Historical Concepts of Ocular Surface Disease
Ocular Surface Disease: Advances in Diagnosis & Medical Management
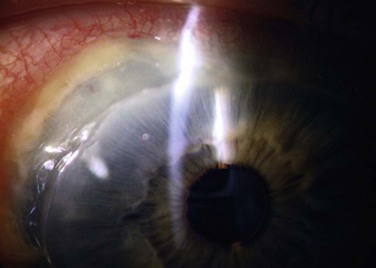
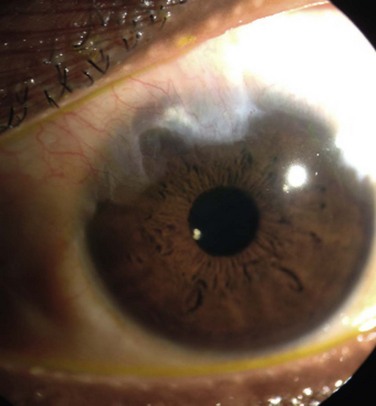
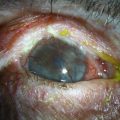
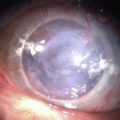
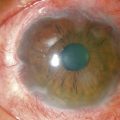
Disorders of the ocular surface include a variety of conditions. Some of the more common conditions encountered in practice include dry eye disease, blepharitis, ocular allergies and pterygia. In addition, less common but more challenging conditions include limbal stem cell deficiency, and ocular surface disease (OSD) from systemic disease (Fig. 1.1). As our understanding of OSD has expanded, the availability of advanced diagnostic tools, medical and surgical therapeutic options, and treatment algorithms for various conditions has enhanced success with OSD. There are classic diagnostic tools for diagnosis of OSD, such as impression cytology, Schirmer testing, tear break-up time, and vital dye staining of the cornea and conjunctiva. These remain valuable tools, however, new diagnostic devices have emerged (Fig. 1.2). Devices, such as tear osmolarity analysis, matrix metalloproteinase-9 analysis, rapid antigen detection for various ocular infectious diseases, and comprehensive analysis of the tear film and lipid are just some of the new diagnostic devices available. Additional advanced diagnostic tools include confocal microscopy, optical coherence tomography (OCT) of the anterior segment, and Scheimpflug imaging of the cornea for advanced diagnosis of various OSD states.1,2 Confocal microscopy enables a detailed investigation of the tarsal and palpebral conjunctiva, central and peripheral cornea, tear film, and eyelids, while affording evaluation of the ocular surface at the cellular level. The device has been particularly useful as a diagnostic tool for cases of atypical keratitis and as a tool to detect phenotypic alterations of the conjunctival epithelium in dry eye disease.1–3
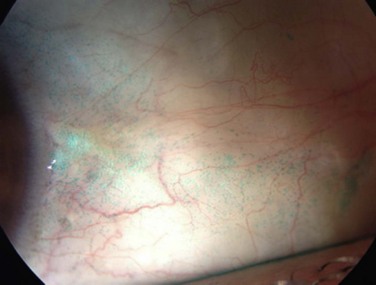
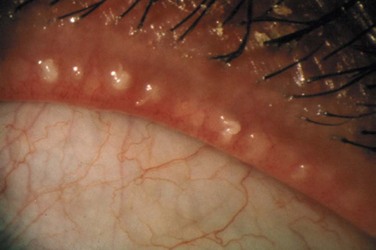
Two of the most common OSD challenges remain dry eye disease and blepharitis. Our knowledge of both of these conditions has expanded over the last few decades with both clinical and basic science research to support the key role of inflammation as a major factor in the development of symptoms and clinical findings of these diseases. The combination of factors leading to dry eye states, often referred to as ‘dysfunctional tear syndrome,’ refers to the compilation of lid margin disease, altered tear film composition, decreased tear volume, diminished corneal sensation, and the presence of anti-inflammatory factors in the tear film.4 The International Dry Eye Workshop (DEWS) included a panel of international ocular surface disease experts challenged to update and review new concepts of dry eye disease. The group developed current concepts of dry eye disease including definition and classification, diagnosis, epidemiology, treatment and management, and research. A fundamental change in our understanding of dry eye is evident in its current definition: ‘Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.’4 DEWS provided levels of disease severity with regard to symptoms and signs of dry eye followed by evidence and consensus-based treatment recommendations for dry eye treatment based on new research linking dry eye disease to inflammation.4 Similarly, the Meibomian Gland Workshop involved a panel of international experts challenged to expand our understanding of meibomian gland disease (MGD) (Fig, 1.3). The group developed a contemporary definition and classification of MGD, reviewed methods of diagnosis and evaluation, developed recommendations for the management and therapy of MGD, and presented recommendations for study designs and future research in MGD.5 The treatment recommendations from these workshops have afforded a better understanding of the underlying pathology of dry eye disease, dysfunctional tear syndrome and blepharitis.
