoxicity. Furthermore, a number of patients with HIV infection have concomitant hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and may have gastrointestinal manifestations of illness from these causes as well. End-stage liver disease is now a common cause of mortality in HIV-infected patients.57
The routes for transmission of HIV, HBV, and HCV are similar for HIV-infected and noninfected patients. Coinfection is common; approximately 25% of individuals with HIV infection are coinfected with HCV, and another 8% are infected with HBV.58 Coinfection is more common in patients who are injection drug users or have hemophilia. HCV-HIV coinfection is associated with poor socioeconomic status and substance abuse. Now that HAART has decreased mortality from AIDS-related illness, end-stage liver disease has become a common cause of death in HIV-infected patients in industrialized countries.58 In addition, concomitant alcohol use contributes to the rapid progression to hepatic failure.
Central Nervous System
Cryptococcus neoformans is the most common cause of meningitis in patients with AIDS.59 As with other opportunistic infections, HAART, where it is readily available, has dramatically decreased the incidence of cryptococcal meningitis. However, it remains a leading cause of mortality for people with HIV infection in most of the developing world.59 It usually affects patients who are profoundly immunosuppressed with CD4+ counts below 100 cells/ µL. The disease is subacute, and patients present with fevers, malaise, and headache. Later in the course, because of increased intracranial pressure, patients experience vomiting and altered mental status. Cryptococcus often does not cause a significant inflammatory response, and meningeal signs are frequently absent. The diagnosis is suggested by the presence of cryptococcal antigen (CRAG) in the CSF or the serum and low white blood cell count in the CSF (typically <50/µL in the CSF). A lumbar puncture is diagnostic; elevated opening pressures during lumbar puncture are typical (70% with pressures >200 mm Hg), and evidence of encapsulated yeast forms on staining of the CSF with India ink can be found.60 Poor prognostic factors for cryptococcal meningitis are altered mental status, absence of CSF pleocytosis, CSF antigen titers greater than 1 : 1024, and positive serum fungal culture.59,61 These signs are indicative of a high organism burden, elevated CSF pressure, and lack of an inflammatory response. If it is left untreated, cryptococcal meningoencephalitis is fatal. The therapy involves three phases: induction, consolidation, and maintenance. If the patient can tolerate it, aggressive initial treatment with 2 weeks of amphotericin B and flucytosine is recommended. Fluconazole is used for the consolidation phase, and it is continued until immune reconstitution (CD4+ counts >100 cell/µL for more than a year). Elevated intracranial pressure is treated with repeated lumbar punctures; on occasion, a lumbar drain is needed. Cryptococcal meningitis immune reconstitution syndrome can cause rapid clinical deterioration. Thus, ART is often started after 4 weeks of antifungal treatment.59
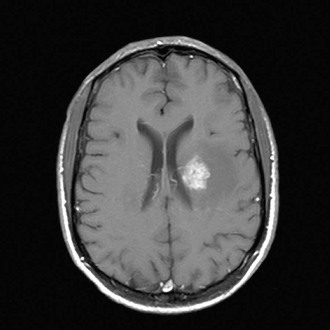
Patients may present with focal CNS lesions. Severely immunocompromised hosts (CD4+ counts <200 cells/µL) are likely to have opportunistic infections or AIDS-associated tumors (Table 132-7). In developed countries, common causes of mass effect are toxoplasmosis and EBV-related primary CNS lymphoma; in developing countries, the etiology is more likely to be tuberculomas. Toxoplasmosis is caused by reactivation of latent infection by the parasite Toxoplasma gondii. The majority of infected patients have a CD4+ count below 100 cells/µL. Patients present with signs of increased intracranial pressure, such as headaches, confusion, lethargy, and seizures. Lesions are typically multiple and ring enhancing on CT. Although the definitive diagnosis is made after a brain biopsy, patients who are serologically positive for toxoplasmosis are usually treated empirically with pyrimethamine and sulfadiazine, keeping in mind that toxoplasmosis is much less common in patients who have been receiving TMP-SMZ prophylaxis for PCP. The majority will show radiographic improvement in 2 weeks; response to treatment will obviate the need for a brain biopsy (Fig. 132-8).
