Chapter 2 History of Spinal Instrumentation
The Modern Era
Dorsal Thoracolumbar Instrumentation
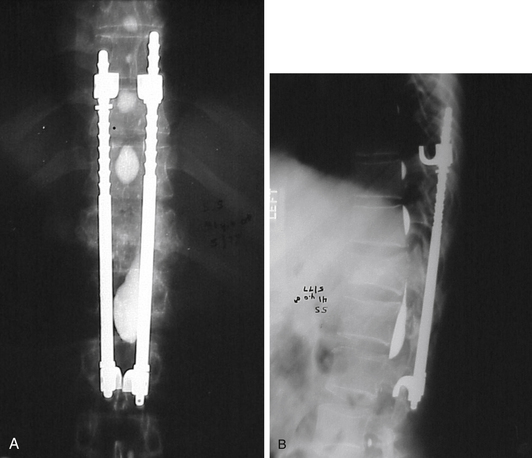
In 1975, the Harrington rod represented the state of the art in spinal instrumentation. The rod system, originally developed by Paul Harrington for the correction of spinal deformities, was soon used in the treatment of traumatic injuries1,2 (Fig. 2-1), degenerative disease,3 and metastatic disease.4,5 The system provided distraction rods as well as compression rods and hooks. Over the years, however, their widespread use led to recognition of their limitations. The use of a distraction system provided excellent correction of coronal plane deformities (scoliosis). Unfortunately, the use of distraction as the sole correction tool resulted in the loss of normal sagittal plane alignment. The loss of normal lumbar lordosis was associated with “flat back syndrome.”6,7 Hook dislodgement and rod breakage also proved to be troublesome complications.8,9 In addition, casting or bracing was generally required in the postoperative period, which proved to be difficult or impractical in some patients.10
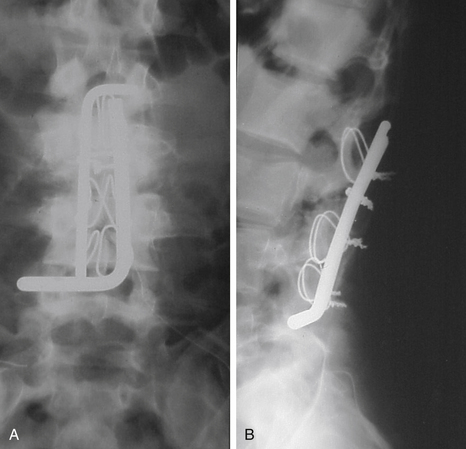
Luque popularized the use of a 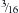 -inch steel rod secured at each spinal level with sublaminar wires (Fig. 2-2). Luque reasoned that increasing the number of fixation points along a construct would reduce the force placed upon each individual point and obviate the need for a postoperative cast or brace. Additional beneficial effects of segmental fixation were that it increased the potential corrective power of instrumentation, reduced the potential for construct failure, and resulted in improved fusion rates.
-inch steel rod secured at each spinal level with sublaminar wires (Fig. 2-2). Luque reasoned that increasing the number of fixation points along a construct would reduce the force placed upon each individual point and obviate the need for a postoperative cast or brace. Additional beneficial effects of segmental fixation were that it increased the potential corrective power of instrumentation, reduced the potential for construct failure, and resulted in improved fusion rates.
Although the corrective power of sublaminar wires was well-appreciated, many surgeons had reservations in using them because of reports of neurologic injury resulting either from direct trauma or from epidural hematoma.11,12 In addition, revision surgery to alter sublaminar wiring is problematic because scarring may preclude the passage of new wires at the same laminae. In response to these concerns, Drummond et al.13 developed a method for segmental fixation using a button-wire implant passed through the base of the spinous process. This technique does not provide as strong fixation as do sublaminar wires. It avoids, however, passing anything into the spinal canal and thus reduces the risk of direct neurologic injury. This compromise of fixation for less risk of neurologic injury was seen as a prudent choice by many surgeons operating on healthy, neurologically normal adolescents with idiopathic scoliosis. Nevertheless, some pundits referred to the procedure as the “chicken-Luque” procedure.
Increasingly sophisticated multiple hook-rod systems appeared in the 1980s that provided much of the strength of wire fixation but with greater flexibility to address deformities in both the sagittal and the coronal dimensions. The Cotrel Dubousset (CD) system was introduced into the United States in 1986 using a ¼-inch rough-surfaced rod.14 Multiple hooks allowed spinal surgeons to apply compression and distraction over different areas within the same rod. The multiple-hook design applied the principles of segmental fixation without the need for sublaminar wires. Significantly, the system provided for a unique mechanism for deformity correction: rod rotation. This proved a powerful force in the correction of scoliosis. Further stability was provided by cross-linking the two parallel rods together.
The advantages of the CD system were partially offset, however, by the difficulty of removing it. The locking mechanism of the hooks was irreversible without destroying the hook or cutting the rod. The Texas Scottish Rite Hospital (TSRH) system was a design advance that addressed the issue of revision surgery. It was similar to the CD system in its use of multiple hooks and cross links but was designed to allow for the removal of the system’s individual components if necessary. Although the features of the TSRH system simplified revision surgery, the top-loading side-tightened system was not universally appreciated. After maturation of the fusion mass, the side-tightened bolts were not always accessible. The following decade saw the introduction of numerous, similar dual-rod systems like Moss-Miami and Isola.6,15 The major variations revolved around the leading and locking mechanisms: side loading, top loading, side tightening, or top tightening. The last decade has seen the introduction of numerous systems that operate with the same design principles, with a shift toward the use of polyaxial screws that make coupling of the fixation points to the rods easier. Today’s systems often have a wide range of screw choices, including monoaxial, polyaxial, uniaxial (screws that are mobile in only one plane to allow for better derotation), as well as monaxial, polyaxial, and uniaxial reduction screws (screws with extended tabs, which allow gradual reduction of the rod into the body of the screw).