With expanded diagnostic tools and a better understanding of the pathophysiology of various forms of OSD, we have seen an explosion of new therapeutic strategies from novel medication classes to new therapeutic devices. In the past, treatment options for various conditions, such as dry eye disease were limited to environmental modifications, artificial tears, and punctal plugs. Current medical treatment advances for OSD include new topical and oral therapies for allergic eye disease, limbal stem cell deficiency, and dysfunctional tear syndrome. Topical nonsteroidal anti-inflammatory agents, cyclosporine A, mast cell stabilizer/antihistamine agents, and various new formulations of corticosteroids can aid in difficult inflammatory eye conditions, such as severe atopic keratoconjunctivitis and dysfunctional tear syndrome. Medical management of limbal stem cell deficiency includes therapeutic agents from topical vitamin A formulations to autologous serum, various topical growth factors, oral omega 3 fatty acid supplementation, and topical vascular endothelial growth factor (VEGF) inhibitors to counteract corneal neovascularization. In addition, new therapeutic devices, such as meibomian gland probing, intense pulse light therapy, and LipiFlow® can be additive to topical and oral medication regimens for relief of signs and symptoms of various types of OSD.5
Origins of the Surgical Management of Severe Ocular Surface Disease
An early concept for the surgical treatment of ocular surface disease (OSD) appeared in 1940 with use of amniotic membrane for the repair of conjunctival defects and symblepharon by De Rotth.6 In 1951, Hartman suggested the use of a free conjunctival graft for correction of pterygium, pseudopterygium, and symblepharon.7 This report suggested the benefit of using conjunctiva for grafting procedures and introduced the notion of harvesting conjunctiva from the contralateral eye in selected cases for the surgical treatment of unilateral disease.7 While Jose Barraquer is credited as the first surgeon to describe stem cell transplant techniques in ocular surface chemical burns,8 Thoft’s description of conjunctival transplantation for monocular chemical burns stands as the basis for the contemporary understanding of ocular surface disease and its treatment.9 Thoft employed autologous ‘conjunctival transplantation’ for the treatment of five cases involving unilateral chemical burns of the cornea. The technique required a complete lamellar keratectomy with removal of the epithelium and pannus formation on the corneal surface followed by 360 degrees of limbal conjunctival resection. Four conjunctival grafts were next harvested from the four bulbar conjunctival quadrants in the uninvolved eye, and each graft was fixated to an analogous quadrant of the diseased eye and secured with sutures.9 The autologous conjunctival graft has stood the test of time and remains the procedure of choice for unilateral stem cell disease as well as contemporary pterygium surgery.