Table 132-7
Differential of Focal Central Nervous System Lesions in Patients with HIV Infection
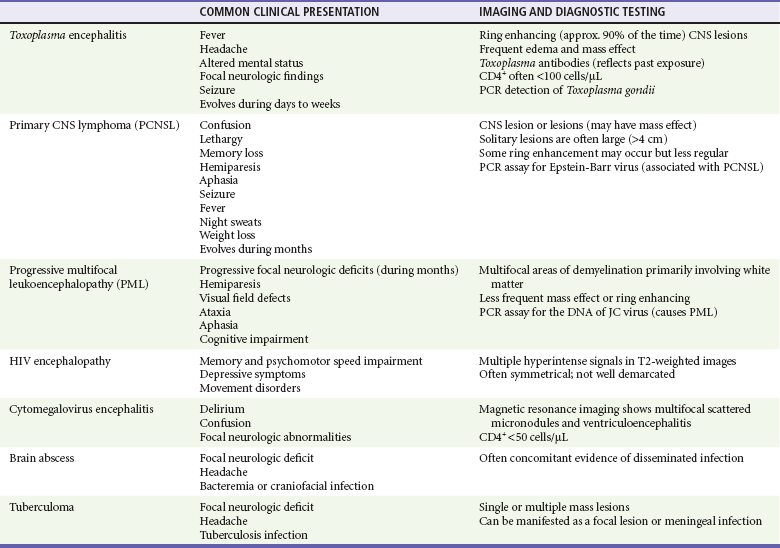
CNS, central nervous system; PCR, polymerase chain reaction.
Primary CNS lymphoma often looks identical to toxoplasmosis on magnetic resonance imaging (MRI) or CT. It also occurs with profound immunosuppression (CD4+ counts <50 cells/µL). EBV is the cause of primary CNS lymphoma, and a polymerase chain reaction (PCR) analysis of CSF looking for the virus has become an integral step in the workup of mass lesions.62 Treatment is with ART and chemotherapy.
The “gold standard” for the diagnosis of CNS mass lesions remains the brain biopsy.62 Corticosteroid therapy can cause false-negative results on brain biopsies in patients with lymphoma, and therefore the use of corticosteroids should be limited to patients with life-threatening mass lesions.
Renal
Renal disorders in patients with HIV infection are common, occurring in up to 30% of infected patients.63 HIV-infected individuals often have other high-risk conditions for renal disease, such as hepatitis B and C, opportunistic infections, hypertension, and injection drug use. ART can also affect the kidney. Indinavir, although less commonly used in industrialized countries, is associated with renal calculi. Tenofovir can cause acute renal failure, a Fanconi-like syndrome, and nephrogenic diabetes insipidus.64 Medications used to treat opportunistic infections can cause acute renal failure; amphotericin, pentamidine, and foscarnet are especially notorious.
HIV-associated nephropathy (HIVAN), a form of focal glomerulosclerosis, usually occurs when the CD4+ count is below 200 cells/µL. Proteinuria, often severe, and elevated creatinine concentration occur. Overall, treated patients with HIVAN are experiencing less mortality, but the treatment is problematic, involving immunosuppressive medications such as steroids and cyclosporine. Some patients recover renal function with HAART, but others progress to end-stage renal disease and require dialysis. Transplantation is now widely accepted for patients with HIV infection, and it is available at certain centers in the United States.63,65
Rheumatologic and Orthopedic
Unusual and atypical infections occur in late-stage AIDS. Bacillary angiomatosis is caused by Bartonella henselae and Bartonella quintana. In addition to causing disseminated disease affecting the skin, lymph nodes, liver, and CNS, the organism is also a common agent of long bone osteomyelitis.66
Patients infected with HIV have lower bone mineral density than that of age-matched controls. Fractures of the hip, spine, and wrist are more common in HIV-infected individuals. Osteonecrosis, especially of the femoral head, is common. Predisposing factors include corticosteroid use, ethanol abuse, and hypertriglyceridemia.67 Benign and mundane musculoskeletal complaints are common in HIV-infected patients, but osteonecrosis should be considered in HIV-infected patients with severe and persistent joint pain.
HIV-related polymyositis can occur at any stage of infection. These patients may have proximal muscle weakness, myalgias, and fatigue. Medications, especially nucleotide reverse transcriptase inhibitors (NRTIs) such as azidothymidine (AZT), can be toxic to the mitochondria and are common causes of polymyositis. Myopathies, spondylarthritis, pyomyositis, and HIV-associated arthritis are also common musculoskeletal problems.68 Reactive arthritis and other seronegative arthropathies are common. It is unclear if this relationship is because of sexual activity, generalized immune suppression, or the inflammatory response from the virus itself.