A major advance provided by these spinal systems was the exploitation of the pedicle as a site for segmental fixation. This innovation is generally credited to Roy-Camille of Paris. Roy-Camille performed his first operation in 1963 but did not publish the results until 1970.16 Pedicle screws presented many advantages when compared with other tools for spinal fixation. Pedicle screws are biomechanically superior as a point of fixation17 compared with hook- or wire-rod constructs and can be placed into the sacrum, an area to which fixation is otherwise difficult. In addition, they can be placed even after a laminectomy has been performed and can be positioned without entering the spinal canal.18 This advantage allowed for the massive proliferation of spinal instrumentation into the area of degenerative spinal disorders. Prior to the advent of pedicle-screw instrumentation systems, there had been only sporadic reports of the use of instrumentation for degenerative spinal disorders. The Knodt rod (a small distraction rod system) had been used previously in degenerative disease but was associated with localized loss of lordosis and device dislodgement. In addition, the system needed some lamina for device fixation. Pedicle-screw systems, however, can be used after a total laminectomy.
Arthur Steffee popularized the use of pedicle screws in the United States in 1984 using a contourable plate. At about the same time, a screw-rod system, developed by Yves Cotrel of France, was in use in Europe that became incorporated into the “Universal” CD system. Controversy soon followed, with both the screw-plate and screw-rod constructs developing a group of proponents.19 Proponents of plates noted that plates were stronger. Most surgeons were ultimately attracted, however, to rods because their use provides greater flexibility, reduces encroachment upon the adjacent facet joints, and leaves more surface area for fusion. The marriage of the long dual-rod constructs to lumbar pedicle screws was an important development that enhanced the surgeon’s ability to accomplish increasingly difficult and complex spinal reconstructions. The use of the polyaxial pedicle screw has further advanced the ease of spinal reconstructions.
In recent years, there has been an interest in developing dynamic stabilization systems for degenerative diseases. The impetus for these systems arises from clinical evidence suggesting that 100% spinal fusion does not correlate with good clinical outcomes, which may range from 60% to 80%.20 Spinal fusion may also have kinematic and kinetic consequences at adjacent segments that may increase the rate of adjacent level degeneration.21 Dynamic stabilization systems aim to restore functional stability while maintaining intersegmental motion.
The most notable advancements in pedicle screw-rod based systems are the Graf ligmentoplasty system, the Isobar TTL Semi-rigid spinal system (Scient’X, West Chester, PA), and the Dynesys system (Zimmer Spine, Minneapolis, MN). The Isobar TTL and the Dynesys have Food and Drug Administration (FDA) approval as an adjunct to fusion, but to date none of these systems have been approved as a dynamic stabilizer (i.e., without fusion).20–24 The Dynesys system was the only device to undergo an FDA Investigational Device Exemptions (IDE) study as a dynamic stabilizer. The system is composed of titanium pedicle screws connected via a terephthalate cord and polycarbonate spacer. Several authors have recently reported mostly favorable results with a variable incidence of complications.25–31 At the time of this writing, no system has demonstrated enough evidence to justify widespread use or to be the gold standard.
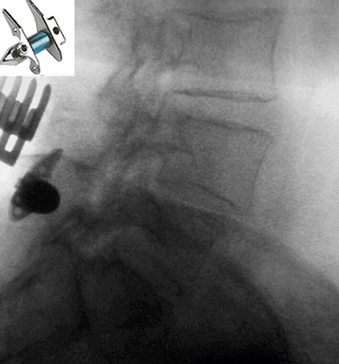
Interspinous devices that increase the intervertebral space have also been developed to treat a myriad of degenerative conditions. These devices can be categorized as static or dynamic.32 The most noteworthy static devices include the X STOP (Saint Francis Medical Technologies Inc., Alameda, CA), ExtenSure (NuVasive Inc., San Diego, CA), and Wallis implants (Abbott Spine, Austin, TX). Of these, the X STOP and the ExtenSure implants have been FDA approved for general use.33 The X STOP has an oblong central core that is stabilized by two lateral wings (Fig. 2-3). The primary indication is mild or moderate neurogenic claudication from spinal stenosis. For dynamic interspinous devices, the Diam (Medtronic Sofamor Danek, Memphis, TN), Coflex (Paradigm Spine, New York, NY), and CoRoent (Nuvasive Inc., San Diego, CA) have been investigationally studied for use in the United States.34 Of these, only the Diam device has been FDA approved. It attaches at the spinous processes and behind the supraspinous ligament. At the time of writing, numerous dorsal thoracolumbar dynamic systems have been approved by the FDA for investigational use. Outcome data for these systems, however, has been extremely limited.