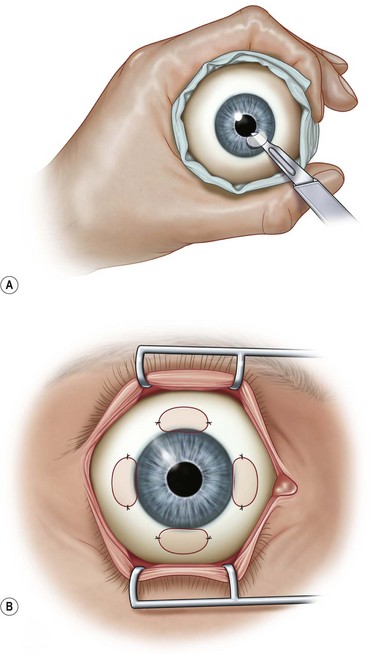
Thoft later described the first allograft procedure, which he termed ‘keratoepithelioplasty,’ in patients with bilateral OSD. This procedure laid the groundwork for contemporary limbal stem cell transplantation techniques (Fig. 1.4).10 Keratoepithelioplasty employed four lenticules which included epithelium and a thin layer of stroma harvested from the peripheral cornea of a donor globe. Each lenticule was secured at the corneoscleral limbus of the surface-damaged eye in each of the four quadrants.10 While keratoepithelioplasty was the first attempt at transplantation of corneal epithelial stem cells in patients with severe bilateral OSD, neither the origin and location of the corneal limbal stem cells nor their functional physiology were clearly understood at that time.
Corneal Stem Cell Theory and Early Clinical Applications
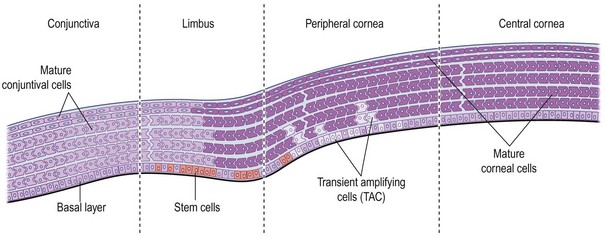
Corneal epithelial stem cells are the progenitor cells and the source of epithelial regeneration after demise or loss of the corneal epithelium. Throughout the body, adult stem cells are found in limited numbers with long life spans, slow cell cycling capabilities, and less differentiation.11–15 Despite these characteristics, they do possess the ability to regenerate and repair tissue after injury. Upon activation, stem cells produce progeny, referred to as ‘transient amplifying cells’ that are responsible for proliferation, differentiation and migration in response to normal physiologic renewal or repair after injury. Daughter cells, in contrast, have short life spans, rapid cell cycling, and high mitotic activity. After epithelial injury, transient amplifying cells migrate centripetally from the limbus and vertically from the basal epithelial layers forward to promote epithelial renewal.15–19 This process of epithelial cell migration is critical in maintenance of the corneal epithelial mass and its ability to regenerate after injury. The limbus serves as a functional ‘barrier,’ preventing encroachment of the conjunctival epithelium onto the cornea during normal homeostasis.19 When this barrier function is impaired, conjunctival epithelium together with blood vessels and fibrous tissue encroach onto the cornea (Fig. 1.5). Loss of this barrier function is one of the first signs in corneal epithelial stem cell deficiency and may result in significant abnormality of the ocular surface.