Hematologic
Hematologic disease is common in patients with HIV infection, and HIV infection can cause anemia, thrombocytopenia, and leukopenia. Thrombocytopenia can occur in any stage of HIV infection, and thus its presence in an undiagnosed patient should prompt consideration of screening. The cause is often immune related, presenting as a disease process similar to idiopathic thrombocytopenic purpura, and the treatment is ART. Thrombotic thrombocytopenic purpura is also well described in HIV-infected patients; it tends to occur at later stages of disease.69
Anemia and leukopenia tend to occur in later stages of HIV infection. Various medications can cause bone marrow toxicity; AZT, TMP-SMZ, and ganciclovir are common offenders. Systemic fungal infections and mycobacterial disease such as disseminated MAC disease can infect the bone marrow and decrease all three cell lines. Nutritional deficiencies, such as folate and vitamin B12, are common.69
AIDS-related lymphoma (Hodgkin’s and non-Hodgkin’s) occurs more frequently in patients with advanced HIV infection. The majority of non-Hodgkin’s lymphomas are of B-cell origin, and they tend to be more aggressive in HIV-infected than in noninfected patients. On occasion, it is the first AIDS-defining illness. CNS lymphomas and Burkitt’s lymphoma are nearly always associated with EBV, and primary effusion lymphoma is associated with human herpesvirus 8. The treatment involves standard chemotherapy and ART.69
Cutaneous
Dermatologic manifestations of HIV infection are extremely common, and up to 90% of HIV-infected patients will have a skin disorder after seroconversion.25 Skin findings occur throughout the course of HIV infection. Some skin findings are manifested early in the disease; others, found later, can be suggestive of profound immunosuppression (Box 132-3). Skin problems increase as HIV infection progresses. Recognition of HIV-related dermatologic conditions can lead to early diagnosis and can help the clinician gauge the immune status of the patient (Box 132-4).
Acute HIV infection often is manifested with a generalized maculopapular or morbilliform rash shortly after the onset of fevers. Oral ulcers, lesions on the palms and soles, and mucosal lesions can all be present.31 Aside from HIV itself, a variety of viruses can involve the skin. An outbreak of herpes zoster can be the first clue that a patient has HIV infection, and affected individuals often have CD4+ counts above 200 cells/µL. Herpes simplex virus infections are often more severe and recur frequently. Chronic ulcerating herpes simplex occurs later in the disease course and is an AIDS-defining opportunistic infection. Other common viral diseases include molluscum contagiosum, human papillomavirus infection, and oral hairy leukoplakia.
Kaposi’s sarcoma, a vascular neoplasm, is the most common AIDS-related malignant disease in the United States, and the skin is the most commonly involved organ. Lesions are violaceous patches, nodules, or plaques (Fig. 132-9). Bacillary angiomatosis is manifested with lesions that resemble those of Kaposi’s sarcoma.
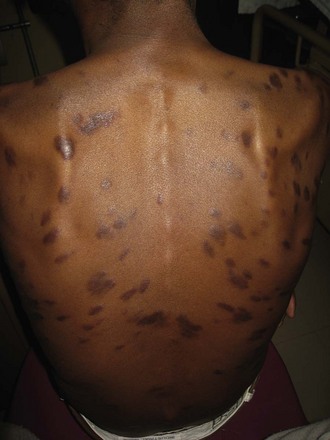
Figure 132-9 Kaposi’s sarcoma lesions.
Diagnostic Strategies
The diagnosis of HIV infection involves the detection of either specific antibodies or viral antigens. Multiple commercial tests are available. Laboratory detection of HIV infection is a two-step process. The first step is a screening test; if the result is positive, a confirmatory test is performed (Box 132-5).
The balance between public health and patient confidentiality has been an issue surrounding HIV testing and reporting. Most states and hospitals have developed policies related to these concerns. Regardless, testing should be done in a confidential manner with appropriate follow-up and counseling. Testing for CD4+ cell levels and HIV viral load is an ill-advised way of circumventing appropriate HIV testing when consent is refused or not attainable; in certain states, there is a requirement to report low CD4+ counts, and this circumvents the patient’s right of refusal of formal HIV testing.70
Emergency Department Testing for HIV
In the past, HIV testing in the ED has been primarily reserved for diagnosis of acute cases of presumed infection; routine screening has not been common practice. Reasons for this include the time it takes to do the test, the risk of not being able to locate the patient after the ED visit to share test results, and the difficulties in providing adequate pretest and post-test counseling.71–73 With the advent of enhanced testing capabilities as well as the push to diagnose existing cases of HIV infection in difficult-to-reach populations, some experts have started to reevaluate this dogma.74 Advantages of testing in the ED include increased detection of HIV infection in difficult-to-reach populations and earlier diagnosis of HIV infection, allowing earlier ART implementation and therefore decreased viral transmission.