Ventral Thoracolumbar Instrumentation
Successful use of the Harrington instrumentation kindled interest in developing a ventral system to address neuromuscular scoliosis. Dwyer developed a ventral system for internal fixation using screws connected by a cable.35 Winter attempted a combined ventral and dorsal approach with Harrington and Dwyer instrumentation to treat painful adult idiopathic scoliosis.36 This concept was of particular interest in that these patients were at high risk for pseudarthrosis and tended to tolerate bracing less well than adolescents.36
The Dunn device was a ventral implant that consisted of two rods that spanned the distance between two vertebral body bridges: one placed ventrolaterally with a vertebral body staple and the other placed more dorsolaterally with an intervertebral body screw.37 This system was not widely accepted because it was bulky and was associated with vascular complications.38
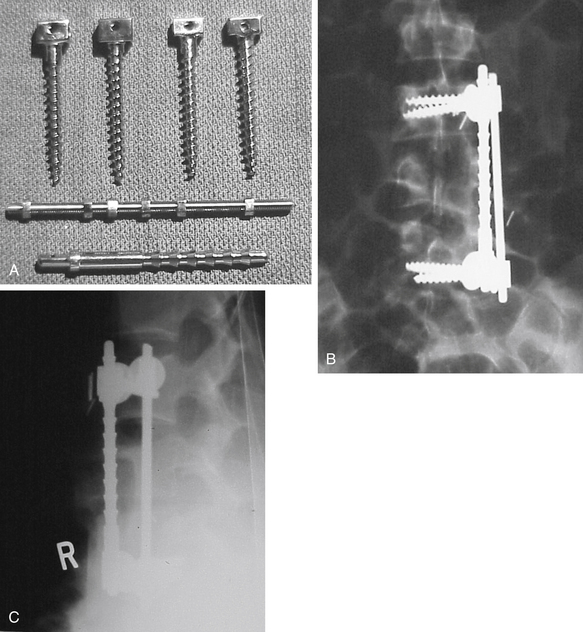
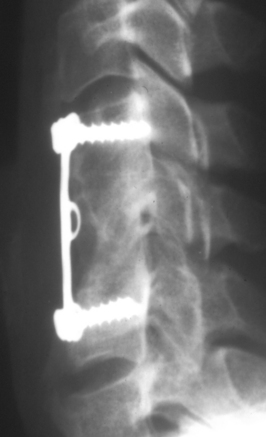
The ventral Kostuik-Harrington instrumentation was an adaptation of short Harrington rods used in conjunction with a pedicle screw developed by Paul Harrington for use in treating myelomeningocele. Introduced by John Kostuik in the early 1980s, it was an innovative short-segment ventral fixation device. The screw, when placed ventrolaterally in the vertebral bodies, allows for short-segment ventral correction of the kyphotic deformity associated with burst fractures. A second neutralization rod was placed parallel to the first rod to enhance stability (Fig. 2-4). Over time, cross-fixators were added in an attempt to further enhance stability. Two parallel rods rigidly cross-linked are the biomechanical equivalent of a plate. Most ventral short-segment constructs subsequently used plates with vertebral body screws.
Several other plate designs soon followed that had a lower profile. Ryan introduced a plate secured by a rostral and caudal bolt inserted through the vertebral body. The single-bolt design, however, offered less resistance to rotation than the designs that used two screws or bolts above and below.39 The Yuan I-Plate was an alternative design that consisted of a 3.5-mm stainless steel plate secured with transvertebral screws.40 Black et al.41 published their experience with a low-profile, rectangular, stainless steel plate with multiple holes that allowed for the placement of three screws at each vertebral level. The Kaneda device represented another stage in the development of ventral thoracolumbar instrumentation because it allowed reduction of kyphotic deformities after ventral decompression while providing good strength without incidence of vascular injury.38
Dorsal Cervical Instrumentation
Several instrumentation systems were devised as adjuncts or alternatives to wiring. The Daab plate was a stainless-steel implant shaped like an elongated H that could be compressed at either end to fixate it to a spinous process.42,43 This instrumentation represented no significant advantage over the available wiring techniques, and it was probably inferior, considering that it typically needed the resection of an intervening spinous process and the associated interspinous ligaments.
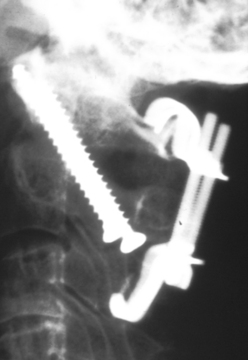
Halifax clamps are a pair of upgoing and downgoing sublaminar hooks tightened together with a screw that is then secured in position with a locking mechanism (Fig. 2-5).43,44 Halifax clamps have the advantage of relatively simple and rapid application. In addition, the area of bone contact is broader than that with wiring and is less likely to pull out of soft bone. They offer C1-2 fixation comparable to that achieved with the Brooks technique.45 Relative disadvantages of the system are that hooks are introduced into the spinal canal and the implant is relatively “high profile” and has limited application when stabilization is needed over multiple segments.
In the mid-1980s, Magerl introduced transarticular screw placement for internal fixation of C1-2. This is a technically demanding procedure compared with wiring, which achieves better C1-2 stability to flexion-extension and rotation46 than wiring procedures and is associated with the highest published C1-2 fusion rates.47 This technique is not always feasible if there is anatomic variation in the course of the vertebral artery, although there is still benefit in unilateral placement.38 Many practitioners supplement transarticular screws with dorsal instrumentation because broken screws have been seen when used as a stand-alone procedure.
Lateral mass plate fixation with screws was introduced by Roy-Camille et al.48 This technique of internal fixation is ideal in instances in which the laminae and spinous processes have been removed or fractured. The first technique for screw placement was modified by Magerl and Seeman,49 Heller et al.,50 and An et al.51 The original lateral mass plates were an application of preexisting bone plates with a distance of 13 mm between plate holes. The Haid Plate and Synthes reconstruction plates were soon marketed, each offering a choice of two interhole distances. These systems all suffered from insufficient versatility in accommodating the wide variety of interhole differences often needed.52 The AXIS system (Medtronic/Sofamor Danek, Memphis, TN) offers plate holes at intervals of 11, 13, and 15 mm and a slotted hole design to allow for limitless interhole variations as well as improved ability to contour the plates.