While several surgical advancements had been made in the treatment of OSD in the late twentieth century, the pivotal breakthrough occurred with the understanding of the anatomic location and function of the limbal stem cells. Our knowledge of corneal epithelial stem cell location and function is relatively new, having been elaborated over the last three decades. One of the most important initial observations of stem cell presence and function was the observation by Friedenwald that the corneal epithelium regenerated fully after total de-epithelialization.20 In the 1970s and 1980s, researchers determined that the palisades of Vogt were the location of corneal epithelial stem cells.21,22 While additional research supported the palisades of Vogt as the anatomic location of corneal epithelial stem cells, several studies have co-located these stem cells in the limbal basal epithelium by identification of cornea-specific keratins (Fig. 1.6).23–26 Other laboratories provided evidence that stem cells reside at the limbus using tritiated thymidine incorporation into limbal basal cells, demonstrating higher rates of mitotic activity, as well a senhanced cell culture growth from limbal basal epithelium.27,28 Moreover, other studies demonstrated that limbal stem cells are less differentiated than epithelial cells found elsewhere in the cornea and that stem cells, as well as transient amplifying cells (TAC), constitute the proliferating cells of the epithelium that are responsible for repair after injury.29,30
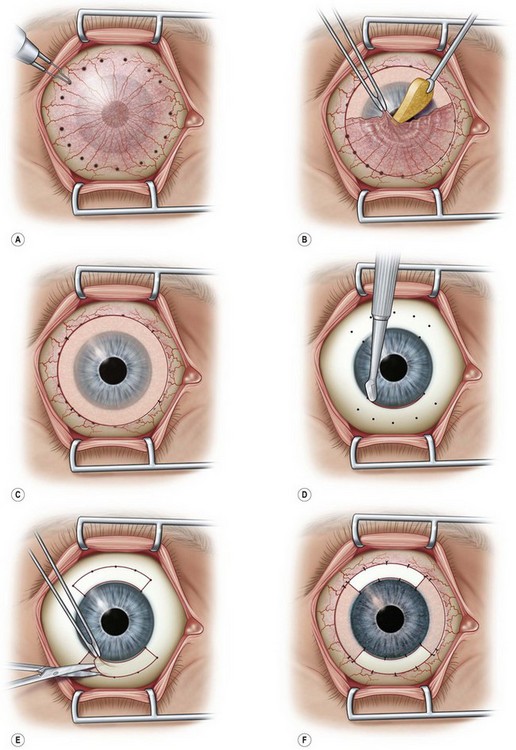
With clarification of the location and function of corneal stem cells, Kenyon and Tseng31 were the first to provide clinical translational applications of stem cell theory. In 1989, they modified Thoft’s original procedure to include limbal stem cells in the conjunctival transplantation procedure. This represented the first programmatic clinical use of transplanted limbal stem cells for severe OSD and represents the initiation of true stem cell autografting techniques (Fig. 1.7).31
In 1994, Tsai and Tseng32 modified Thoft’s keratoepithelioplasty technique and called it ‘allograft limbal transplantation,’ using a donor whole globe to provide a keratolimbal graft for the treatment of severe OSD. The cadaveric keratolimbal ring was divided into three equal pieces and was transferred to the recipient eye. The authors employed oral cyclosporine in additional to topical immunosuppression for postoperative treatment. This represented the first keratolimbal allograft (KLAL) with adjunct systemic immunosuppression in limbal stem cell transplant for treatment of severe OSD. Tsubota and colleagues33 further modified the KLAL procedure and were the first to report use of stored corneoscleral rims for stem cell transplantation in OSD. The concept of stored tissue for ocular surface reconstruction engendered new considerations in eye banking that established the groundwork for modified procedures in tissue procurement and delivery for transplant.
Kwitko and colleagues34 developed the concept of using living-related ocular tissue as allografts for the treatment of bilateral OSD in 1995. They described a technique referred to as ‘allograft conjunctival transplantation’ in which harvested conjunctival tissue (not limbal tissue) was obtained from siblings or a parent and transplanted to the recipient eye of the affected relative. Kenyon and Rapoza35 expanded this concept to include conjunctival and limbal tissue in a technique similar to Kenyon’s earlier report of limbal autografting. However, their procedure utilized donor tissue from a living relative rather than the contralateral eye. This technique formed the basis for using living-related limbal tissue for transplantation to a relative with bilateral severe OSD, in which the contralateral eye cannot be used for limbal autografting techniques. Topical and systemic immunosuppression were employed as adjuncts in all of the living-related allograft cases.35
Ocular Surface Disease: Contemporary Advances in Surgical Management
A major landmark in the surgical treatment of OSD occurred with the development of a uniform classification system to describe the variety of proposed surgical techniques for restoration of the ocular surface. Holland and colleagues developed a nomenclature that included a standardization of surgical techniques based on the donor and the tissue transplanted with corresponding acronyms. In addition, the nomenclature was linked to treatment algorithms for the implementation of specific techniques based on the severity and laterality of OSD.36–39 Moreover, in conjunction with corneal surgeons interested in ocular surface disease, the eye banking system developed eye banking criteria and the establishment of procurement and tissue processing regimens specific to the delivery of corneoscleral limbal tissue to surgeons treating OSD.37 Further advances in eye banking protocols for the harvesting and delivery of limbal tissue for transplantation followed the development of surgical treatment classifications for OSD.