In 2006, the CDC published revised recommendations for HIV testing in health care settings, including hospital EDs.75 This report recommends the use of diagnostic HIV testing and opt-out HIV screening in routine clinical care. Routine screening is recommended for 13- to 64-year-old patients, all patients who require TB treatment, those seeking treatment for sexually transmitted diseases, and all pregnant women. Repeated annual screening is recommended for people thought to be at high risk. Providers are to encourage patients to have HIV screening before initiation of sexual relationships and after occupational exposures. Recommendations specify that consent should be obtained for HIV testing, that pretest information should be shared with patients, and that persons responsible for the patient’s care should be notified verbally of the planned testing.75
Since their publication in 2006, efforts to implement these CDC recommendations and to characterize the implications of these changes to ED practice have been under way.76–82 Thus far, identified barriers to HIV screening in the ED include insufficient time, burdensome consent processes, lack of knowledge or training, lack of patient acceptance, pretest counseling requirements, competing priorities, and inadequate reimbursement.71,72,83 Whereas studies of ED providers reflect that the majority of providers are supportive of routine HIV testing,71,84 it is clear that many gaps remain in determining how best to implement routine screening into practice.85–87
Management
HAART has led to dramatic reductions in morbidity and mortality from opportunistic diseases and non-AIDS conditions.87 The goal of therapy is to suppress viral replication and to reconstitute the immune system. Typically in patients receiving appropriate HAART, the viral load is undetectable, and there is a restoration of immune function. Primary and secondary prophylaxis against opportunistic infections can be safely stopped with a return of CD4+ cells. Treatment options in industrialized nations continue to evolve, and the regimens are now easier to tolerate and are less toxic. The best evidence for successful treatment is in those patients with CD4+ counts below 350 cells/µL. However, CD4+ counts below 500 cells/µL indicate that there is an impairment of immune function, and recent guidelines recommend treatment of asymptomatic patients at this level.88 In addition, even patients with nearly normal CD4+ counts with ongoing viral replication are at elevated risk for a number of conditions, such as cardiovascular disease, kidney and liver disease, and malignant neoplasms. As a result, initiation of treatment for HIV-positive patients at any CD4+ count can be considered.89
Antiretroviral therapies target the major viral enzymes (reverse transcriptase, protease, and integrase) and their attachment and fusion sites. HAART involves the use of three active drugs, usually two NRTIs and another agent, often a protease inhibitor or NNRTI. Zidovudine, an NRTI, was the first drug released. Several NRTIs are currently available and approved by the Food and Drug Administration for treatment of HIV infection. Protease inhibitors, released in 1995, revolutionized the treatment of HIV infection and were the beginning of HAART. NNRTIs are also commonly a part of the three-drug regimen. Recently, new classes of antiretroviral therapies have been developed; these include integrase inhibitors and CCR5 inhibitors, allowing further options for patients who suffer side effects or have drug-resistant virus.88,89 The initial regimen of ART needs to be individualized. Factors to consider in choosing a regimen include tolerability, viral resistance, dosing frequency, cost, and comorbidities.
Antiretroviral treatment failure can occur for many reasons. Commonly, it is from poor medication adherence,90,91 although studies show exceptional rates of adherence in sub-Saharan Africa.92 Fixed-dose regimens are thought to increase adherence, and the combination of tenofovir, emtricitabine, and efavirenz is currently available as a once-daily medication. Resistance testing is critical in treatment-experienced patients. Ideally, at least two active medications should be used. Newer therapies, such as advanced-generation NNRTIs (etravirine), integrase inhibitors (raltegravir), CCR5 inhibitors (maraviroc), and new protease inhibitors (darunavir), provide choices for patients.
