Chapter 1 History of Spine Surgery
Antique Period and Spine Surgery
Srimad Bhagwat Mahapuranam, an ancient Indian epic (3500–1800 bce), depicts the oldest documentation of spinal traction. In a passage from this document, it is described that Lord Krishna applied axial traction to correct a hunchback in one of his devotees.1
The Edwin Smith Papyrus (2600–2200 bce) is the most well-known document on Egyptian medicine. This document reports 48 cases. Imhotep (2686–2613 bce), a late second-dynasty surgeon, authored this papyrus, which reported six cases of spinal trauma. Hence, nearly 4600 years ago, vertebral subluxation and dislocation and traumatic quadriplegia and paraplegia were described.2 Recently, it was reported that Egyptian physicians described the “spinal djet column concept.”3
Antique medicine was also influenced by the Greco-Roman period physicians.4 Hippocrates (460–375 bce) addressed the anatomy and pathology of the spine, describing the normal curvatures of the spine, its structure, and the tendons attached to it. He defined tuberculous spondylitis, posttraumatic kyphosis, scoliosis, spinal dislocation, and spinous process fracture. He addressed the relationship between spinal tuberculosis and gibbus. According to Hippocrates, spinous process fracture was not dangerous. However, fractures of the vertebral body were more important. He described two frames for reduction of the dislocated spine, including the Hippocratic ladder and the Hippocratic board.5 The details of Hippocratic treatment were recorded by Aulus Cornelius Celsus (25 bce–50 ce).
Aristotle (384–322 bce) focused on kinesiology. His treatises—“parts of animals, movement of animals, and progression of animals—described the actions of the muscles.” He analyzed and described walking, in which rotatory motion is transformed into translational motion. Although his studies were not directly related to the spine, they were the first to address human kinesiology and, in fact, biomechanics.6
Galen of Pergamon (130–200 ce), another physician of the antique era, worked as a surgeon and anatomist. He studied the anatomy of animals and extrapolated his findings to human anatomy. His anatomic doctrines became the basis for medical education for more than 1200 years. He used the terms kyphosis, scoliosis, and lordosis, and he attempted to correct these deformities. He also worked as the official surgeon of gladiators in amphitheaters. Because of this position, he was accepted as “the father of sports medicine.” He confirmed the observations of Imhotep and Hippocrates regarding the neurologic sequences of cervical spine trauma. Nevertheless, to the best of our knowledge, he did not operate for spinal trauma.6,7
Oribasius (325–400 ce), another physician of the antique period, added a bar to the Hippocratic reduction device and used it to treat both spinal trauma and spinal deformity.8
One of the most important figures dealing with spinal disorders during the end of this period was Paulus of Aegina (625–690 ce). He collected what was known from the previous 1000 years in a seven-volume encyclopedia. Paulus of Aegina not only used the Hippocratic bed, but also worked with a red-hot iron. He is credited with performing the first known laminectomy. This was performed for a case of spinal fracture resulting in spinal cord compression. He emphasized the use of orthoses in spinal trauma cases.6,9
Medieval Period and Spine Surgery
The studies and reports of Paulus of Aegina are the most important source of information regarding this period of medicine. This age was followed by the Dark Ages (ca. 500–1000 ce) in Europe. Although Western medicine showed no progress during the Dark Ages, the Eastern world developed the science. The early Islamic civilizations realized the importance of science and scientific investigation. The most important books of the antique age were translated into Syrian, Arabic, and Persian. Therefore, using the Western doctrines, the Islamic civilizations discovered new information and were able to contribute further. In terms of spine medicine, several important contributors, including Avicenna and Abulcasis, added to this movement.
Avicenna (981–1037 ce), a famous physician from present-day Uzbekistan, worked in all areas of medicine (Fig. 1-1). His famous book, the Canon of Medicine, was a seminal textbook until the 17th century in Europe. He described the biomechanics-related anatomy of the spine, as well as flexion, extension, lateral bending, and axial rotation of the spine.10 Avicenna also used a traction system similar to the system described by Hippocrates.
Abulcasis (936–1013 ce), a famous Arabian surgeon of the 11th century, wrote a surgery treatise, “At-Tasnif.” He described several surgical disorders, including low back pain, sciatica, scoliosis, and spinal trauma. He recommended the use of chemical or thermal cauterization for several spinal disorders. He also developed a device to reduce the dislocated spine.11
Serefeddin Sabuncuoglu (1385–1468 ce), a Turkish physician of the 15th century, wrote an illustrated atlas of surgery,12 in which he described scoliosis, sciatica, low back pain, and spinal dislocations. He delineated a technique for reduction of spinal dislocations, using a frame similar to that designed by Abulcasis.
Renaissance and Spine Surgery
The works of Leonardo Da Vinci (1452–1519 ce) are of importance in this regard. Da Vinci worked on the philosophy of mechanics and on anatomy in De Figura Humana. He described spine anatomy, the number of vertebrae, and the joints in detail. By studying anatomy in the context of mechanics, da Vinci gained some insight into biomechanics. He considered the importance of the muscles for stability in the cervical spine. However, his work was unpublished for centuries, and his brilliant daydreaming had a limited scientific influence on biomechanics.13,14
Andreas Vesalius (1514–1564 ce), an anatomist and physician, wrote his famous anatomy book, De Humani Corporis Fabrica Liberi Septum, which changed several doctrines described by Galen. Actually, it took several centuries for the world to accept that Galen had made errors that were corrected by Vesalius. Because he described and defined modern anatomy, he is commonly accepted as the father of anatomy. He described the spine, intervertebral disc, and intervertebral foramina. His biomechanical point of view regarding the flexion extension of the head was similar to that of Avicenna.15
The early anatomic studies and observations were followed by biomechanical advancements. Prominent among the contributors to those advancements was Giovanni Alfonso Borelli (1608–1679 ce), who described the biomechanical aspects of living tissue. He is the founder of the “iatrophysics” concept—a term that subsequently became known as biomechanics. He is accepted as the “father of spinal biomechanics.” His book, De Motu Animalium, describes the movements of animals. He wrote that the intervertebral disc is a viscoelastic material that carries loads. This is so because he observed that muscles could not bear the loads alone. He concluded that the intervertebral discs should have function during load bearing. He was the first scientist to describe the human weight center (center of gravity).16,17
Early Modern Period and Spine Surgery
Spinal Decompression and the Early Modern Period
Spinal decompression in the early modern period was primarily via laminectomy. Throughout most of the 19th century, laminectomy was developed and its utility debated as the only surgical approach to all spinal pathologies, including tumor, trauma, and infection. At the dawn of the 20th century, the indications for laminectomy were extended to the decompression of spinal degenerative disease, an understanding of which had eluded 19th-century surgeons because they failed to appreciate the connection between its clinical and pathologic manifestations.
Thus, during the 19th century, the indications for spinal surgery were limited to the treatment of tumor, trauma, and infection.18 Although each of these conditions posed unique clinical and surgical problems, they shared the need for surgical decompression. Throughout the early modern period, surgical decompression of the spine was the single most common reason to undertake the risks of spinal surgery, and laminectomy was the most commonly used technique to achieve it.
Birth and Development of the Laminectomy
H. J. Cline, Jr., and the Argument against Spinal Surgery
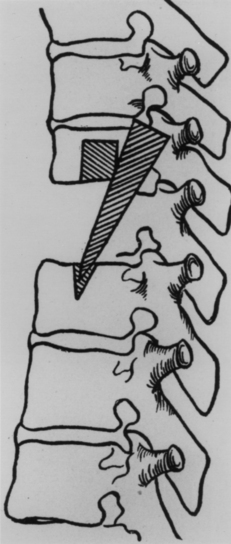
In 1814, Cline performed a multilevel laminectomy for a thoracic fracture-dislocation associated with signs of a complete paraplegia (Fig. 1-2).19 The patient was a 26-year-old man who fell from the top of a house. “He was bled previous to his admission” to the St. Thomas’s Hospital in London, “and some imprudent attempts were made to relieve him by pressing the knees against the injured part, which only increased the pain and inflammation.”19 Upon admission to the hospital the patient was examined by Cline, who “ascertained that some of the spinous processes . . . were broken off and were pressing upon the spinal marrow . . . [and] who resolved to cut down and remove the pressure from the spinal marrow.”19

FIGURE 1-2 First page of H. J. Cline Jr.’s historic laminectomy, as reported by G. Hayward.
(From Cline HJ Jr [cited by Hayward G]: An account of a case of fracture and dislocation of the spine. N Engl J Med Surg 4:13, 1815.)
The patient was observed overnight in the hospital, and on the day following admission, Cline performed his proposed operation. Although the operation was performed within 24 hours of injury, Cline was unable to reduce the dislocation or to achieve a complete decompression of the neural elements. The patient survived for 3 days after surgery, with increasing pain and a steadily increasing pulse. On postoperative day 4, however, the patient died, “and on an examination of the body by Mr. Cline, it was found that the spinal marrow was entirely divided.”19 Despite the severity of the neural injury, and the complexity of the fracture-dislocation, the untoward outcome of this unfortunate case would remain a topic of conversation for almost a century, providing ample ammunition for the opponents of spinal surgery.