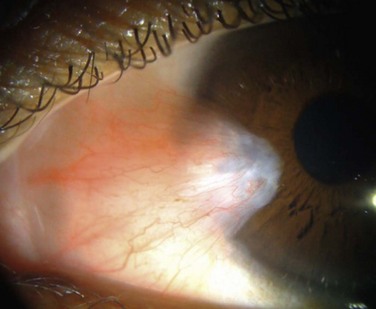
Pterygium surgery represents one of the most common examples of an OSD that requires surgical intervention for a cure (Fig. 1.8). This is hardly surprising given the relatively recent understanding of its pathophysiology that demonstrates a localized stem cell dysfunction in combination with genetic factors and inflammation play a key role in its development. A recent review of the surgical treatment of pterygia demonstrated a wide variety of surgical approaches exist, owing to the difficulty in curing this condition.40 The review recommendations reported that the bare sclera excision of pterygium results in a significantly higher recurrence rate than excision accompanied by use of certain adjuvants. Additional adjuvants utilized in pterygium surgery include amniotic membrane, conjunctival autografts, fibrin glue for graft adherence, and antifibrotic agents, such as mitomycin C. Conjunctival or limbal autograft was superior to amniotic membrane graft surgery in reducing the rate of pterygium recurrence in the review of adjunvants.40 Advanced surgical techniques corroborate the findings of the review, suggesting conjunctival or limbal autografts are associated with very low recurrence rates.41
In conditions with more diffuse OSD or limbal stem cell deficiency, KLAL modifications have improved surgical outcomes and ultimate success in the surgical treatment of severe OSD. Croasdale and Holland37,38 expanded on the KLAL technique of Tsubota by employing two stored corneoscleral rims rather than one. The two rims were each bisected, creating four harvested 180-degree crescents of limbal tissue. Three of the four pieces of cadaveric tissue were transplanted to the recipient eye. This technique allowed for complete coverage of the recipient limbus by donor tissue and delivered one-and-a-half times the transplanted limbal stem cells than could be derived from a single corneoscleral limbal rim.36,37
Another modification to the KLAL procedure was developed for patients with severe conjunctival deficiency in conditions, such as Stevens–Johnson syndrome or ocular cicatricial pemphigoid.42 The technique has been referred to as the ‘Cincinnati procedure’ and employs the use of living-related conjunctival and limbal tissue harvested from a sibling or parent. The allograft tissue (lr-CLAL) is applied to the surface deficient eye of the recipient/relative in the superior and inferior four hours after epithelial debridement and a 360-degree conjunctival peritomy. Following this, a cadaveric KLAL is applied to the nasal and temporal limbus of the diseased eye with a technique similar to that described by Croasdale et al.37 (with the exception of using a single donor corneoscleral rim), making sure to avoid any gap areas in donor tissue at the recipient limbus.42
Another significant advance in ocular surface transplantation has been the development of techniques for ex vivo expansion of autologous or living-related stem cells. While the idea of cultured corneal epithelial stem cells was considered as early as 1982,43 the first clinical reports of cultured autologous limbal stem cell transplantation did not appear until 1996 and 1997.44,45 Torfi and Schwab first reported success with cultured autologous grafts delivered to the damaged eye and demonstrated improvement in ocular surface function in three of four patients with severe unilateral disease.44 Similarly, Pellegrini and colleagues described ocular surface restoration in two patients with severe unilateral stem cell deficiency using autologous cultured corneal epithelial stem cells expanded in the laboratory and delivered to the diseased eye as a cultivated corneal epithelial sheet attached to a therapeutic bandage lens.45 Both groups confirmed that a small 1–2-mm2 limbal biopsy provides sufficient amounts of cultured corneal epithelial cells to restore the entire corneal–limbal surface after expansion in culture.44,45 Techniques of ex vivo expansion of both autologous and living-related stem cells continue to evolve, with successful ex vivo expansion of limbal stem cells for grafting.45–49
A critical concept that has evolved in ocular stem cell transplantation is the use of adjunct immunosuppression. Immunosuppression has been employed to enhance the outcomes of ocular surface transplantation including the use of both topical as well as oral immunosuppressive agents. Holland and colleagues50 have stressed the importance of approaching systemic immunosuppression in ocular surface transplantation in a fashion similar to solid organ transplantation. In addition, these authors have demonstrated the safety and efficacy of immunosuppression in ocular surface patients.50 Studies have demonstrated that ocular surface transplantation in the absence of systemic immunosuppression leads to high failure rates when compared with procedures accompanied by systemic immunosuppression.38,51,52