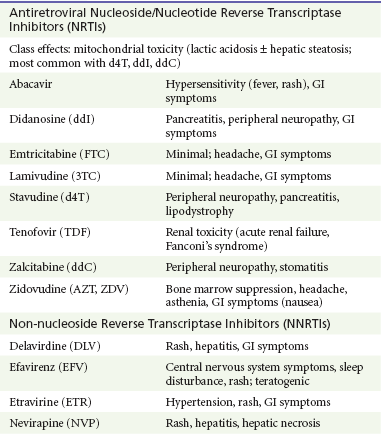
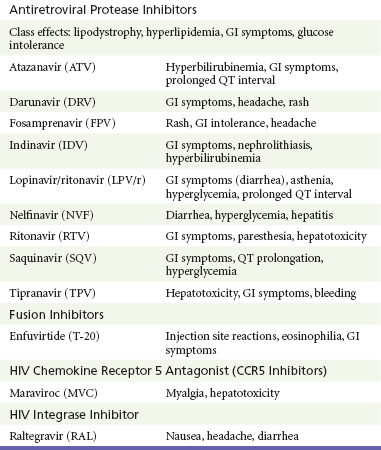
Side effects from antiretroviral medications are extremely common. Protease inhibitors are notorious for gastrointestinal side effects; most cause nausea and diarrhea. The NRTIs are mitochondrial toxic and can cause pancreatitis and hepatitis. Nevirapine, an NNRTI, can cause hepatic necrosis. Atazanavir causes a Gilbert-like syndrome. Efavirenz is commonly associated with self-limited neuropsychiatric problems (Table 132-8).
Availability of ART to resource-limited countries is an important priority. Presently, of the 15 million people living with HIV/AIDS in low- and middle-income countries, only 5.2 million have access to ART.1 HAART must be continued for life. The development of an effective vaccine continues to be a top research priority.
Postexposure Prophylaxis
All efforts should be made to minimize the potential for occupational exposure to body fluids. When exposure to body fluids occurs, however, the risk of acquiring HIV infection is low. Before ART, a large study examining HIV transmission from needle-stick injuries to health care workers showed that transmission occurred in only 1 of 300 cases (0.33%), and there were no known cases of transmission through intact skin.93 Now with postexposure prophylaxis (PEP) using ART, the risk of transmission is thought to be greatly reduced, especially with two or three drugs.
The recommendations for whether to use PEP and which medications to use are based on the type of injury and the body fluid involved. Body fluids of concern include semen, vaginal secretion, and any fluid contaminated with visible blood. Potentially infectious body fluids include CSF and synovial, pleural, peritoneal, pericardial, and amniotic fluids. Unless they contain blood, the following fluids are not considered infectious for HIV: vomitus, feces, nasal secretions, saliva, sputum, sweat, tears, and urine. Low-risk injuries are those involving solid needles (such as suture needles), those that are superficial, and those involving a low-risk source patient or body fluid. High-risk injuries include those involving hollow-bore needles with visible blood and percutaneous injury from a needle that was in an artery or vein of the source patient. Unless a mucocutaneous exposure involves large volumes of blood from a source patient with a plasma HIV viral load of more than 1500 copies/µL, mucocutaneous exposures are considered to be low risk. Transmission is estimated to be as low as 0.09% (1 in 1000) for a splash of infectious body fluid to mucous membranes or broken skin.94
Recommendations for PEP are based on the HIV status of the source patient. If the serostatus of the source patient is unknown, PEP should be initiated until the source patient is tested. The source patient may have tested seronegative in the past and could have contracted HIV since the last test; testing of HIV RNA in the source patient should be considered if this is a possibility. If the exposed person is to receive PEP, the goal is to initiate therapy within 1 to 2 hours after exposure; the efficacy of PEP greatly decreases after 24 to 36 hours. Follow-up HIV testing should occur at 6 weeks, 3 months, and 6 months; an additional test at 12 months can be considered for those exposed to source patients coinfected with HIV and HCV.94
If the potential benefit of PEP is deemed to outweigh the risk of the medication side effects, persons with low-risk exposures should be treated with two nucleosides. Those with high-risk exposures should be treated with two nucleosides plus a boosted protease inhibitor.94 The most common two-drug nucleoside regimens are zidovudine and lamivudine (available in a fixed-dose combination) and tenofovir and emtricitabine (available in a fixed-dose, once-daily formulation). For high-risk exposures, the third drug can be ritonavir-boosted lopinavir (lopinavir/r) or an alternative protease inhibitor. The decisions about which medications to use should be made with use of local hospital guidelines and infectious disease specialist consultation, if needed. The patient should be observed closely to monitor for drug toxicity. PEP should be continued for 4 weeks.
Nonoccupational Exposure
Some patients may present to the ED after possible exposure to HIV/AIDS and are concerned about the potential of transmission. Possible means of exposure include sexual contact, injection drug use, and exposure to body fluids through broken skin or mucous membranes. The risk of transmission varies with the means of exposure. For sexual exposures, receptive anal intercourse carries a higher risk of transmission among men who have sex with men compared with other sexual contact exposures because of the potential for mucosal breakdown and rectal bleeding. Similarly, the presence of genital ulcerative disease increases the likelihood of HIV transmission through sexual contact.17 Among heterosexual contact, the likelihood of HIV transmission is greatest after receptive anal intercourse, followed by receptive vaginal intercourse and insertive vaginal intercourse.17 Male-to-female transmission of HIV is more common than female-to-male transmission. The risk of transmission after use of injection needles is greater after percutaneous injection of a contaminated needle into an artery or vein. Other important factors influencing the risk of HIV transmission include the HIV status and viral load of the source.