Of course, the case of Cline was not an isolated mortality. In 1827, for example, Tyrell20 reported a 100% mortality for a small series of patients with surgically treated spinal dislocation and neurologic injury. Other reports (e.g., Rogers21 in 1835) were often equally discouraging. Looking back on these early years of the debate about spinal surgery, the early 20th century British surgeon Donald Armour22 described the controversy this way:
Of course, surgical fatalities in this period were due as much to septic complications and anesthetic inadequacies as they were to surgical technique. The lack of an effective means of pain control during surgery intensified the problem of intraoperative shock and made speed essential. Furthermore, the problems of wound infection and septicemia were both predictable and frequently fatal. These hindrances to surgery were not ameliorated until the introduction of general anesthetic agents (i.e., nitrous oxide, ether, and chloroform) in the mid-1840s and the adoption of Listerian techniques (using carbolic acid) in the 1870s.23
A. G. Smith and the First Successful Laminectomy
Despite these risks, a little-known surgeon named Alban G. Smith from Danville, Kentucky, performed a laminectomy in 1828 on a patient who had fallen from a horse and sustained a traumatic paraplegia. To Smith’s credit, his patient not only survived the operation but achieved a partial neurologic recovery. The operative technique and surgical results were reported in the North American Journal of Medicine and Surgery in 1829 (Fig. 1-3).24 Smith’s procedure comprised a multilevel laminectomy through a midline incision, involving removal of the depressed laminae and spinous processes, exploration of the dura mater, and closure of the soft tissue incision. Although the report of this landmark case appears to have attracted little attention at the time, it is a significant technical achievement and places Smith among the pioneers of the early modern period in spinal surgery.
Laminectomy for Extramedullary Spinal Tumor
During the half century after Smith’s historic operation, the primary indication for laminectomy was spinal trauma. In the latter part of the 19th century, the indications for laminectomy were extended to tumor and infection.25 The first and most celebrated surgical case for spinal tumor in the 19th century, that of Captain Gilbey, was also the first successful one, and it played an important role in the rehabilitation of the laminectomy as a safe and effective procedure.
Captain Gilbey was an English army officer who suffered the misfortune of losing his wife in a carriage accident in which he also was involved. Although Gilbey himself escaped serious injury, he soon began to experience progressive dull back pain, which he attributed to the accident. As the pain became relentless, Gilbey sought the advice of a series of physicians, all of whom were unable to identify the source of his pain. Eventually, Gilbey was referred to the eminent London neurologist, William Gowers, who elicited from the patient a history of back pain, urinary retention, paraplegia, and loss of sensation below the thoracic level (Fig. 1-4).26
The neurologist’s diagnosis was immediate and unequivocal: the cause of Gilbey’s symptoms was located in his spine, where a tumor was causing compression of the thoracic spinal cord. Although no intraspinal tumor had ever been resected successfully, Gowers referred the patient to his London surgical colleague, Victor Horsley (Fig. 1-5). After all, Gowers had himself asserted, in his authoritative textbook, Manual of Diseases of the Nervous System, that removal of an intradural spinal cord tumor was “not only practicable, but actually a less formidable operation than the removal of intracranial tumors.”27
Horsley acted quickly. Within 2 hours of the initial consultation, a skin incision was made at 1 pm, June 9, 1887, at the National Hospital, Queens Square, London. Despite his precipitous decision to undertake this dangerous operation, Horsley did not approach the operation unprepared. Although the Act of 1876 made it a criminal offense to experiment on a vertebrate animal for the purpose of attaining manual skill, Horsley had repeatedly practiced the proposed procedure in the course of his surgical experimentation. Despite some initial difficulty in locating the tumor, an intradural neoplasm in the upper thoracic spine causing compression of the spinal cord was identified and safely resected. The pathologic diagnosis was “fibromyxoma of the theca.”
Laminectomy for Intramedullary Spinal Tumor
In 1890, Fenger attempted to remove an intramedullary spinal tumor in an operation that resulted in the patient’s death.28 In 1905, Cushing29,30 also attempted to remove an intramedullary spinal cord tumor but decided to abort the procedure after performing a myelotomy in the dorsal column. To Cushing’s surprise, the patient improved after surgery. In 1907, von Eiselsberg31 successfully resected an intramedullary tumor.
The unexpected improvement that was observed in the patient reported by Cushing attracted the attention of the New York surgeon Charles Elsberg. Elsberg32 described Cushing’s technique, which he aptly named the “method of extrusion.” The technique was intended to remove an intramedullary tumor by spontaneous extrusion of the tumor through a myelotomy made in the dorsal column. The rationale for this method was predicated on the theory that an intramedullary tumor was associated with an increase in intramedullary pressure. Release of this pressure by a myelotomy that extended from the surface of the spinal cord to the substance of the tumor was expected to provide sufficient force to spontaneously extrude the tumor. According to Elsberg, the advantage of this procedure over a standard tumor resection was that it required minimal manipulation of the spinal cord and therefore minimal spinal cord tissue injury.
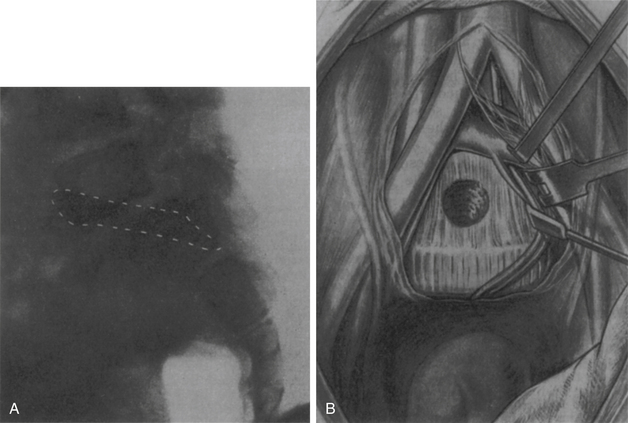
Because the spontaneous extrusion of an intramedullary tumor occurred slowly, Elsberg performed these procedures in two stages. In the first stage, a myelotomy was fashioned in the dorsal column, extending from the surface of the spinal cord to the tumor (Fig. 1-6A).
When the tumor was identified and observed to begin to bulge through the myelotomy incision, the operation was concluded, the dura mater was left opened, and the wound closed. In the second stage of the procedure, which was performed approximately 1 week after the first stage, Elsberg reopened the wound and inspected the tumor (Fig. 1-6B). Typically, the tumor was found outside the spinal cord, and the few adhesions that remained between the spinal cord and the tumor were sharply divided. After the tumor was removed, the wound, including the dura mater, was closed.
Variations in Laminectomy Technique
By the last decade of the 19th century, after the case of Captain Gilbey, the possibility of safely performing a spinal operation was established in the collective surgical consciousness. Furthermore, new anesthetic techniques and aseptic methods had become available to most practicing surgeons.33 All of these factors served to increase the appeal of the laminectomy to surgeons and to widen its range of application. For example, after Horsley’s widely publicized success for resecting a spinal tumor, many similar operations were soon described in the literature,34–39 and in 1896, Makins and Abbott40 reported 24 cases of laminectomy for vertebral osteomyelitis.
In 1889, Dawbarn41 described an osteoplastic method of laminectomy that addressed this concern. Instead of a midline incision, Dawbarn described two lateral incisions that were carried down to the transverse processes. The lateral incisions were connected in an H-like fashion, and a superior and inferior flap—including skin, muscle, fascia, and bone—was then turned. In closing the wound, the intact flaps were reflected back and reapproximated in their normal anatomic positions.
Although not all surgeons subscribed to the osteoplastic method, many turn-of-the-century surgeons were largely preoccupied with modifications of this procedure.42 At the same time, however, a more important innovation in laminectomy technique, the hemilaminectomy, was developed independently in both Italy43,44 and the United States.44
Charles A. Elsberg: The Laminectomy in Stride
Charles A. Elsberg was one of the most influential writers on spinal decompression (Fig. 1-7). Working at the Neurological Institute of New York, which he had helped to found, Elsberg45 published his first series of laminectomies in 1913. In 1916, he published his classic text, Diagnosis and Treatment of Surgical Diseases of the Spinal Cord and Its Membranes.46 Although these publications represent landmarks in the history of spinal surgery, they constitute more of a culmination than an innovation in spinal surgery. Elsberg’s work on spinal surgery, coming as it did at the end of a century of evolution of the decompressive laminectomy, effectively codified 19th and early 20th century developments.
Unlike degenerative disease, tumor, trauma, and infection were already well-known in antiquity. Although the concept of localization of function in the nervous system was undeveloped during the 19th century, the diagnosis and localization of tumor, trauma, and infection, particularly in their late stages, was not especially difficult. Degenerative disease, on the other hand, possessed a more subtle pathophysiology that was not as easily characterized, especially without the help of radiography. Thus, recognition of degenerative spine disease eluded the 19th-century surgeon. This tardy appreciation for the clinical, surgical, and pathologic importance of degenerative spine disease deserves further mention.
Laminectomy for Intervertebral Disc Herniation
Intervertebral disc pathology was first described by Rudolph Virchow47 in 1857 (Fig. 1-8). Virchow’s description of a fractured disc was made at autopsy on a patient who had suffered a traumatic injury.