Amniotic membrane transplantation (AMT) has been a useful adjunct to ocular surface transplantation when used in conjunction with limbal stem cell transplant. AMT can provide a scaffold for amplification and delivery of stem cells in ex vivo expansion techniques. While AMT is not used alone in conditions of limbal stem cell deficiency, several studies have shown that it can facilitate epithelial growth and reduce ocular surface inflammation when used in conjunction with other techniques, such as KLAL or ex vivo expansion of stem cells.53,54
Just as there have been both advancements in disease classification and a proliferation of new surgical techniques for OSD, immunosuppressive therapy has advanced in parallel. Earlier, adjunct immunosuppressive therapy typically included the use of oral cyclosporine and corticosteroids. Treatment models for immunosuppression have expanded with the development of new systemic anti-inflammatory agents and new classes of immunosuppressive agents, which will be elaborated upon in later chapters. Medication classes, such as immunophilin binders and antimetabolites include agents with decreased systemic side effects. In addition, we have seen the emergence of new drug classes with potent systemic immunosuppressive effects, such as polyclonal and monoclonal antibodies. Topical cyclosporine has been another useful adjunct for postoperative treatment after ocular surface transplantation. Immunosuppressive drugs are now typically combined with topical corticosteroids and topical cyclosporine following limbal stem cell transplantation. This is most effectively accomplished with a multi-disciplinary team approach involving the ocular surface specialist, internal medicine and transplant services for the monitoring of graft success and potential medication-induced local and systemic side effects.50
The next advances in ocular surface transplantation will involve the continued development, standardization, and enhancement of ex vivo stem cell expansion techniques for treatment of OSD. A number of materials have been employed as stem cell carriers for ex vivo expansion techniques ranging from collagen and de-epithelialized amniotic membrane to therapeutic soft contact lenses, fibrin gel, oral mucosal cells and silk fibroin.44–49,55–57 No ‘gold standard’ has been developed to date. Investigators are exploring additional sources of stem cells, including stem cells from hair follicles, embryonic stem cells, conjunctival epithelial stem cells, dental pulp, umbilical cord lining, and bone marrow-derived mesenchymal stem cells.58 Despite these advances, a multitude of challenges with ex vivo stem cell expansion persist. These challenges include the development of the ideal carrier for stem cells from the laboratory to the diseased ocular surface, the lack of a definite limbal epithelial stem cell marker to monitor graft quality and the likelihood of a successful expansion and transplantation, and methods of assessment of cultured stem cell therapy in limbal stem cell deficiency without a known marker. Regardless of the challenges, several reports cite improved outcomes for treatment of limbal stem cell deficiency, including a recent meta-analysis performed by Baylis et al.59 which included the outcomes of cultured limbal epithelial cell therapy published since 1997 (583 patients). The overall success rate of cultured ex vivo expanded stem cell transplantation at the time of the review was 76%.59 Individual centers have also reported success using cultivated oral mucosal epithelial transplantation to deliver autologous stem cells for the treatment of severe OSD with successfully restored ocular surfaces in patients as long as 35 months after surgery.56–58 Rama et al.60 have reported outcomes in 112 patients with corneal damage due to limbal stem cell deficiency who underwent autologous cultivated stem cell transplants using a fibrin carrier, the largest series of patients to date. The study observed permanent restoration of the ocular surface in 77% of patients undergoing autologous cultivated (ex vivo expanded) stem cell transplantation, with the majority of OSD cases resulting from chemical ocular surface burns.60