In recent years, numerous manufacturers have introduced lateral mass plate fixation systems. The most commonly used systems include the Cervifix system (Synthes Spine, Paoli, PA), Starlock instrumentation (Synthes Spine, Paoli, PA), Summit system (Depuy Acromed, Rayham, MA), and others. Advancements and design variations in lateral mass plate fixation have allowed the surgeon flexibility to address variations in anatomy beyond that offered by the AXIS system. Hybrid plate-rod implants are also available for occipital screw placement in occipito-cervical fusions.
Ventral Cervical Instrumentation
Since the first system was developed by Bohler in the mid-1960s, ventral cervical plating has become a popular means of supplementing a ventral cervical fusion.53 Early in the development of this instrumentation, the potential for screw backout was recognized as a possible cause of serious complications, including tracheal or esophageal erosion. The first systems widely available were the Caspar (Aesculap, San Francisco, CA) and the Orozco (Fig. 2-6) (Synthes, Paoli, PA). Both of these systems consisted of simple plates with slots or holes but without any locking devices. Constraint of the screws depended on obtaining bicortical purchase and “blocking” backout by screw angulation.
The rate of screw backout or breakage and graft subsidence was high with the first generation of ventral cervical plates. This led to the development of the Cervical Spine Locking Plate (CSLP) (Synthes, Paoli, PA),54 first introduced in North America in 1991. The CSLP used a titanium expansion screw that secured the screwhead to the plate and, thus, allowed for unicortical purchase without the risk of screw backout. The substitution of titanium for stainless steel allowed for postoperative magnetic resonance imaging. The CSLP reduced the incidence of screw backout55; however, its limitations were a rigid screw trajectory and the fact that the plate was wide and difficult to contour.
The Orion ventral cervical plate (Medtronic/Sofamor Danek, Memphis, TN) represented the next major product introduction for ventral cervical plating. The plate was manufactured “prelordosed” with a wide variety of screw lengths to allow for unicortical or bicortical purchase. The drill guide was fixed to the plate, providing 15 degrees of rostral and caudal angulation and 6 degrees of medial angulation. Locking screws were added to fix the screws to the plate by overlapping the screw heads. Although the Orion plate was widely used and had good reported surgical results,56,57 some surgeons felt that the system was too rigid and shielded the graft from stress, thereby promoting a significant rate of pseudarthrosis.58
In response to the perceived deficiencies of rigid ventral plates, dynamic cervical plates have evolved to lower the incidence of graft subsidence and plate failure, while still limiting movement across the diseased segment.59 There are three main types of dynamic plates: longitudinal, translational, and telescoping. The longitudinal plate allows for toggling of the screw at the plate screw interface, but has the potential for screw loosening and pull-out. Longitudinal plates include the ACCS (Synthes Spine, Paoli, PA), Acufix (Abbott Spine, Austin, TX), Atlantic (Medtronic Sofamor Danek, Memphis, TN), Reflex (Stryker, Allendale, NJ), Slim-LOC (DePuy Spine, Raynham, MA), and Zephir (Medtronic Sofamor Danek, Memphis, TN).60 In contrast, translational plates have slotted screw holes that allow each screw and vertebra to slide in the axial plane. If improperly placed or with settling over time, translational plates may overlap adjacent disc spaces, leading to ossification and degeneration. Examples include the ABC (Aesculap, Tuttlingen, Germany), C-Tek (Biomet, Warsaw, IN), and Premier (Medtronic Sofamor Danek, Memphis, TN).60 Telescoping plates are designed to allow axial movement internally (Fig. 2-7). The most notable devices of this type are the DOC and the Swift (Depuy Spine, Raynham, MA). Although thicker in profile, these devices remain rigidly fixed to bone and therefore do not overlap with adjacent disc spaces. Lastly, hybrid constructs can be created in several ways by utilizing variable or rigidly fixed screws in combination with slotted or nonslotted plate holes.
Ventral fixation of odontoid fractures can be achieved with the placement of one or multiple screws. Although the technique was published in 1971 by Barbour,61 it did not achieve popularity until the late 1980s.62 Controversy developed over whether one or two screw placements is optimal for fixation.63 Several recent papers, however, have not shown improved results with multiple screw placement.64,65 Some surgeons have advocated the application of cannulated screws placed over Kirschner wires (K-wires) in this procedure, citing improved accuracy and the ability to redirect the screw trajectory as technical advantages. Other surgeons, however, prefer the original noncannulated screws, noting the potential risks of K-wire breakage as well as unintended K-wire advancement during screw placement.66
Total Disc Arthroplasty: Cervical and Lumbar
Cervical arthrodesis has been one of the most successful operations in orthopaedic surgery, with 95% of patients reporting significant improvement of symptoms following surgery.67 However, the rate of adjacent segment disease—as reported by Hilibrand et al.—is 2.9%/year, leading to a significant reoperation rate over the long term.68 Especially for younger patients with single- or two-level disc disease, motion preservation technology has emerged with hopes to improve outcomes and reduce the incidence of adjacent segment disease.