In 1896, the Swiss surgeon T. Kocher48,49 identified and described a traumatic disc rupture at autopsy of a patient who had fallen 100 feet and landed on her feet. Although Kocher recognized that the L1-2 disc was displaced dorsally, no clinical correlation was suggested.
The first transdural intervertebral discectomy was reported by Oppenheim and Krause50 in 1908. However, they reported the disc as “enchondroma.”
In 1911, George Middleton,51 a practicing physician, and John Teacher, a Glasgow University pathologist, described two cases of ruptured intervertebral disc observed at autopsy. Like Virchow and Kocher before them, however, Middleton and Teacher, although they described the pathology, failed to postulate its connection with radiculopathy or back pain.
In 1911, Joel Goldthwaite52 made this connection. In an article on the lumbosacral articulation, Goldthwaite described and illustrated how weakening of the annulus fibrosus could result in dorsal displacement of the nucleus pulposus. The nucleus pulposus, he argued, could in turn result in low back pain and paraparesis. What eluded Goldthwaite and the surgeons before him, however, was the connection between a herniated disc and radiculopathy.
In a 1929 issue of the Archives of Surgery, Walter E. Dandy53 published a description of two cases of herniated lumbar discs causing a cauda equina syndrome (Fig. 1-9). Dandy correctly described how “loose cartilage from the intervertebral disc” produced the symptoms of cauda equina compression that were relieved alter surgical decompression. He considered that in the second decade of the 20th century, more than 20 years after the first spinal fusion operations, intervertebral disc disease could be added to the list of indications for decompressive laminectomy.
In June of 1932, Barr attempted to treat a patient with an extruded disc herniation. Following a 2-week unsuccessful course of nonoperational treatment, Barr consulted with Mixter. Mixter recommended a myelogram. The myelogram revealed a filling defect. Mixter subsequently operated on the patient and removed the “tumor.” Barr studied the “tumor” specimens. Because he contributed to Schmorl’s study published in German, Barr remembered the microscopic appearances in Schmorl’s study and realized that the specimen from this index patient was the nucleus pulposus. After this finding, Mixter, Barr, and Mallory (pathologist) reevaluated all the cases that were diagnosed (or misdiagnosed) as chondroma in recent years at Massachusetts General Hospital. They retrospectively diagnosed most of these cases as ruptured intervertebral discs. Mixter and Wilson operated on the first ruptured disc herniation diagnosed preoperatively on December 31, 1932. Mixter and Barr reported the case in New England Surgical Society in September 30, 1933.8,54,55
In the late 1930s Love56 from the Mayo Clinic reported on an extradural laminectomy technique. In 1967, Yasargil57 used the microscope for discectomy. The first results of the lumbar microdiscectomy were reported by Yasargil57 and Caspar.58
Laminectomy for Cervical Disc Herniation
In 1905 Watson and Paul59 performed a negative exploration for cervical spinal cord tumor. They found an anterior extradural mass in the intervertebral disc at autopsy. This may be the first reported case of cervical disc herniation. The first dorsal approach was performed by Elsberg60 in 1925. He found a “chondroma” in a quadriparetic patient.
Laminectomy for Spinal Stenosis
Unlike the herniated intervertebral disc, the stenotic spinal canal was described comparatively early in the 19th century. Portal,61 in 1803, observed that a small spinal canal may be causally related to spinal cord compression, leading to paraplegia. No clinical reports of this entity were published, however, until 1893 when William A. Lane62 described the case of a woman aged 35 years with a progressive paraplegia and a degenerative spondylolisthesis. The patient improved after a decompressive laminectomy.
Further demonstration of the efficacy of decompressive laminectomy for spinal stenosis came from Sachs and Frankel63 in 1900. They published an account of a man aged 48 years with neurogenic claudication and spinal stenosis whose symptoms improved after a two-level laminectomy. Recognition of the degenerative nature of the clinical entity of spinal stenosis was established by Bailey and Casamajor64,65 in 1911 in a report on a patient who was successfully decompressed by Charles Elsberg. In his 1916 textbook, Elsberg46 later wrote, “a spinal operation may finally be required in some cases of arthritis or spondylitis on account of compression of the nerve roots or the cord by new-formed bone. . . .”
In 1945, Dr. Sarpyener, a Turkish orthopedic surgeon, described congenital lumbar spinal stenosis.66 This report was followed by a report on adult spinal stenosis from Dr. Verbiest.67 In 1973 Hattori68 described the technique of laminoplasty.
Approaches to the Spine
Dorsolateral Approaches to the Spine
In 1779, Percival Pott described a condition involving spinal kyphosis and progressive paraplegia in a now-classic monograph titled “Remarks on that kind of palsy of the lower limbs which is frequently found to accompany a curvature of the spine and is supposed to be caused by it; together with its method of cure; etc.’’ (Fig. 1-10). For the management of this condition, which now bears his name, Pott recommended the use of a paraspinal incision to drain pus from the invariably present paraspinal abscess. For almost a century, this simple surgical procedure became a standard part of the treatment of Pott’s paraplegia.
By the late 19th century, however, the laminectomy had received widespread acceptance as a safe and effective method of spinal decompression.69 This was in part related to the decrease in surgical mortality associated with the adoption of the Listerian methods beginning in the 1870s, and it was only natural then that the laminectomy would play a role in the management of Pott’s disease. As in many of its applications, however, disenchantment arose with the results of laminectomy, and alternative approaches were therefore sought.70 The most promising of these approaches was the so-called “costotransversectomy” of Ménard.
Ménard’s Costotransversectomy
Like many surgeons at the beginning of the 20th century, Ménard71 was disappointed by the surgical results from the laminectomy. In 1894, he described the costotransversectomy as an alternative method for achieving the goal of Pott, namely, drainage of the paraspinal abscess. The advantage of the costotransversectomy over the laminectomy lay in the improved exposure it provided of the lateral aspect of the vertebral column. The procedure was also known as the “drainage latéral,” emphasizing that the goal of the procedure was to drain the lateral, paravertebral tubercular abscess.
As described by Ménard, the costotransversectomy involved an incision overlying the rib that was located at the apex of the kyphos. The rib was then skeletonized and divided about 4 cm distal to the articulation with its corresponding vertebra, from which it was disarticulated and removed. These maneuvers provided access to the tuberculous focus, which was exposed and then decompressed directly (Fig. 1-11). Ménard did not intend to totally remove the lesion, but rather to simply decompress the abscess.
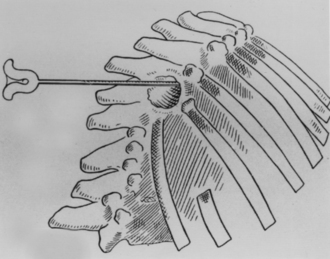
FIGURE 1-11 Drainage of a tubercular abscess via the costotransversectomy of Ménard.
(From Ménard V: Causes de la paraplegia dans le mal de Pott. Son traitement chirurgical par I’ouverture direct du foyer tuberculeux des vertebras. Rev Orthop 5:47–64, 1894.)
The surgical results of Ménard’s costotransversectomy far surpassed the results obtained with the laminectomy. Ménard experienced several successes among his first few 23 cases, including significant motor improvement.72 Regrettably, these promising initial surgical results began to sour with time, as it became increasingly clear that two major complications were occurring with increasing frequency: postoperative development of secondary infection and the postoperative formation of draining sinus tracts. Both problems resulted from the opening up of the abscess. Because no antitubercular chemotherapeutic agents were available at the time, the consequences of the infections that ensued after surgery were frequently disastrous, resulting in significant surgical mortality. As Calot73 grimly put it in 1930, “The surgeon who, so far as tuberculosis is concerned, swears to remove the evil from the very root, will only find one result waiting him: the death of his patient.” The operation of Ménard thus fell into disrepute, and in time even Ménard abandoned it.
Capener’s Lateral Rhachotomy
Like Ménard, the English surgeon Norman Capener attempted to find a surgical solution to the problem of Pott’s paraplegia. Capener modified Ménard’s costotransversectomy in a procedure that he developed and began using in 1933, which was first reported by H. J. Seddon74 in 1935. Departing from the emphasis of Pott and Ménard, who simply decompressed the tubercular abscess, Capener attempted to directly remove the lesion, which typically consisted of a ventral mass of hardened material. To achieve his more radical goal of spinal decompression, Capener required a more lateral or ventral view of the vertebrae than was afforded by Ménard’s approach.