The CDC recommends PEP for persons presenting within 72 hours after an exposure to a source known to be HIV positive if contact of body fluid contaminated with blood (including semen, vaginal secretions, rectal secretions, and breast milk) was made with the vagina, rectum, eye, mouth or other mucous membrane, or nonintact skin or by percutaneous injection.95 Efforts should be made to determine the current HIV status of the source.
Disposition
The ED management of patients with HIV/AIDS can be difficult, and the complexities in initiation of treatment and in discerning the appropriate disposition for these patients should not be underestimated. The widespread use of HAART among HIV-positive individuals has dramatically changed the course of the disease; individuals often have sustained and lasting immune reconstitution, living relatively normal lives. Knowledge of their immune status is critical in disposition and treatment; patients with normal or nearly normal CD4+ counts should be treated like non–HIV-infected patients. Drug interactions are common. Patients with AIDS, unlike immunocompetent patients, often suffer from multiple, simultaneous underlying pathologic processes, making workup and treatment decisions even more difficult; a unifying diagnosis is not the norm.96 These patients are at greater risk of morbidity and mortality from common disease entities as well as from complications of HIV/AIDS. Clinicians who approach these patients with background knowledge of the potential manifestations of HIV/AIDS will be poised to deliver the best emergent care.
References
1. Joint United Nations Programme on HIV/AIDS. UNAIDS Report on the Global AIDS Epidemic 2010. Geneva: UNAIDS; 2010.
2. Centers for Disease Control and Prevention. The global HIV/AIDS pandemic, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:841–884.
3. Simon, V, Ho, DD, Abdool Karim, Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet. 2006;368:489–504.
4. HIV in the United States. An Overview. Atlanta, Ga: Centers for Disease Control and Prevention; 2010.
5. Hall, HI, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529.
6. Gomez, C, Hope, TJ. The ins and outs of HIV replication. Cell Microbiol. 2005;7:621.
7. Levy, JA. HIV pathogenesis: 25 years of progress and persistent challenges. AIDS. 2009;23:147.
8. Stevenson, M. HIV-1 pathogenesis. Nat Med. 2003;9:853–860.
9. Dorak, MT, et al. Transmission of HIV-1 and HLA-B allele-sharing within serodiscordant heterosexual Zambian couples. Lancet. 2004;363:2137–2139.
10. Quinn, TC, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929.
11. Gray, RH, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1–discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153.
12. Wawer, MJ, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409.
13. Auvert, B, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 Trial. PLoS Med. 2005;2:e298.
14. Bailey, RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet. 2007;369:643–656.
15. Gray, RH, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: A randomised trial. Lancet. 2007;369:657–666.
16. Landovitz, RJ, Currier, JS. Clinical practice. Postexposure prophylaxis for HIV infection. N Engl J Med. 2009;361:1768–1775.
17. Powers, KA, Poole, C, Pettifor, AE, Cohen, MS. Rethinking the heterosexual infectivity of HIV-1: A systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563.
18. Brenner, BG, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195:951.
19. Pilcher, CD, et al. Brief but efficient: Acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785.
20. Fiebig, EW, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879.
21. Roland, ME, et al. HIV RNA testing in the context of nonoccupational postexposure prophylaxis. J Infect Dis. 2004;190:598–604.
22. Crum, NF, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200.
23. Jean-Jacques, M, Walensky, RP, Aaronson, WH, Chang, Y, Freedberg, KA. Late diagnosis of HIV infection at two academic medical centers: 1994-2004. AIDS Care. 2008;20:977.
24. Napoli, AM, et al. Absolute lymphocyte count in the emergency department predicts a low CD4 count in admitted HIV-positive patients. Acad Emerg Med. 2011;18:385–389.
25. Garman, ME, Tyring, SK. The cutaneous manifestations of HIV infection. Dermatol Clin. 2002;20:193.
26. Dube, M, Fenton, M. Lipid abnormalities. Clin Infect Dis. 2003;36:S79–S83.
27. Grunfeld, C, et al. Preclinical atherosclerosis due to HIV infection: Carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–1849.