References
1. Tu, EY, Joslin, CE, Sugar, J, et al. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea. 2008;27:764–772.
2. Lee, WB, Gotay, A. Bilateral Acanthamoeba keratitis in synergeyes contact lens wear: clinical and confocal microscopy findings. Eye & Contact Lens. 2010;36:164–169.
3. Kieval, JZ, Karp, CL, Abou Shousha, M, et al. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology. 2012;119:481–486.
4. Pflugfelder, S. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). (committee chairman). Ocul Surf. 2007;5:163–178.
5. Geerling, G, Tauber, J, Baudouin, C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2050–2064.
6. De Rotth, A. Plastic repair of conjunctival defects with fetal membrane. Arch Ophthalmol. 1940;23:522–525.
7. Hartman, DC. Use of free grafts in correction of recurrent pterygia, pseudopterygia and symblepharon. California Med. 1951;75:279–280.
8. Holland, EJ, Schwartz, GS. The Paton Lecture: ocular surface transplantation: 10 year’s experience. Cornea. 2004;23:425–431.
9. Albert, DM, Miller, JW, Azar, DT, et al. Albert & Jakobiec’s Principles and practice of ophthalmology. Saunders. 2008:871–880. [Figure 65.4].
10. Thoft, RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97:1–6.
11. Lajtha, LG. Stem cell concepts. Differentiation. 1979;14:23–34.
12. Leblond, CP. The life history of cells in renewing systems. Am J Anat. 1981;160:114–158.
13. Tseng, SCG. Concept and application of limbal stem cells. Eye. 1989;3:141–157.
14. Kinoshita, S, Adachi, W, Sotozono, C, et al. Characteristics of the human ocular surface epithelium. Prog Ret Eye Res. 2001;20:639–673.
15. Dua, HS, Azuara-Blanco, A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425.
16. Lehrer, MS, Sun, TT, Lavker, RM. Strategies of epithelial repair: modulation of stem cell and transient amplifying cell proliferation. J Cell Sci. 1998;111:2867–2875.
17. Thoft, RA, Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443.
18. Dua, HS, Gomes, JA, Singh, A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78:401–408.
19. Dua, HS, Miri, A, Alomar, T, et al. The role of the limbal stem cells in corneal epithelial maintenance. Ophthalmology. 2009;116:856–863.
20. Friedenwald, JS. Growth pressure and metaplasia of conjunctival and corneal epithelium. Doc Ophthalmol. 1951;5:184–192.
21. Davanger, M, Evensen, A. Role of the periocorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561.
22. Goldberg, MF, Bron, AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–171.
23. Schermer, A, Galvin, S, Sun, TT. Differentiation-related expression of major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62.
24. Cotsarelis, G, Cheng, SZ, Dong, G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209.
25. Kasper, M, Moll, R, Stosiec, P, et al. Patterns of cytokeratin and vimentin expression in the human cycle. Histochemistry. 1988;89:369–373.
26. Pellegrini, G, Golisano, O, Paterna, P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782.
27. Cotsarelis, G, Dong, G, Sun, TT, et al. Differential response of limbal and corneal epithelia to phorbol myristate acetate (TPA). ARVO Abstracts. Invest Ophthalmol Vis Sci. 1987;28(suppl.):1.