Capener’s solution was to adopt Ménard’s costotransversectomy but with this difference: whereas Ménard approached the spine via a trajectory that was medial to the erector spinae muscles, Capener75 transversely divided the muscles and retracted them rostrally and caudally (Fig. 1-12). He named his new approach the lateral rhachotomy to distinguish it from Ménard’s costotransversectomy. The simple change in dissection planes distinguishes these two techniques by producing a significantly different trajectory and surgical exposure. Although the operation was designed for the surgical treatment of Pott’s paraplegia, Capener later drew attention to the versatility of the approach and its appropriateness for a variety of pathologic processes, including “the exploration of spinal tumors, the relief of certain types of traumatic paraplegia, and the drainage of suppurative osteitis of the vertebral bodies.”75
It was perhaps unfortunate that for 19 years the only description of Capener’s lateral rhachotomy was in a single case report published by another surgeon.74 Not until 1954 did Capener himself describe the procedure, and even then he still chose not to publish the results of his 23 cases.75
Larson’s Lateral Extracavitary Approach
In 1976, Sanford J. Larson et al.76 at the Medical College of Wisconsin published an influential article that helped to popularize Capener’s lateral rhachotomy, which they modified and renamed the lateral extracavitary approach (Fig. 1-13). This approach has been used more for trauma and tumor than for tuberculosis. The technical difference that distinguishes the lateral rhachotomy from the lateral extracavitary approach lies primarily in the treatment of the paraspinous muscles.
Whereas the procedure of Capener involves transversely dividing these muscles and reflecting them rostrally and caudally, Larson’s procedure uses a surgical exposure with a trajectory ventral to the paraspinous muscles, which are then reflected medially to expose the ventrolateral aspect of the spine. Later in the procedure these muscles are redirected laterally to provide access for instrumentation of the dorsal aspect of the spine using the same surgical exposure as that for the ventrolateral approach. Although neurosurgeons, as spine surgeons, had traditionally emphasized spinal decompression over spinal stabilization, an essential aspect of the significance of Larson’s overall contribution to the discipline of spinal surgery lies in the fact that, as a neurosurgeon, he dedicated his career to the advancement of reconstructive spinal surgery.
Spinal Stabilization and Deformity Correction
Recognition of the idea that compression of the neural elements, in cases of tumor, trauma, and infection, could be responsible for neurologic compromise was the crucial first step needed to develop the idea that spinal decompression could improve neurologic outcome. The invention of a technical means to achieve decompression, namely by laminectomy, represented the next necessary step in bringing this concept into clinical practice. Similarly, the idea of spinal stabilization arose from the observation that the unstable spine was at risk for the development of progressive deformity and that surgical intervention might prevent such deformities. Of course, bringing this concept into practice depended on achieving an adequate technical means. And, indeed, two technical advances were developed around the beginning of the 20th century that provided a means for spinal stabilization that would revolutionize the practice of modern spinal surgery.77
Birth and Development of Spinal Fusion and Spinal Instrumentation
Both spinal fusion and spinal instrumentation were born around the turn of the 20th century as methods of stabilizing the unstable spine. For many years, these two technical advances were developed and applied essentially independently, with results that were often complicated by pseudarthrosis. Early attempts at spinal instrumentation in particular failed to gain popularity because of their inability to maintain more than immediate spinal alignment. Spinal fusions were often used to achieve stabilization, but these also frequently suffered a similar fate: pseudarthrosis.78
Spinal Fusion
The idea of using spinal fusion for stabilization is attributed to Albee79 and Hibbs,80 who, in 1911, independently reported its use (Fig. 1-14). Although these early operations were performed to prevent progressive spinal deformation in patients with Pott’s disease, the procedure was later adopted in the management of scoliosis and traumatic fracture. The method of Hibbs, which was most frequently used, comprised harvesting an autologous bone graft from the laminae and overlaying the bone dorsally. Despite later improvements in this technique, however, such as the use of autologous iliac crest graft, the rate of pseudarthrosis, particularly in scoliosis, remained unacceptably high.81
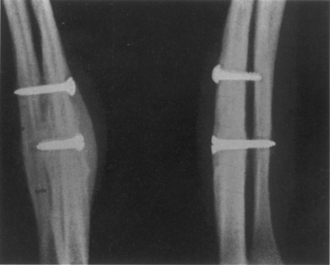
In the 1920s Campbell82 described trisacral fusion and iliac crest grafting. In 1922, Kleinberg83 used xenograft for spinal fusion. Anterior lumbar interbody fusion (ALIF) was described by Burns84 in 1933, and posterior lumbar interbody fusion (PLIF) was performed by Cloward85 in 1940. In the late 1990s transforaminal lumbar interbody fusion (TLIF) was described. In 1959 Boucher described a different spine fusion method.86 In 1977 Callahan et al.87 used bone for lateral cervical facet fusion.
Several ventral cervical fusion techniques were described in the 1950s. Robinson and Smith88 described their technique in 1955, and Cloward89 described his cervical fusion technique in 1958.
Spinal Instrumentation and Clinical Biomechanics
With the introduction of vitallium by Venable and Stuck90 in the 1930s, a metal was found that was previously used successfully as a dental filling material and that had proven resistant to electrolysis (Fig. 1-15).91 Further attempts at internal fixation during the 1930s and 1940s included fixed-moment arm cantilever constructs. These also failed to maintain alignment.42,92,93
F. W. Holdsworth
In the 1950s, the British orthopedic surgeon Sir Frank W. Holdsworth94 performed perhaps the first large systematic study of the problem of internal fixation for the treatment of posttraumatic fracture. Although the constructs he used, which employed cantilever beams attached to the spinous processes, were traditional, Holdsworth’s emphasis on patient selection brought the process of surgical spinal stabilization to a new, more sophisticated, level. His rationale for patient selection was based on a biomechanical definition of instability that he had derived from a study of a large number of spinal-injured patients.
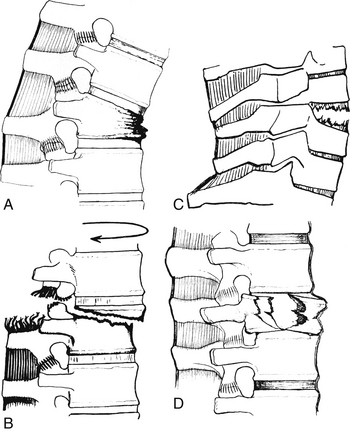
In 1963, Holdsworth95 published his results and proposed a classification scheme of subaxial spinal fractures based on a two-column model of spinal stability. Four categories of fractures were identified on the basis of the mechanism of injury and on the presence or absence of spinal stability. The latter determination rested significantly on the integrity of the dorsal ligaments. Holdsworth categorized the fractures as follows:
1. Pure flexion: A pure flexion mechanism is usually associated with an intact dorsal ligamentous complex and no evidence of spinal instability. The vertebral body absorbs the greater part of the impact, and the result is a wedge compression fracture (Fig. 1-16A).
2. Flexion-rotation: A rotation or flexion-rotation mechanism causes disruption of the dorsal ligamentous complex and results in an unstable fracture-dislocation. It is usually associated with paraplegia (Fig. 1-16B).
3. Extension: An extension mechanism, which is usually stable, most frequently occurs in the cervical spine. It may be associated with a fracture of the dorsal elements, with an intact dorsal ligamentous complex (Fig. 1-16C).
4. Compression: A compression, or “burst,” fracture is caused by forces transmitted directly along the line of the vertebral bodies. All of the ligaments are usually intact, and the fracture tends to be stable (Fig. 1-16D).
Holdsworth’s classification was important, as he himself observed, not as a biomechanical theory (although it was this too), but because it had implications for treatment. At around the same time that Holdsworth’s article appeared, several other classifications of spinal fractures were proposed. With the introduction of modern spinal biomechanics, a new era in spinal surgery had begun.96,97
Paul Harrington and the Birth of Modern Surgical Stabilization
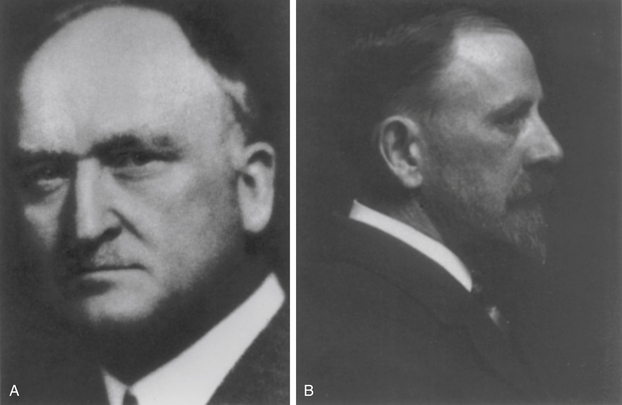
In his 1891 report of a case of interspinous wiring for cervical fracture, Berthold Hadra98 considered in what circumstances his newly described procedure would be indicated (Fig. 1-17). Hadra concluded that his procedure might be indicated for “any deviation of a vertebra.”98 Despite the prescience of his innovation, the substance of Hadra’s comment is remarkable, not so much for what it contains, as for what is missing from it; namely, any hint of consideration of biomechanical principles. When one considers the importance of biomechanical principles in Holdsworth’s 1963 classification of spinal fractures, Hadra’s early 20th-century approach to spinal stabilization serves to underline how much progress was made in the interval. The significance of this new (biomechanical) approach to spinal stabilization, which was heralded by Holdsworth, was brought home in the 1960s with the work of the father of modern spinal stabilization, Paul Harrington (Fig. 1-18).