28. Khunnawat, C, Mukerji, S, Havlichek, D, Jr., Touma, R, Abela, GS. Cardiovascular manifestations in human immunodeficiency virus–infected patients. Am J Cardiol. 2008;102:635–642.
29. Tershakovec, AM, Frank, I, Rader, D. HIV-related lipodystrophy and related factors. Atherosclerosis. 2004;174:1–10.
30. Friis-Møller, N, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735.
31. Barbaro, G. Highly active antiretroviral therapy–associated metabolic syndrome: Pathogenesis and cardiovascular risk. Am J Ther. 2006;13:248–260.
32. Grinspoon, S, Carr, A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62.
33. Torriani, FJ, et al. Endothelial function in human immunodeficiency virus–infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576.
34. Baker, JV, et al. Untreated HIV infection and large and small artery elasticity. J Acquir Immune Defic Syndr. 2009;52:25–31.
35. de Gaetano Donati, K, et al. Increased soluble markers of endothelial dysfunction in HIV-positive patients under highly active antiretroviral therapy. AIDS. 2003;17:765–768.
36. Kuller, LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203.
37. Ross, AC, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49:499–506.
38. Dillmon, MS, Saag, MS, Hamza, SH, Adler, BK, Marques, MB. Unusual thromboses associated with protein S deficiency in patients with acquired immunodeficiency syndrome: Case reports and review of the literature. AIDS Res Hum Retroviruses. 2005;21:753–756.
39. Micieli, E, et al. Venous and arterial thrombosis in patients with HIV infection. Blood Coagul Fibrinolysis. 2007;18:259–263.
40. Sullivan, PS, Dworkin, MS, Jones, JL, Hooper, WC. Epidemiology of thrombosis in HIV-infected individuals. The Adult/Adolescent Spectrum of HIV Disease Project. AIDS. 2000;14:321–324.
41. Saber, AA, Aboolian, A, LaRaja, RD, Baron, H, Hanna, K. HIV/AIDS and the risk of deep vein thrombosis: A study of 45 patients with lower extremity involvement. Am Surg. 2001;67:645–647.
42. Lijfering, WM, Sprenger, HG, Georg, RR, van der Meulen, PA, van der Meer, J. Relationship between progression to AIDS and thrombophilic abnormalities in HIV infection. Clin Chem. 2008;54:1226–1233.
43. Calmy, A, et al. HIV increases markers of cardiovascular risk: Results from a randomized, treatment interruption trial. AIDS. 2009;23:929–939.
44. El-Sadr, WM, et al. CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296.
45. Fontas, E, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: Are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189:1056–1074.
46. Strategies for Management of Anti-Retroviral Therapy/INSIGHT; DAD Study Groups. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:F17–F24.
47. Beck, JM, Rosen, MJ, Peavy, HH. Pulmonary complications of HIV infection. Report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med. 2001;164:2120–2126.
48. Wolff, AJ, O’Donnell, AE. Pulmonary manifestations of HIV infection in the era of highly active antiretroviral therapy. Chest. 2001;120:1888–1893.
49. Thomas, CF, Jr., Limper, AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498.
50. Hidalgo, A, et al. Accuracy of high-resolution CT in distinguishing between Pneumocystis carinii pneumonia and non–Pneumocystis carinii pneumonia in AIDS patients. Eur Radiol. 2003;13:1179–1184.
51. Holmes, CB, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: Implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–469.
52. Wood, R, Maartens, G, Lombard, CJ. Risk factors for developing tuberculosis in HIV-1–infected adults from communities with a low or very high incidence of tuberculosis. J Acquir Immune Defic Syndr. 2000;23:75–80.
53. Sonnenberg, P, et al. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150–158.
54. Corbett, EL, et al. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med. 2004;170:673–679.
55. Lopez-Gatell, H, et al. Effect of tuberculosis on the survival of women infected with human immunodeficiency virus. Am J Epidemiol. 2007;165:1134–1142.
56. Yang, Z, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38:199–205.
57. Bica, I, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497.
58. Lewden, C, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalité 2000 and 2005” surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr. 2008;48:590–598.
59. Jarvis, JN, Harrison, TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–2129.
60. Graybill, JR, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30:47–54.
61. Perfect, JR, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50:291–322.
62. Skiest, DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:103–115.
63. Gupta, SK, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40:1559–1585.
64. Karras, A, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus–infected patients: Three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 2003;36:1070–1073.
65. Qiu, J, Terasaki, PI, Waki, K, Cai, J, Gjertson, DW. HIV-positive renal recipients can achieve survival rates similar to those of HIV-negative patients. Transplantation. 2006;81:1658–1661.