28. Ebato, B, Friend, J, Thoft, RA. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1988;29:1533–1537.
29. Lathja, LG. Stem cell concepts. Differentiation. 1979;14:23–34.
30. Kinoshita, S, Friend, J, Thoft, RA. Biphasic cell proliferation in trans-differentiation of conjunctival to corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1983;24:1008–1014.
31. Kenyon, KR, Tseng, SCG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–723.
32. Tsai, RJF, Tseng, SCG. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389–400.
33. Tsubota, K, Toda, I, Saito, H, et al. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486–1495.
34. Kwitko, S, Raminho, D, Barcaro, S, et al. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995;102:1020–1025.
35. Kenyon, KR, Rapoza, PA. Limbal allograft transplantation for ocular surface disorders. Ophthalmology. 1995;102:101–102.
36. Holland, EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;19:677–743.
37. Croasdale, CR, Schwartz, GS, Malling, JV, et al. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52–58.
38. Holland, EJ, Schwartz, GS. Changing concepts in the management of severe ocular surface disease over twenty-five years. Cornea. 2000;19:688–698.
39. Daya, SM, Chan, CC, Holland, EJ, et al. Cornea Society nomenclature for ocular surface rehabilitative procedures. Cornea. 2011;30:1115–1119.
40. Kaufman, SC, Jacobs, DS, Lee, WB, et al. Options and adjuvants in surgery for pterygium. Ophthalmology. 2013;120:201–208.
41. Hirst, LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008;115:1663–1672.
42. Biber, JM, Skeens, HM, Neff, KD, et al. The Cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30:765–771.
43. Friend, J, Kinoshita, S, Thoft, RA, et al. Corneal epithelial cell cultures on stroma carriers. Invest Ophthalmol Vis Sci. 1982;23:41–49.
44. Torfi, H, Schwab, IR, Isseroff, R. Transplantation of cultured autologous limbal stem cells for ocular surface disease (abstract). In Vitro. 1996;32:47A.
45. Pellegrini, G, Traverso, CE, Franzi, AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993.
46. Schwab, IR, Reyes, M, Isseroff, RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426.
47. Shimazaki, J, Aiba, M, Goto, E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290.
48. Han, B, Schwab, IR, Madsen, TK, et al. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea. 2002;21:505–510.
49. Sangwan, VS, Matalia, HP, Vemuganti, GK, et al. Early results of penetrating keratoplasty after cultivated limbal epithelium transplantation. Arch Ophthalmol. 2005;123:334–340.
50. Holland, EJ, Mogilishetty, G, Skeens, HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31:655–661.
51. Rao, SK, Rajagopal, R, Sitalakshmi, G, et al. Limbal allografting from related live donors for corneal surface reconstruction. Ophthalmology. 1999;106:822–828.
52. Daya, SM. Living-related conjunctivo-limbal allograft (lr-CLAL) for the treatment of stem cell deficiency: an analysis for long-term outcomes. Ophthalmology. 1999;106(suppl.):243.
53. Tsubota, K, Satake, Y, Ohyama, M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens–Johnson syndrome. Am J Ophthalmol. 1996;122:38–52.
54. Tseng, SC, Prabhasawat, P, Barton, K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441.
55. Harkin, DG, George, KA, Madden, PW, et al. Silk fibroin in ocular tissue reconstruction. Biomaterials. 2011;32:2445–2458.
56. Nishida, K, Yamato, M, Hayashida, Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196.
57. Nakamura, T, Inatomi, T, Sotozono, C, et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–1284.
58. O’Callaghan, AR, Daniels, JT. Concise review: limbal epithelial stem cell therapy: controversies and challenges. Stem Cells. 2011;29:1923–1932.
59. Baylis, O, Figueiredo, F, Henein, C, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112:993–1002.
60. Rama, P, Matuska, S, Paganoni, et al. Limbal stem cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155.