In 1945, after military service in World War II, Paul Harrington99 entered into orthopedic practice in Houston, Texas. Within 2 years, Harrington was faced with the orthopedic problems of a large population of poliomyelitis patients, which at that time had reached epidemic proportions. The involvement of the trunk, which afflicted many of these patients, often resulted in scoliotic spinal deformity in association with cardiopulmonary compromise. The presence of cardiopulmonary compromise in a patient with scoliosis often meant that the standard cast corrective measures could not be applied safely. Furthermore, in 1941, the American Orthopaedic Association100 published a report on the results of treatment in 425 cases of idiopathic scoliosis. The report was quite discouraging. Among those patients treated by exercises and braces, but without spinal fusion, 60% progressed in their deformity and 40% remained unchanged. In another group of patients who underwent surgical correction and fusion, 25% (54 of 214) developed pseudarthrosis and 29% had lost all correction. Among the entire group, the end result for 69% was considered fair or poor, and only 31% were rated good to excellent. It was against this backdrop of dismal results from nonoperative treatment and dorsal spinal fusion that Harrington began his seminal work.
After an initial (unsuccessful) trial of internal fixation with facet screw instrumentation,101 the method was abandoned in favor of a combination of compression and distraction hooks and rods made of stainless steel. The advantages of these instruments in the establishment of deformity correction became obvious: for the first time in the history of spinal stabilization, spinal instruments provided compression, distraction, and three-point bending forces, which proved equally useful in deformity correction as they did in the maintenance of posttraumatic stability. Nineteen patients were observed during the early phase of Harrington’s investigation of dorsal instrumentation. The results of this investigation were published in 1962.99 The longevity of Harrington’s spinal instrumentation system, which remains in use today, is a testimony to both its safety and its efficacy.
Nevertheless, despite a frequent and gratifying correction of the poliomyelitis curvature, the loss of that correction was commonly discovered within 6 to 12 months after surgery. In part, the failure to maintain the alignment achieved at surgery was the result of frequent instrument failure, most commonly instrument fracture and disengagement of the hooks. However, more fundamentally, Harrington recognized that the concept of a dynamic correction system was inherently flawed: the complication of instrument failure would be far less significant if a spinal fusion could maintain the deformity correction achieved by the placement of the implant.102
Ventral Approaches to the Spine
Nothing, however, has been said so far about the achievement of these goals in the ventral spine, where a significant portion of spinal pathology is located. As it happens, the first successful interventions for stabilization of the ventral spine were achieved in the same time frame as the dorsal ones (i.e., in the first half of the 20th century). What is peculiar about surgery of the ventral spine is that a decompressive procedure must be accompanied almost invariably by simultaneous stabilization, which often includes measures taken to obtain deformity correction. Therefore, the history of the major goals of ventral spine surgery—that is, decompression, stabilization, and deformity correction—has been one of parallel developments, not serial ones, as was the case for the dorsal spine. In other words, the history of stabilization and deformity correction of the dorsal spine developed in the half century following the establishment of dorsal decompression. All three goals, in the ventral spine, were achieved during the same 50 years.
Ventral Decompression and Stabilization
The primary difficulty in applying ventral techniques to the spine was in the surgical approach. The relative technical ease and low morbidity associated with a dorsal approach to the dorsal spine provided ample opportunity for the early development of dorsal spinal techniques. By contrast, ventral approaches to the ventral spine required transgression of the abdomen or chest, which (similar to the head) up until the 1880s remained sanctuaries not to be opened, except by accident.18
In part, the late development of abdominal and thoracic surgery was a product of the problem of infection: cognizant of the morbidity and mortality related to hospital-acquired gangrene, few of those who entered a surgical ward in the 19th century did so with the hope of leaving alive. The reluctance to adopt the principles of antisepsis as first enunciated by Lister103 in 1867 and a slowness to accept its theoretical foundation—the germ theory of disease—meant delays for the development of abdominal and thoracic surgery. However, even after the practice of antiseptic surgery became generally accepted, early 20th-century surgeons still approached abdominal surgery with trepidation.
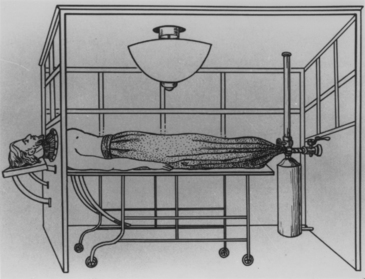
Anyone who would contemplate surgically violating the thoracic cavity had to grapple with the technical problem of the pressure relationships in the chest.104 Beginning in 1903, Ferdinand Sauerbruch of Breslau conducted a series of experiments that led to the development of an apparatus in which negative pressure for the open thorax could be maintained, and around 1910, endotracheal or insufflation anesthesia became available (Fig. 1-19). This alleviated one of the major technical difficulties confronted by would-be thoracic surgeons, but even then, good control of respiration by a reliable apparatus was not widely available until the late 1930s.
W. Müller
The first report of a successful attempt to approach the ventral thoracic or lumbar spine is attributed to Müller.105 In 1906, Müller performed a transperitoneal approach to the lumbosacral spine in a patient with a suspected sarcoma. At operation, Müller found tuberculosis. Alter curetting the infected bone, Müller applied iodoform powder and closed. The surgical result was excellent. Notwithstanding the success of this initial operation, however, later attempts at the same procedure failed miserably. After several misadventures that ended in disaster, Müller was forced to abandon further attempts at a ventral exposure.
B. H. Burns
Perhaps the next published report of a successful ventral exposure did not appear until 1933, when the British surgeon B. H. Burns84 performed a ventral interbody fusion of the lumbosacral spine for an L5-S1 spondylolisthesis (Fig. 1-20). Before the Burns procedure, the only method available to stabilize an unstable spondylolisthesis was a dorsal fusion. However, the results of dorsal fusion for ventral instability, as Burns himself learned firsthand, proved unsound both in theory and in practice. Faced with a high incidence of failed dorsal fusions, Burns chose to take a transabdominal, transperitoneal approach to the lumbosacral spine, which he first investigated on three cadavers before operation. The first operation involved a 14-year-old boy who presented with low back pain and neurogenic claudication after jumping from a height. A radiograph of the lumbosacral spine showed an L5 spondylolysis and a grade II, L5-S1 spondylolisthesis. A tibial autograft was taken and was tamped into a hole drilled obliquely from L5 to S1. Convalescence was uneventful, and pain relief was achieved, even on ambulation at 2 months postoperatively.
Ito and Others
Like the landmark operations of Albee and Hibbs, the first reported series of ventral spinal operations comprised a group of surgical treatments for spinal tuberculosis. In their 1934 article, “A New Radical Operation for Pott’s Disease,” Ito et al.106 observed that the surgical stabilization procedure described by Albee and Hibbs did not differ significantly from nonoperative immobilization; the goal in both instances was to rest and unload the diseased spine. Ito, on the other hand, a professor of orthopedic surgery from Kyoto, Japan, proposed a decompressive procedure, which he believed provided a definitive surgical treatment.
Of course, the obstacles that Ito confronted in devising a ventral approach to the spine were considerable. In addition to the obvious anatomic obstacles, all early 20th-century spine surgeons faced the seemingly intractable problem of infection. Although postoperative infections posed major difficulties for the development of (clean) abdominal and thoracic surgical procedures, these difficulties were compounded when the surgical indication was infection, as in the case of Pott’s disease. Indeed, previous attempts to surgically decompress tuberculosis of the ventral spine via a lateral approach (i.e., a costotransversectomy) met with a high incidence of complications from postoperative secondary infection, permanent fistulas, or persistent spinal tuberculosis resulting from incomplete removal of infected bone.49,71,107,108
In part, these operations failed because they were performed prior to 1910, in the age of antiseptic, rather than aseptic, surgery. Perhaps they also failed in part because they predated the introduction of antimicrobial chemotherapy. However, the unsatisfactory results that these operations yielded was also importantly attributed to the poor surgical exposure of the vertebral bodies that the lateral approach provided. Recognizing this, Ito proposed a decompression operation that would adequately resect infected vertebrae in order to fully eradicate the presence of tuberculosis in the spine. Drawing on experience with the transabdominal approach, which he had previously used for another purpose, Ito reported his operative technique and surgical results on 10 patients with moderately advanced Pott’s disease. The possibility of approaching the ventral spine occurred to Ito et al. after repeated operations using their original technique for lumbosacral sympathetic ganglionectomy. In 1923, Ito and Asaini109 originated this technique for the purpose of improving lower extremity circulation and reported their results to the Japanese Surgical Society in 1925. The technique was subsequently modified to provide an extraperitoneal approach to the lumbar spine and was adopted for their radical operation for Pott’s disease (Fig. 1-21).
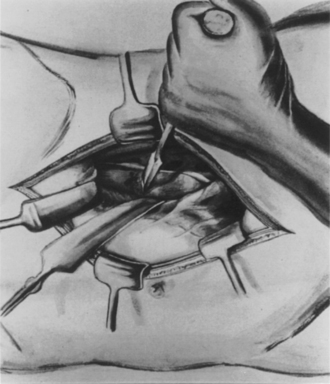
FIGURE 1-21 Extraperitoneal exposure of the body of the lumbar vertebra and resection of the body with a chisel.
(From Ito H, Tsuchiya J, Asaini G: A new radical operation for Pott’s disease. J Bone Joint Surg 16:499–515, 1934.)