The Ulf Fernstrom ball bearing device was the first disc replacement method introduced in 1966, which was implanted in the cervical and lumbar spine with disappointing results. To date, the Bryan cervical disc, Prestige disc, and Pro-disc-C are the only cervical disc replacements that have received FDA approval in the United States. The Bryan cervical disc (Medtronic Sofamor Danek, Memphis, TN) consists of a two titanium shells separated by a polyurethane nucleus. Prospective randomized trials demonstrated some benefit of the Bryan disc over one- or two-level fusion in terms of reduced reoperation rate, improved outcome scores, and improved motion at the diseased segment. Complications were related to surgical technique, increased segmental kyphosis, or device failure.69–73 The newest Prestige ST implant (Medtronic Sofamor Danek, Memphis, TN) underwent a prospective randomized study comparing it with one-level spinal fusion. For patients implanted with the Prestige ST, the study reported improved resolution of neurologic symptoms, less revision procedures, and less revision surgery for adjacent segment disease.74 The Pro-disc-C (Synthes Spine, Paoli, PA) consists of two end plates of cobalt-chromium-molybdenum alloy with a central keel projecting into each end plate for stability (Fig. 2-8). A prospective randomized trial found no complications, fewer revision procedures, and improved clinical outcomes with the Pro-disc-C compared with spinal fusion for single-level disease.74 The newest cervical disc replacements with ongoing FDA IDE trials include the Porous Coated Motion (PCM) device (NuVasive, San Diego, CA) and the CerviCore device (Stryker, Allendale, NJ).74 The PCM is a two-piece device consisting of a cobalt-chrominium-molybdenum end plate with a polyethylene inner core. The CerviCore device, however, is a semiconstrained metal on metal prosthesis. The articulating surface is saddle-shaped, with two keels containing spikes on each end plate.
Total disc replacements for the lumbar spine have also been developed for the same purpose. These devices may be constrained, semiconstrained, or unconstrained. Currently, two lumbar total disc prostheses—the ProDisc-L (Synthes Spine, Paoli, PA) and the SB Charite (DePuy Spine, Raynham, MA)—have FDA approval.75 The ProDisc-L, designed by Thierry Marnay, a French orthopaedic surgeon, is a semiconstrained device with a fixed center of rotation. The SB Charité disc was designed by Shellnac and ButtnerJans to have a sliding polyethylene core that moved with flexion and extension. At the time of writing, the Maverick (Medtronic Sofamor Danek, Memphis, TN) and the Flexicore (Stryker, Allendale, NJ) devices are awaiting FDA approval. The Maverick was designed by Le Huec et al. as a metal-on-metal ball-and-socket configuration.76 The Flexicore is also a metal-on-metal ball-and-socket joint with a semiconstrained designed and fixed center of rotation.
Cage Technology: Horizontal and Vertical
The development of cages to promote interbody fusion traces back to the veterinary work of Bagby in which stainless-steel baskets filled with bone were used to treat wobbler-neck syndrome in race horses.76 Bagby subsequently pioneered the development of a cage for use in human lumbar interbody fusions.77,78 The implantation of cages as an interbody device through either a ventral or dorsal approach has become a widely performed procedure. Horizontal titanium-threaded cages include the BAK cage (Sulzer Spine-Tech, Minneapolis, MN) and the Ray Threaded Fusion Cage (Surgical Dynamics, Norwalk, CT), both of which were designed to be stand-alone devices for the ventral column. Brantigan et al.79 Introduced cages composed of a radiolucent carbon fiber that allowed for improved postoperative imaging. It is also argued that the carbon fiber material has a modulus closer to that of native bone and, thus, should theoretically be a better fusion substrate than metal.79
Although the initial cage development was done for the cervical spine, the technology was first widely implemented in the lumbar spine. In April 2002, the FDA approved the use of the BAK-C device (Sulzer Spine-Tech, Minneapolis, MN) for cervical fusion.80 Recent experience with these implants has indicated that fusion rates are comparable to those seen after procedures using uninstrumented allograft.46
To facilitate ventral vertebral reconstruction after ventral and middle column resection, Harms developed a vertical titanium mesh cage that can be packed with bone and is seated into the end plates.50 This implant has found application in cases of vertebral body destruction resulting from metastatic disease, degenerative conditions, and trauma. The Harms cage was considered a valuable innovation even to those surgeons who preferred using struts made of allograft or autograft because a suitable bone graft is sometimes unavailable. At the time of writing, numerous manufacturers have now developed vertical cage implants of various sizes and materials. These vertical cage implants are most commonly used as ventral column support in combination with dorsal pedicle fixation either inserted ventrally or through a dorsal based interbody approach.
Minimally Invasive Approaches Utilizing Instrumentation
Recently, surgeons have employed minimally invasive approaches to the spine to minimize soft tissue disruption, recovery time, and scar appearance. Transforaminal lumbar interbody fusion (TLIF) has been used most commonly for grade 1 or grade 2 spondylolisthesis with radiculopathy, but also for discogenic back pain.75,81 Using tubular retractors, METRx (Medtronic Sofamor Danek, Memphis, TN), the technique allows for decompression of the ispilateral exiting and traversing nerve roots. Another emerging technique, extreme lateral interbody fusion (XLIF), has been employed for treating axial back pain but also spondylolisthesis and degenerative scoliosis. From the lateral approach, the spine is accessed through the retroperitoneal fat and psoas muscle using MaXcess tubular retractors (NuVasive Inc, San Diego, CA). For both procedures, preliminary results are promising, but longer-term results have yet to be reported. As with many novel techniques, these approaches have been limited by a steep learning curve.82 Numerous less invasive spinal instrumentation systems have also been developed for thoracic and lumbar dorsal spinal fusion. Rather than disrupting the paraspinal musculature, pedicle screws have been successfully placed percutaneously with a variety of systems. Under fluoroscopic visualization, tubular retractors are used to spread the paraspinal musculature over the pedicle. Placement of pedicle screws is generally accomplished with cannulated screws over a small guide wire. Placement of the rod or longitudinal connector may vary with the system. Many surgeons may also choose to use stimulated electromyographic neuromonitoring for additional safety.83
Kyphoplasty and vertebroplasty are minimally invasive percutaneous procedures that stabilize vertebral compression fractures. These have been indicated for chronic pain secondary to osteoporosis or osteolytic changes within a vertebral body. Vertebroplasty stabilizes a vertebral body fracture with injection of polymethylmethacrylate (PMMA) into the vertebral body, while kyphoplasty may also restore vertebral height by injecting the material within an inflatable device (KyphX, Medtronics Kyphon, Sunnyvale, CA) (Fig. 2-9).84 Kyphoplasty may afford a smaller risk for cement leakage and associated complications, but advocates of vertebroplasty have claimed that high-viscosity cement may also alleviate these risks at a much lower cost. Both options have been debated in terms of clinical efficacy and complication rate, which has been reported as 1% to 2% for osteoporotic fractures and 5% to 10% for metastatic lesions with either procedure. Complications related to cement extravasation include new fractures of adjacent levels, cord/root compression, subdural hematoma, and embolization.85
Instrumentation through video-assisted thoracoscopic surgery (IVATS) has been used recently for thoracic scoliosis. The procedure allows for less disruption of the thoracic rib cage for removal of the disc spaces, release of ligamentous structures, and instrumentation with vertebral body screws and rods. Endoscopic hardware includes variable-angled thoracoscopes and specialized thoracospinal instruments (Medtronic Sofamor Danek, Memphis, TN). Although reducing blood loss and improving scar appearance, IVATS has been limited by increased operative times, ICU stays, and complication rates compared with dorsal spinal fusion.86,87 However, ventral release procedures without instrumentation remain a useful adjunct for scoliosis surgery.