66. Biviji, AA, Paiement, GD, Steinbach, LS. Musculoskeletal manifestations of human immunodeficiency virus infection. J Am Acad Orthop Surg. 2002;10:312–320.
67. Brown, P, Crane, L. Avascular necrosis of bone in patients with human immunodeficiency virus infection: Report of 6 cases and review of the literature. Clin Infect Dis. 2001;32:1221–1226.
68. Reveille, JD, Williams, FM. Infection and musculoskeletal conditions: Rheumatologic complications of HIV infection. Best Pract Res Clin Rheumatol. 2006;20:1159–1179.
69. Sloand, E. Hematologic complications of HIV infection. AIDS Rev. 2005;7:187–196.
70. Jansen, LA. HIV exceptionalism, CD4+ cell testing, and conscientious subversion. J Med Ethics. 2005;31:322–326.
71. Arbelaez, C, et al. Emergency provider attitudes and barriers to universal HIV testing in the emergency department. J Emerg Med. 2012;42:7–14.
72. Burke, RC, et al. Why don’t physicians test for HIV? A review of the US literature. AIDS. 2007;21:1617–1624.
73. McKenna, M. HIV testing: Should the emergency department take part? Ann Emerg Med. 2007;49:190–192.
74. Franco-Paredes, C, Tellez, I, del Rio, C. Rapid HIV testing: A review of the literature and implications for the clinician. Curr HIV/AIDS Rep. 2006;3:169–175.
75. Branson, BM, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17.
76. Arbelaez, C, et al. Rapid HIV testing program implementation: Lessons from the emergency department. Int J Emerg Med. 2009;2:187–194.
77. Leider, J, Fettig, J, Calderon, Y. Engaging HIV-positive individuals in specialized care from an urban emergency department. AIDS Patient Care STDS. 2011;25:89–93.
78. Chen, JC, et al. A provider participatory implementation model for HIV testing in an ED. Am J Emerg Med. 2010;29:418–426.
79. Haukoos, JS, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304:284–292.
80. Brown, J, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention Guidelines: Results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007;46:395–401.
81. Haukoos, JS, et al. Development and implementation of a model to improve identification of patients infected with HIV using diagnostic rapid testing in the emergency department. Acad Emerg Med. 2007;14:1149–1157.
82. Lyons, MS, Lindsell, CJ, Ledyard, HK, Frame, PT, Trott, AT. Emergency department HIV testing and counseling: An ongoing experience in a low-prevalence area. Ann Emerg Med. 2005;46:22–28.
83. Ubhayakar, ND, et al. Risk, reasons for refusal, and impact of counseling on consent among ED patients declining HIV screening. Am J Emerg Med. 2011;29:367–372.
84. Sohoni, A, Gordon, DM, Vahidnia, F, White, DA. Emergency department staff satisfaction with rapid human immunodeficiency virus testing. Acad Emerg Med. 2010;17:561–565.
85. Millen, JC, Arbelaez, C, Walensky, RP. Implications and impact of the new US Centers for Disease Control and Prevention HIV testing guidelines. Curr Infect Dis Rep. 2008;10:157–163.
86. Rothman, RE, Merchant, RC. Update on emerging infections from the Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. Ann Emerg Med. 2007;49:575–579.
87. Egger, M, et al. Prognosis of HIV-1–infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet. 2002;360:119.
88. Thompson, MA, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society–USA panel. JAMA. 2010;304:321.
89. Sax, PE, Baden, LR. When to start antiretroviral therapy—ready when you are? N Engl J Med. 2009;360:1897–1899.
90. Gross, R, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114.
91. Wood, E, et al. Impact of baseline viral load and adherence on survival of HIV-infected adults with baseline CD4 cell counts > or = 200 cells/microl. AIDS. 2006;20:1117–1123.
92. Mills, EJ, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis. JAMA. 2006;296:679–690.
93. Henderson, DK, et al. Risk for occupational transmission of human immunodeficiency virus type 1 (HIV-1) associated with clinical exposures. A prospective evaluation. Ann Intern Med. 1990;113:740–746.
94. Panlilio, AL, Cardo, DM, Grohskopf, LA, Heneine, W, Ross, CS. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1–17.
95. Smith, DK, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States. MMWR Recomm Rep. 2005;54:1–20.
96. Hilliard, AA, Weinberger, SE, Tierney, LM, Jr., Midthun, DE, Saint, S. Clinical problem-solving. Occam’s razor versus Saint’s triad. N Engl J Med. 2004;350:599.