Ito et al.’s work was beneficial for several reasons. First, they recognized the need to address the pathology directly, despite the technical difficulties that such an approach presented. Second, at a time when the major surgical treatment for Pott’s disease was dorsal fusion, Ito proposed a radical new surgical therapy: decompression. An attempt to eradicate spinal infection by surgical decompression represented an alternative approach to the standard stabilization procedure originated by Albee and Hibbs. In another sense, the idea of decompression harkened back to the 19th-century laminectomy for Pott’s disease, which was largely abandoned because of disappointing results, after the introduction of dorsal spinal fusion.110 Finally, Ito recognized the need, and established the technique, for stabilizing the spine, which, if not already unstable, was certainly rendered unstable by resection of the major load-bearing element. He accomplished this goal by fashioning a ventral interbody fusion, which both provided significant stability and facilitated spinal fusion (Fig. 1-22). However, despite Ito’s successes—all except 2 of his 10 cases showed a healing by primary intention and despite his acknowledgment of the inadequacies of the dorsolateral approach—Ito himself used the costotransversectomy approach in the 2 cases of thoracic Pott’s disease included in his series.
Hodgson and Stock
Thus, it fell to another group of surgeons treating Pott’s disease to develop a true ventral approach to the thoracic spine. In 1956, Hodgson and Stock111 published their first report on ventral spinal fusion for Pott’s disease. These authors acknowledged the contributions of Ito et al., and they repeated Ito’s assessment of the restricted field of view afforded by the costotransversectomy. They noted that this field of view provided insufficient exposure to determine the extent of the lesion or to confidently undertake its complete resection. What is more, the limited exposure of the costotransversectomy left no room to accurately insert a ventral bone graft, which they considered offered the best chance for fusion because the bone graft would be placed in a compression mode.
Ventral Deformity Reduction and the Development of Ventral Instrumentation
Harrington’s method of scoliosis reduction was based on the principle of lengthening the short (concave) side of the curve. After the introduction of a meticulous fusion technique to supplement the immediate rigid internal fixation achieved by the implant, the Harrington instrumentation system proved both a safe and effective corrective measure, an assessment that is corroborated by its long and successful history of clinical application. Nevertheless, the principle of simple dorsal distraction had its drawbacks. First, the Harrington method requires that the fusion be extended at least two levels above and below the extent of the spinal curvature, thus decreasing mobility in otherwise normal spinal motion segments. Second, in most instances, the distribution of force application with the Harrington instrumentation system is uneven, such that the total force applied is borne only by the two vertebrae attached to the upper and lower hooks. Finally, for patients who require a simultaneous ventral decompression and dorsal stabilization procedure, this could be accomplished only through a two-stage operation involving two separate incisions and surgical exposures. Thus, the arrival of a ventral instrumentation system, introduced by Dwyer et al.112 in 1969, proved an important addition to the spinal surgeon’s surgical armamentarium.
A. F. Dwyer
A. F. Dwyer was an orthopedic surgeon from Australia who appears to have originated his method in an effort to provide an alternative to the Harrington technique for treating scoliotic deformity reduction. In his initial report of 1969, Dwyer described a method of ventral instrumentation in which compressive forces are applied to the convex side of the curve at each segmental level. The technique comprises excision of the discs at the motion segments involved and the insertion of vertebral body screws into the convex aspect of the curve. A titanium cable is then threaded through the heads of the inserted screws and tension is applied, providing corrective bending moments at the intervertebral spaces. The tension is maintained by swaging the threaded cable on the screw heads (Fig. 1-23).
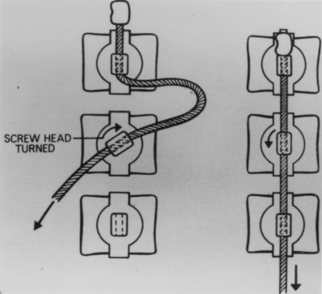
FIGURE 1-23 Dwyer’s ventral short segment fixation device.
(From Dwyer AF, Schafer MF: Anterior approach to scoliosis. Results of treatment in fifty-one cases. J Bone Joint Surg [Br] 56:218–224, 1974.).
In a follow-up article published in 1974, Dwyer and Schafer113 reported their results of treatment in 51 cases, which demonstrated a generally favorable record of deformity correction and only a 4% rate of pseudarthrosis.79 Furthermore, some of the disadvantages of the Harrington dorsal instrumentation system were overcome—fusion could be restricted to the motion segments of the curve only; the load borne by the instrumentation device was evenly distributed over the curve; and the exposure necessary for ventral decompression, stabilization, and deformity correction was achieved using a single incision. Although the initial enthusiasm for the Dwyer device was later diminished by the recognition that it encouraged the tendency of the spine toward progressive kyphosis and that it provided no resistance to axial loading, the generally successful application of this ventral instrumentation system stimulated the development of additional ventral implants, such as the instrumentation systems of Zielke and Pellin114 and Kaneda and associates.115
Spine Imaging
X-rays were discovered by Conrad Roentgen (1845–1923).116 Roentgen, working in Würzburg University, invented the x-ray tube on 8 November 1895. This introduced a new era in the field of medicine. Radiographic imaging using x-rays is now a routine part of diagnostic techniques worldwide. Roentgen was awarded the first Nobel Prize in Physics for his discovery.
The invention of plain film radiography quickly changed diagnostic algorithms. Sicard and Forestier were injecting the radiopaque contrast medium Lipiodol into facet joints during the first World War.117 In 1920, an incidental injection of contrast medium into the dural sac (instead of the facet joint) provided the first myelogram. In 1942, Steinhausen recommended the use of iodophenylundecylic acid (Pantopaque). Hence, Pantopaque myelography was used routinely for the diagnosis of spinal tumors and disc disorders for decades.118 Since the 1970s, new contrast media such as Thorotrast, Conray, Dimeray, and Metrizamid have been used for myelography.
Discography has been used since its introduction by Lindblom.119 It was widely used for both lumbar and cervical imaging throughout 1950s and 1960s. The invention of CT decreased its popularity. After the introduction of spine MRI, however, discography had a resurgence with an increased interest in the black disc, high-intensity zones, and discogenic pain.
In 1972, Oldendorf, Hounsfield, and Ambrose reported the successful use of CT for diagnosing spinal disorders.120,121 With this invention, Hounsfield was awarded the Nobel Prize for Physiology or Medicine in 1979. Soon thereafter, Damadian invented the MRI scanner.122
Summary
The technical accomplishment of performing surgery on the ventral spine provides perhaps a useful marker for the endpoint of the history of “early modern” spinal surgery. By 1970, it may be argued, the basic groundwork had been laid for the subsequent advances, particularly in spinal instrumentation, that have been made over the last 25 years. These advances include an emphasis on location-appropriate decompression; the development of segmental spinal instrumentation by E. R. Luque in the early 1970s,123–126 the refinement and proliferation of pedicular instrumentation techniques, first described by Harrington in 1969,127,128 the introduction of universal spinal instrumentation by Cotrel and associates,129 the further development of ventral thoracolumbar instrumentation by Zielke, Kostuik,130 and Kaneda; the introduction of ventral cervical instrumentation by Caspar and associates in 1989;131 and, most recently, the application of endoscopic techniques.132
Gruber P., Boeni T. History of spinal disorders. In: Boos N., Aebi M., editors. Spinal disorders: fundamentals of diagnosis and treatment. Berlin: Springer; 2008:1-35.
Markham J.W. Surgery of the spinal cord and vertebral column. In: Walker A.E., editor. A history of neurological surgery. New York: Hafner Publishing Company; 1967:370-371.
Naderi S., Andalkar N., Benzel E.C. History of spine biomechanics. Part I. The pre-Greco-Roman, Greco-Roman, and Medieval roots of spine biomechanics. Neurosurgery. 2007;60:382-391.
Naderi S., Andalkar N., Benzel E.C. History of spine biomechanics. Part II. From the renaissance to the 20th century. Neurosurgery. 2007;60:392-404.
Wiltse L.L. The history of spinal disorders. In: Frymoyer J.W., editor. The adult spine: principles and practice. Philadelphia: Lippincott-Raven; 1997:3-40.
1. Kumar K. Historical perspective: spinal deformity and axial traction. Spine. 1996;21:653-655.
2. Brasted J.H. The Edwin Smith Papyrus. In: Wilkins R.H., editor. Neurosurgical classics. Park Ridge, Ill: American Association of Neurologic Surgeons; 1992:1-5.
3. Lang J.K., Kolenda H. First appearance and sense of the term “spinal column” in ancient Egypt: historical vignette. J Neurosurg. 2002;97(Suppl 1):152-155.
4. Brok A.J. Greek medicine. London: J.M. Dent & Sons; 1929.
5. Adams F. The genuine works of Hippocrates. Baltimore: Williams & Wilkins; 1939.
6. Naderi S., Andalkar N., Benzel E.C. History of spine biomechanics. Part I. The pre-Greco-Roman, Greco-Roman, and Medieval roots of spine biomechanics. Neurosurgery. 2007;60:382-391.