Bono C.M., Lee C.K. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29:455-463.
Cotrel Y., Dnbousset J. [A new technique for segmental spinal osteosynthesis using the posterior approach]. Rev Chir Orthop Reparatrice Appar Mot. 1984;70:489-494.
Denaro V., Papalia R., Denaro L., et al. Cervical spinal disc replacement. J Bone Joint Surg (Br). 2009;91:713-719.
Esses S.T., Bednar D.A. The spinal pedicle screw: techniques and systems. Orthop Rev. 1989;18:676-682.
Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous cervical arthrodesis. J Bone Joint Surg (Am). 1999;81:519-528.
Lagrone M.O., Bradford D.S., Moe J.H., et al. Treatment of symptomatic flatback after spinal fusion. J Bone Joint Surg (Am). 1988;70:569-580.
Zucherman J.F., Hsu K.Y., Hartjen C.A., et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30:1351-1358.
1. Anden U., Lake A., Nordwall A. The role of the anterior longitudinal ligament in Harrington rod fixation of unstable thoracolumbar spinal fractures. Spine. 1980;5:23-25.
2. Wang G.J., Whitehill R., Stamp W.G., et al. The treatment of fracture dislocations of the thoracolumbar spine with halofemoral traction and Harrington rod instrumentation. Clin Orthop Relat Res. 1979:168-175.
3. Morscher E. [Two-stage reposition and fixation of spondyloptosis with Harrington instrumentation and anterior intercorporal spondylodesis (authors transl)]. Arch Orthop Unfallehir. 1975;83:323-334.
4. Livingston K.E., Perrin R.G. The neurosurgical management of spinal metastases causing cord and cauda equina compression. J Neurosurg. 1978;49:839-843.
5. Sundaresan N., Galicich J.H., Lane J.M. Harrington rod stabilization for pathological fractures of the spine. J Neurosurg. 1984;60:282-286.
6. Bridwell K.H. Spinal instrumentation in the management of adolescent scoliosis. Clin Orthop Relat Res. 1997:64-72.
7. Lagrone M.O., Bradford D.S., Moe J.H., et al. Treatment of symptomatic flatback after spinal fusion. J Bone Joint Surg (Am). 1988;70:569-580.
8. Erwin V.V.D., Dickson J.H., Harrington P.R. Clinical review of patients with broken Harrington rods. J Bone Joint Surg (Am). 1980;62:1302-1307.
9. Sturz H., Hinterberger J., Matzen K., et al. Damage analysis of the Harrington rod fracture after scoliosis operation. Arch Orthop Trauma Surg. 1979;95:113-122.
10. Luque E.R. Segmental spinal instrumentation for correction of scoliosis. Clin Orthop Relat Res. 1982;163:192-198.
11. Johnston C.E.2nd, Happel L.T.Jr., Norris R., et al. Delayed paraplegia complicating sublaminar segmental spinal instrumentation. J Bone Joint Surg (Am). 1986;68:556-563.
12. Wilber R.G., Thompson G.H., Shaffer J.W., et al. Postoperative neurological deficits in segmental spinal instrumentation: a study using spinal cord monitoring. J Bone Joint Surg (Am). 1984;66:1178-1187.
13. Drummond D., Guadagni J., Keene J.S., et al. Interspinous process segmental spinal instrumentation. J Pediatr Orthop. 1984;4:397-404.
14. Cotrel Y., Dnbousset J. [A new technique for segmental spinal osteosynthesis using the posterior approach]. Rev Chir Orthop Reparatrice Appar Mot. 1984;70:489-494.
15. Stambough J.L. Posterior instrumentation for thoracolumbar trauma. Clin Orthop Relat Res. 1997;335:73-88.
16. Roy-Camille R., Roy-Camille M., Demeulenaere C. [Osteosynthesis of dorsal, lumbar, and lumbosacral spine with metallic plates screwed into vertebral pedicles and articular apophyses]. Presse Med. 1970;78:1447-1448.
17. Abumi K., Panjabi M.M., Duranceau J. Biomechanical evaluation of spinal fixation devices. Part III. Stability provided by six spinal fixation devices and interbody bone graft. Spine. 1989;14:1249-1255.
18. Dickman C.A., Fessler R.G., MacMillan M., et al. Transpedicular screw-rod fixation of the lumbar spine: operative technique and outcome in 104 cases. J Neurosurg. 1992;77:860-870.