7. Marketos S.G., Skiadas P.K. Galen: A pioneer of spine research. Spine. 1999;24:2358-2362.
8. Wiltse L.L. The history of spinal disorders. In: Frymoyer J.W., editor. The adult spine: principles and practice. Philadelphia: Lippincott-Raven; 1997:3-40.
9. Gurunluoglu R., Gurunluoglu A. Paul of Aegina: landmark in surgical progress. World J Surg. 2003;27:18-25.
10. Naderi S., Acar F., Mertol T., et al. Functional anatomy of the spine by Avicenna in his eleventh century treatise Al-Qanun fi al-Tibb (The Canons of Medicine). Neurosurgery. 2003;52:1449-1453.
11. Spink M.S., Lewis G.L. Albucasis, on surgery and instruments: a definitive edition of the Arabic text with English translation and commentary. London: The Wellcome Institute of the History of Medicine; 1973.
12. Naderi S., Acar F., Arda M.N. History of spinal disorders and cerrahiyetülhaniye: a review of a Turkish treatise written by Serefeddin Sabuncuoglu in 15th century. J Neurosurg. 2002;96:352-356.
13. Naderi S., Andalkar N., Benzel E.C. History of spine biomechanics. Part II. From the Renaissance to the 20th century. Neurosurgery. 2007;60:392-404.
14. Novell J.R. From Da Vinci to Harvey: The development of mechanical analogy in medicine from 1500 to 1650. J R Soc Med. 1990;83:396-398.
15. Benini A., Bonar S.K. Andreas Vesalius. Spine. 1514–1564;21:1388-1393. 1996
16. Maquet P. Iatrophysics to biomechanics. From Borelli (1608–1679) to Pauwels. J Bone Joint Surg (Br). 1885–1980;74:335-339. 1992
17. Middleton W.E.K. A little-known portrait of Giovanni Alfonso Borelli. Med Hist. 1974;18:94-95.
18. Matas R. Surgical operations fifty years ago. Am J Surg. 1951;82:111.
19. Cline H.J.Jr. (cited by Hayward G): An account of a case of fracture and dislocation of the spine. N Engl J Med Surg. 1815;4:13.
20. Tyrell F. Compression of the spinal marrow from displacement of the vertebrae, consequent upon injury. Operation of removing the arch and spinous processes of the twelfth dorsal vertebra. Lancet. 1827;11:685-688.
21. Rogers D.L. A case of fractured spine with depression of the spinous processes, and the operation for its removal. Am J Med Sci. 1835;16:91-94.
22. Armour D. Surgery of the spinal cord and its membranes. Lancet. 1927;1:423-430.
23. Boudorf B.G. Des resection des apopeses transverse des vertebraes. Strausberg. 1864.
24. Smith A.G. Account of a case in which portions of three dorsal vertebrae were removed for the relief of paralysis from fracture, with partial success. North Am Med Surg J. 1829;8:94-97.
25. Simeone F.A. The neurosurgeon as a spine expert: an historical perspective. Clin Neurosurg. 2002;50:77-85.
26. Gowers W.R., Horsley V.A. A case of tumour of the spinal cord. Removal; recovery. Med Chir Trans. 1888;53:379-428.
27. Gowers W. A manual of diseases of the nervous system. London: J & A Churchill; 1886–1888. 2 vols
28. Church A., Eisendrath D.W. A contribution to spinal cord surgery. Am J Med Sci. 1892;103:395-412.
29. Cushing H. The special field of neurological surgery. Bull Johns Hopkins Hosp. 1905;16:77-87.
30. Cushing H. The special field of neurological surgery: five years later. Bull Johns Hopkins Hosp. 1910;21:325-329.
31. von Eiselsberg A.F., Ranzi E. Ueber die chirurgische Behandlung der Him-und Rückenmarkstumoren. Arch Klin Chir. 1913;102:309-468.
32. Elsberg C.A., Beer E. The operability of intramedullary tumors of the spinal cord. A report of two operations with remarks upon the extrusion of intraspinal tumors. Am J Med Sci. 1911;142:636-647.
33. Alessandri R. Processo osteoplastico modificato di laminectomia, con due cast operati. Arch Ed Atti Soc Hal Chir. 1905;18:135-154.
34. Abbe R. Spinal surgery: report of eight cases. Med Rec. 1890;38:85-92.
35. Caponotto A., Pescarolo B. Estirpazione di un tumore intradurale del canale rachideo. Riforma Med. 1892;8:543-549.
36. Cushing H. Intradural tumor of the cervical meninges with early restoration of function in the cord after removal of the tumor. Ann Surg. 1904;39:934-955.
37. Eskridge J.T., Freeman L. Intradural spinal tumor opposite the body of the fourth dorsal vertebra; complete paralysis of the parts below the lesion; operation; recovery; with ability to walk without assistance within three months. Phil Med J. 1898;2:1236-1243.
38. Laquer L. Ueber Compression der Cauda equina. Compressions-Erscheinungen im Gebeite der lumbal und Sacral wurzeln. Eroffnung des Canalis sacralis. Exstirpation eines Lymphangioma cavernosum. Beseitigung fast aller Beschwerden. Neurol Centralbl. 1891;10:193-204.
39. Roy C.D. Report of a case of spinal exsection and removal of a tumor from the cord. South Med Rec. 1890;20:564-566.
40. Makins G.H., Abbott F.C. On acute primary osteomyelitis of the vertebrae. Ann Surg. 1896;23:510-539.
41. Dawbarn R.H.M. A successful case of spinal resection. New York Med J. 1889;49:711-715.
42. Markham J.W. Surgery of the spinal cord and vertebral column. In: Walker A.E., editor. A history of neurological surgery. New York: Hafner Publishing Company; 1967:370-371.
43. Alessandri R. Laininectomia della terza e quarta vertebra lombre per lesione della cauda equina. Riv Patol Nerv. 1905;10:86-92.
44. Taylor A.S. Unilateral laminectomy. Ann Surg. 1910;51:529-533.
45. Elsberg C.A. Experiences in spinal surgery. Surg Gynecol Obstet. 1913;16:117-132.
46. Elsberg C.A. Diagnosis and treatment of diseases of the spinal cord and its membranes. Philadelphia: WB Saunders; 1916.
47. Virchow R. Untersuchungen uber die Entwickelung des Schadelgruendes im gesunden und krankhaften Ziistande, etc. Berlin, G Reimer. 1857.
48. Kocher T. Chirurgische Operationslehre. Jena: G Fischer; 1892.
49. Kocher T. Die Verletzungen der Wirbelsule zugeleieh als Beitrag zur Physiologie des menschlichen Ruckenmarks. Mitt Grenzgeb Med Chir. 1896;1:415-480.
50. Oppenheim H., Krause F. Über Einklemung bzw. Strangulationbder Cauda Equina. Dtsch Med Wochenschrift. 1909;35:697-700.
51. Middleton G.S., Teacher J.H. Injury of the spinal cord due to rupture of an intervertebral disc during muscular effort. Glasgow Med J. 1911;76:1-6.
52. Goldthwaite J.E. The lumbosacral articulation. An explanation of many cases of “lumbago,” “sciatica,” and paraplegia. Boston Med Sci J. 1911;164:365-372.
53. Dandy W.E. Loose cartilage from the intervertebral disc simulating tumour of the spinal cord. Arch Surg. 1929;19:660-672.
54. De Castro I., dos Santos D.P. The history of spinal surgery for disc disease. An illustrated timeline. Arq Neuropsiquiatr. 2005;63:701-706.
55. Mixter W.J., Barr J.S. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
56. Love J.G. Removal of protruded intervertebral discs without laminectomy. Proceedings of staff meeting. Mayo Clin. 1939;14:800-805.
57. Yasargil M.G. Microsurgical operation of herniated lumbar disc. Adv Neurosurg. 1977;4:81.
58. Caspar W. A new surgical procedure for lumbar disc herniation causing less tissue damage through a microsurgical approach. Adv Neurosurg. 1977;4:77-80.
59. Watson G.L., Paul W.E. Contribution to the study of spinal surgery: one successful and one unsuccessful operation for the removal of the tumor. Boston Med Surg J. 1905;153:114-117.
60. Elsberg C.A. The extradural ventral chondromas (enchondroses), their favorite sites, the spinal cord and root symptoms they produce, and their surgical treatment. Bull Neurol Inst N Y. 1931;1:350-388.
61. Portal A. Cours d’Anatomie Medicale ou Elements de l’Anatomie de I’Homme. Paris: Baudouin, 1803;vol 1.
62. Lane W.A. Case of spondylolisthesis associated with progressive paraplegia; laminectomy. Lancet. 1893;1:991.
63. Sachs B., Frankel J. Progressive ankylotic rigidity of the spine. J Nerv Ment Dis. 1900;27:1.
64. Bailey R.W., Badgley C.E. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg (Am). 1960;42:565-594.
65. Bailey P., Casamajor C. Osteoarthritis of the spine as a cause of compression of the spinal cord and its roots. J Nerv Mental Dis. 1911;38:588-609.
66. Sarpyener M.A. Congenital stricture of the spinal canal. J Bone Joint Surg. 1945;27:70-79.
67. Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg (Br). 1954;36:230-237.
68. Hattori S. A new method for cervical laminectomy. Central Jap J Orthop Traum Surg. 1973;16:792-794.
69. Gruber P., Boeni T. History of spinal disorders. In: Boos N., Aebi M., editors. Spinal disorders: fundamentals of diagnosis and treatment. Berlin: Springer; 2008:1-35.
70. Zavaleta M.A. Resecion de vertebras. Ann Circ Med Argent. 1892;15:497-502.
71. Ménard V. Causes de la paraplegia dans le mal de Pott. Son traitement chirurgical par l’ouverture direct du foyer tuberculeux des vertebras. Rev Orthop. 1894;5:47-64.
72. Sorrel-Dejerine Y: Contribution a l’Etude des Paraplegies Pottiques. Paris, 1925.
73. Calot T. Sur le meilleur traitement localdes tuberculoses doses articulations et ganglions lymphatigues. Acta Chir Scand. 1930;67:206-226.
74. Seddon H.J. Pott’s paraplegia: prognosis and treatment. Br J Surg. 1935;22:769.
75. Capener N. The evolution of lateral rhachotomy. J Bone Joint Surg (Br). 1954;36:173-179.
76. Larson S.J., Hoist R.A., Hemmy D.C., Saiices A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
77. Humphreis A.W., Hawk W.A., Berndt A.L. Anterior fusion of the lumbar spine using an internal fixation device. J Bone Joint Surg (Am). 1959;41:371.
78. Thompson W.A.L., Ralston E.L. Pseudoarthrosis following spine fusion. J Bone Joint Surg (Am). 1949;31:400-405.
79. Albee F.H. Transplantation of a portion of the tibia into the spine for Pott’s disease. JAMA. 1911;57:885-886.
80. Hibbs R.A. An operation for progressive spinal deformities. NY Med J. 1911;93:1013-1016.
81. Boehler J. Anterior stabilization for acute fractures and non-unions of the dens. J Bone Joint Surg (Am). 1982;64:18-27.
82. Campbell W.C. An operation for extra-articular fusion of sacroiliac joint. Surg Gynecol Obstet. 1927;45:218-219.
83. Kleinberg S. The operative treatment of scoliosis. Arch Surg. 1922;5:631-645.
84. Burns B.H. An operation for spondylolisthesis. Lancet. 1933;1:1233.
85. Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
86. Boucher H.H. A method of spinal fusion. J Bone Joint Surg (Br). 1959;41:248-259.
87. Callahan R.A., Johnson R.M., Margolis R.N., et al. Cervical facet fusion for control of instability following laminectomy. J Bone Joint Surg (Am). 1977;59:991-1002.
88. Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223.
89. Cloward R.B. The treatment of ruptured intervertebral disc by vertebral body fusion. Ann Surg. 1952;136:987-992.
90. Venable C.S., Stuck W.G. Electrolysis: controlling factor in the use of metals in treating fractures. JAMA. 1939;3:349.
91. Venable C.S., Stuck W.G., Beach A. The effects on bone of metals: based upon electrolysis. Ann Surg. 1937;105:917-938.
92. King D. Internal fixation for lumbosacral fusion. J Bone Joint Surg (Am). 1948;30:560-565.
93. Wilson P.D., Straub L.R. The use of a metal plate fastened to the spinous processes. Ann Arbor, MI: American Academy of Orthopaedic Surgeons Instructional Course Lecture; 1952.
94. Holdsworth F.W., Hardy A. Early treatment of paraplegia from fractures of the thoracolumbar spine. J Bone Joint Surg (Br). 1953;35:540-550.
95. Holdsworth F.W. Fractures, dislocations, and fracture-dislocation of the spine. J Bone Joint Surg (Br). 1963;45:6-20.
96. Bailey R.W. Fractures and dislocations of the cervical spine: orthopedic and neurosurgical aspects. Postgrad Med. 1964;35:588-599.
97. Kelly R.P., Whitesides T.E. Treatment of lumbodorsal fracture-dislocations. Ann Surg. 1968;167:705-717.
98. Hadra B.E. Wiring the spinous processes in Pott’s disease. Trans Am Orthop Assoc. 1891;4:206-210.
99. Harrington P.R. Treatment of scoliosis. J Bone Joint Surg (Am). 1962;44:591-610.
100. American Orthopaedic Association Research Committee. End-result study of the treatment of idiopathic scoliosis. J Bone Joint Surg. 1941;23:963-977.
101. Harrington P.R. The history and development of Harrington instrumentation. Clin Orthop Relat Res. 1973;93:110-112.
102. Harrington P.R., Tullos H.S. Reduction of severe spondylolisthesis in children. South Med J. 1969;62:1-7.
103. Lister J. On the antiseptic principle in the practice of surgery. Br Med J. 1867;2:246.
104. Meyer H.W. The history of the development of the negative differential pressure chamber for thoracic surgery. J Thorac Cardiovasc Surg. 1955;30:114.
105. Müller W. Transperitoneale Freilegung der Wirbelsaule bei Tuberkuloser Spondylitis. Deutsche Ztschr Chir. 1906;85:128.
106. Ito H., Tsuchiya J., Asaini G. A new radical operation for Pott’s disease. J Bone Joint Surg. 1934;16:499-515.
107. Vincent E. Contribution a la chirurgie rachidienne du drainage vertebral dans le mal de Pott. Rev Chir. 1892:1273-1294.
108. Vincent E. Chirurgie rachidienne et mal de Pott. Rev Chir. 1898;18:47-54.
109. Ito H., Asaini G. Lumbosacral sympathetic ganglionectomy. Its value as a therapeutic measure for thromboangiitis obliterans. Am J Surg. 1932;15:26.
110. Ollier L. Traite des resections et des operations conservatrices qu’on peut pratiquer sur le systeme osseux. Paris: G Masson, 1891;vol 3. pp 833–857
111. Hodgson A.R., Stock F.E. Anterior spinal fusion. A preliminary communication on the radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266-275.
112. Dwver A.F., Newton N.C., Sherwood A.A. An anterior approach to scoliosis. A preliminary report. Clin Orthop Relat Res. 1969;62:192-202.
113. Dwyer A.F., Schafer M.F. Anterior approach to scoliosis. Results of treatment in fifty-one cases. J Bone Joint Surg (Br). 1974;56:218-224.
114. Zielke K., Pellin B. Neue Instrumente und Implantate zur Erganzung des Harrington Systems. Z Orthop Chir. 1976;114:534.
115. Kaneda K., Abumi K., Fujiya K. Burst fractures with neurologic deficits of the thoracolumbar spine. Results of anterior decompression and stabilization with anterior instrumentation. Spine. 1984;9:788-795.
116. Roentgen C., Talbott J., editor. A biographical history of medicine. Orlando: Grüne & Stratton, 1970.
117. Sicard J.H., Forestier J. Methode radiographique d’exploration de la cavite epidurale par le lipiodol. Rev Neurol. 1921;37:1264-1266.
118. Steinhausen T.B., Dungan C.E., Furst J.B., et al. Iodinated organic compounds as contrast media for radiographic diagnoses: experimental and clinical myelography with ethyl iodophenylundecylate (Pantopaque). Radiology. 1944;43:230-234.
119. Lindblom K. Diagnostic puncture of intervertebral discs in sciatica. Acta Orthop Scand. 1948;17(Suppl 4):233-239.
120. Hounsfield G. (Quoted by Oldendorf WH): The quest for an image of the brain. New York: Raven Press; 1980.
121. Oldendorf W.H. Displaying the internal structural pattern of a complex object. Trans Bio-Med Elect (BME). 1961;8:68.
122. Damadian R. Tumor detection by nuclear magnetic resonance. Science. 1971;171:1151-1153.
123. Luque E.R., Cassis N., Ramirez-Wiella G. Segmental spinal instrumentation in the treatment of fractures of the thoracolumbar spine. Spine. 1982;7:256-259.
124. Luque E.R. The anatomic basis and development of segmental spinal instrumentation. Spine. 1982;7:256-259.
125. Luque E.R. Interpeduncular segmental fixation. Clin Orthop Relat Res. 1986;203:54-57.
126. Luque E.R. Segmental spinal instrumentation of the lumbar spine. Clin Orthop Relat Res. 1986;203:126-134.
127. Harrington P.R., Tullos H.S. Spondylolisthesis in children. Clin Orthop Relat Res. 1971;79:75-84.
128. Wiltse L.L. History of pedicle screw fixation of the spine. Spine: State of the Art Rev. 1992;6:1-110.
129. Cotrel Y., Dubousset J., Guillaumat M. New universal instrumentation in spinal surgery. Clin Orthop Relat Res. 1988;227:10-23.
130. Kostuik J.P. Anterior fixation for fractures of the thoracic and lumbar spine with or without neurologic involvement. Clin Orthop Relat Res. 1984;189:103-115.
131. Caspar W., Barbier D.D., Klara P.M. Anterior cervical fusion and Caspar plate stabilization for cervical trauma. Neurosurgery. 1989;25:491-502.
132. Kambin P., Gellman H. Percutaneous lateral discectomy of the lumbar spine. Clin Orthop Relat Res. 1983;174:128-132.