19. Esses S.T., Bednar D.A. The spinal pedicle screw: techniques and systems. Orthop Rev. 1989;18:676-682.
20. Bono C.M., Lee C.K. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29:455-463.
21. Cheh F., Bridwell K.H., Lenke L.G., et al. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007;32:2253-2257.
22. Hadlow S.V., Fagan A.B., Hillier T.M., et al. The Graf ligamentoplasty procedure. Comparison with posterolateral fusion in the management of low back pain. Spine. 1998;23:1172-1179.
23. Perrin G., Cristini A. Prevention of adjacent level degeneration above a fused vertebral segment: long-term effect, after a mean follow-up of 8.27 years of the semi-rigid intervertebral fixation as a protective technique for pathological adjacent disc. International Meeting for Advanced Spine Techniques. 2005.
24. Awasthi D. Juxtafusional outcomes with the dynamic posterior lumbar instrumentation. Rio de Janeiro, Brazil: World Spine III; 2005.
25. Grob D., Benini A., Junge A., et al. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: surgical and patient-orientated outcome in 50 cases after an average of 2 years. Spine. 2005;30:324-331.
26. Stoll T.M., Dubois G., Schwarzenbach O., et al. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11(Suppl 2):S170-S178.
27. Putzier M., Schneider S.V., Funk J.F., et al. The surgical treatment of the lumbar disc prolapse: nucleotomy with additional transpedicular dynamic stabilization versus nucleotomy alone. Spine. 2005;30:E109-E114.
28. Cakir B., Ulmar B., Koepp H., et al. Posterior dynamic stabalization as an alternative for dorso-ventral fusion in spinal stenosis with degenerative instability. Z Orthop Ihre Grenzgeh. 2003;141:418-424.
29. Karadimas K., Nicol M., Siddiqui M., et al. Dynesys stabilization system for the treatment of patients with discogenic low back pain. Spine J. 2005;5:S112.
30. Plev D., Sutcliffe J. Outcome and complications using a dynamic neutralization and stabilization pedicle screw system (Dynesys): is this a “soft fusion”? Spine J. 2005;5:141S.
31. Delamarier R., Maxwell J., Davis R., et al. Nonfusion application of the Dynesys system in the lumbar spine: early results. Spine J. 2006;6:S77.
32. Bono C.M., Vaccaro A.R. Interspinous process devices in the lumbar spine. J Spinal Disorders Tech. 2007;20:255-261.
33. Zucherman J.F., Hsu K.Y., Hartjen C.A., et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30:1351-1358.
34. Khoueir P., Kim K.A., Wang M.Y., et al. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22:E3.
35. Schafer M.F. Dwyer instrumentation of the spine. Orthop Clin North Am. 1978;9:115-122.
36. Winter R.B. Combined Dwyer and Harrington instrumentation and fusion in the treatment of selected patients with painful adult idiopathic scoliosis. Spine. 1978;3:135-141.
37. Dunn H.K. Anterior stabilization of thoracolumbar injuries. Clin Orthop Relat Res. 1984;Oct:116-124.
38. Ghanayem A.J., Zdeblick T.A. Anterior instrumentation in the management of thoracolumbar burst fractures. Clin Orthop Relat Res. 1997;Feb:89-100.
39. Ryan M.D., Taylor T.K., Sherwood A.A. Bolt-plate fixation for anterior spinal fusion. Clin Orthop Relat Res. 1986;Feb:196-202.
40. Yuan H.A., Mann K.A., Found E.M., et al. Early clinical experience with the Syracuse I-Plate: an anterior spinal fixation device. Spine. 1988;13:278-285.
41. Black R.C., Gardner V.O., Armstrong G.W., et al. A contoured anterior spinal fixation plate. Clin Orthop. 1988;227:135-142.
42. Bostman O., Myllynen P., Riska E.B. Posterior spinal fusion using internal fixation with the Daab plate. Acta Orthop Scand. 1984;55:310-314.
43. Holness R.O., Huestis W.S., Howes W.J., et al. Posterior stabilization with an interlaminar clamp in cervical injuries: technical note and review of the long term experience with the method. Neurosurgery. 1984;14:318-322.
44. Cybulski G.R., Stone J.L., Crowell R.M., et al. Use of Halifax interlaminar clamps for posterior C1-C2 arthrodesis. Neurosurgery. 1988;22:429-431.
45. Grob D., Criseo J.J.3rd, Panjabi M.M., et al. Biomechanical evaluation of four different posterior atlantoaxial fixation techniques. Spine. 1992;17:480-490.
46. Grob D., Jeanneret B., Aebi M., et al. Atlantoaxial fusion with transarticular screw fixation. J Bone Joint Surg (Br). 1991;73:972-976.
47. Haid R.W.Jr., Subach B.R., McLaughlin M.R., et al. C1-C2 transarticular screw fixation for atlantoaxial instability: a 6-year experience. Neurosurgery. 2001;49:65-68. discussion 69–70
48. Roy-Camille R., Saillant G., Mazel C. Internal fixation of the cervical spine by a posterior osteosynthesis with plates and screws. In: Sherk H., Dunn E., Eisment F., editors. The cervical spine. Philadelphia: JB Lippincott; 1989:390-403.
49. Magerl F., Seeman P. Stable posterior fusion of the atlas and axis by transarticular screw fixation. In: Kehr P., Weidner A., editors. The cervical spine. Wien: Springer Verlag; 1987:322.
50. Togawa D., Bauer T.W., Brantigan J.W., Lowery G.L. Bone graft incorporation in radiographically successful intervertebral body fusion cage. Spine. 2001;26:2744-2750.
51. An H.S., Gordin R., Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine. 1991;16:S548-S551.
52. Cooper P.R. The axis fixation system for posterior instrumentation of the cervical spine. Neurosurgery. 1996;39:612-614.
53. Vaccaro A.R., Balderston R.A. Anterior plate instrumentation for disorders of the subaxial cervical spine. Clin Orthop Relat Res. 1997;335:112-121.
54. Morscher E., Sutter F., Jenny H., et al. [Anterior plating of the cervical spine with the hollow screw-plate system of titanium]. Chirurg. 1986;57:702-707.
55. Spivak J.M., Chen D., Kummer F.J. The effect of locking fixation screws on the stability of anterior cervical plating. Spine. 1999;24:334-338.
56. Mayr M.T., Subach B.R., Comey C.H., et al. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96:10-16.
57. Schukz K.D.Jr., McLaughlin M.R., Haid R.W.Jr., et al. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93:214-221.
58. Lowery G.L., McDonough R.F. The significance of hardware failure in anterior cervical plate fixation: patients with 2- to 7-year follow-up. Spine. 1998;23:181-186. discussion 186–187
59. Epstein N. Anterior approaches to cervical spondylosis and ossification of the posterior longitudinal ligament: review of operative technique and assessment of 65 multilevel circumferential procedures. Surg Neurol. 2001;55:313-324.
60. Rhee J.M., Riew K.D. Dynamic anterior cervical plates. J Am Acad Orthop. 2007;15:640-646.
61. Barbour J. Screw fixation in fracture of the odontoid process. South Anst Clin. 1971;5:20.
62. Bome G.M., Bedou G.L., Pinaudeau M., et al. Odontoid process fracture osteosynthesis with a direct screw fixation technique in nine consecutive cases. J Neurosurg. 1988;68:223-226.
63. El Saghir H., Bohm H. Anderson type H fracture of the odontoid process: results of anterior screw fixation. J Spinal Disord. 2000;13:527-530.
64. Apfelbaum R., Lonser R., Veres R., Casey A. Direct anterior screw fixation for recent and remote odontoid fractures. J Neurosurg. 2000;93:227-236.
65. Arand M., Lemke M., Kinzl L., et al. Incidence of complications of the screw osteosynthesis of odontoid process fractures. Zentralbl Chir. 2001;126:610-615.
66. Fuji T., Oda T., Kato Y., et al. Accuracy of atlantoaxial transarticular screw insertion. Spine. 2000;25:1760-1764.
67. Denaro V., Papalia R., Denaro L., et al. Cervical spinal disc replacement. J Bone Joint Surg (Br). 2009;91:713-719.
68. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous cervical arthrodesis. J Bone Joint Surg (Am). 1999;81:519-528.
69. Goffin J., Casey A., Kohr P., et al. Preliminary clinical experience with the Bryan cervical disc prosthesis. Neurosurgery. 2002;51:840-845.
70. Goffin J., Van Calenbergh F., van Loon J., et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan cervical disc prosthesis: single-level and bi-level. Spine. 2003;28:2673-2678.
71. Pickekett G.E., Mitsis D.K., Sekhon L.H., et al. Effects of cervical disc prosthesis on segmental and cervical spine alignment. Neurosurg Focus. 2004;17:E5.
72. Corrie D., Finger F., Boltes P., et al. Prospective randomized controlled study of the Bryan cervical disc: early clinical results from a single investigational site. J Neurosurg Spine. 2006;4:31-35.
73. Sasso R.C., Smuoker J.D., Haoker R.J., et al. Artificial disc versus fusion: a prospective randomized study with 2-year follow-up on 99 patients. Spine. 2007;32:2933-2940.
74. Mummaneni P.V., Burkus J.K., Haid R.W., et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled trial. J Neurosurg Spine. 2007;6:198-209.
75. Schwender J.D., Holly L.T., Rouben D.P., et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1-S6.
76. DeBowes R.M., Grant B.D., Bagby G.W., et al. Cervical vertebral interbody fusion in the horse: a comparative study of bovine xenografts and autografts supported by stainless steel baskets. Am J Vet Res. 1984;45:191-199.
77. Bagby G.W. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931-934.
78. Kuslich S.D., Ulstrom C.L., Griffith S.L., et al. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine. 1998;23:1267-1278. discussion 1279
79. Brantigan J.W., Steffee A.D., Geiger J.M. A carbon fiber implant to aid interbody lumbar fusion. Mechanical testing. Spine. 1991;16:S277-S282.
80. Matge G. Anterior interbody fusion with the BAK-cage in cervical spondylosis. Acta Neurochir. 1998;140:1-8.
81. Frelinghuysen P., Huang R.C., Girardi F.P., et al. Lumbar total disc replacement. Part I: rationale, biomechanics, implant types. Orthop Clin North Am. 2005;36:293-299.
82. Ozgur B.M., Aryan H.E., Pimenta L., et al. Extreme lateral interbody fusion (XLIF): a novel technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
83. Anderson G.D., Samartzis D., Shen F.H., et al. Percutaneous instrumentation of the thoracic and lumbar spine. Orthop Clin North Am. 2007;38:401-408.
84. Denaro L., Longo U.G., Denaro V., Vertebroplasty and kyphoplasty. reasons for concern? Orthop Clin North Am. 2009;40(4):465-471.
85. Eichholz K.M., O’Toole J.E., Christie S.D., et al. Vertebroplasty and kyphoplasty. Neurosurg Clin North Am. 2006;17(4):507-518.
86. Lonner B.S., Dimitry K., Farhan S., et al. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg (Am). 2006;88:1022-1034.
87. Reddi A., Clarke D.V., Arlet V., et al. Anterior thoracoscopic instrumentation in adolescent idiopathic scoliosis. A systematic review. Spine. 2008;33:1986-1994.