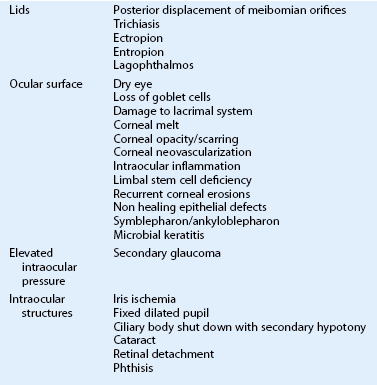
Chemical and Thermal Injuries to the Ocular Surface
Introduction
Chemical or thermal injury to the eye constitutes an ophthalmic emergency, due to the potential for permanent visual impairment and the threat to the structural integrity of the eye. The prognosis for severe injury is typically poor and may result in widespread damage to the ocular surface epithelium, cornea and anterior segment.2 However, in recent years, the prognosis of severe ocular burns has improved, with advances in the understanding of the physiology of the cornea and the resultant development of enhanced medical and surgical treatments. The final visual prognosis is influenced by the nature of the chemical insult, the extent of ocular damage, and the timing and efficacy of treatment.
Epidemiology
Chemical or thermal burns represent 7.7% to 18% of cases of ocular trauma.1,3 Responsible chemicals are numerous and include cleaning agents, fertilizers, refrigerants, cement, preservatives and fireworks.2 Alkali injuries occur more frequently than acid injuries, as a result of their more ubiquitous presence in household and industrial products.2 Ocular burns caused by detergents and thermal agents are less common.2,4,5
Fortunately, the majority of chemical injuries are classified as mild2,5,6 and the estimated incidence of severe chemical injuries in the United Kingdom is approximately 0.02 per 100 000. Injured parties are characteristically young and male5–8 and exposure most commonly occurs in a variety of agricultural, industrial and domestic settings, or less commonly in association with a criminal assault.2,5,8 Unfortunately, studies report an increasing number of patients presenting with chemical eye injuries resulting from assault.6
Ocular Chemical Injury
Etiology of Chemical Injury: Causative Agents
Alkalis
More than 25,000 chemical products with the potential to cause chemical eye injuries have been identified, many of which may be classified as acids or bases, oxidizing or reducing agents, or corrosives. The most frequently implicated chemical agents are acids and bases. The severity of the injury is related to the nature, concentration, quantity and pH of the chemical involved, and the duration and surface area of exposure.3 In particular, a history of a high-velocity (explosive) chemical or thermal injury should always raise suspicion of an associated intraocular foreign body.
Wet and dry cement, ammonia, lye, potassium hydroxide, magnesium hydroxide, and lime, constitute the most common causes of alkali injury to the eye.2,5,8 The severity of an alkali injury is governed by the pH, rather than the properties of the cation.2 Therefore, the most severe injuries are typically caused by ammonia and lye8 which are both capable of rapid penetration into the eye. The damage inflicted by lime injuries is reduced by the formation of calcium soaps that precipitate and hinder further penetration.2 Firework injuries deserve special mention as the presence of magnesium hydroxide results in a combined chemical and thermal injury. The most important agents causing alkali and acid injuries to the eye are summarized in Table 29.1.
Table 29.1
Common Causes of Alkali and Acid Injuries2
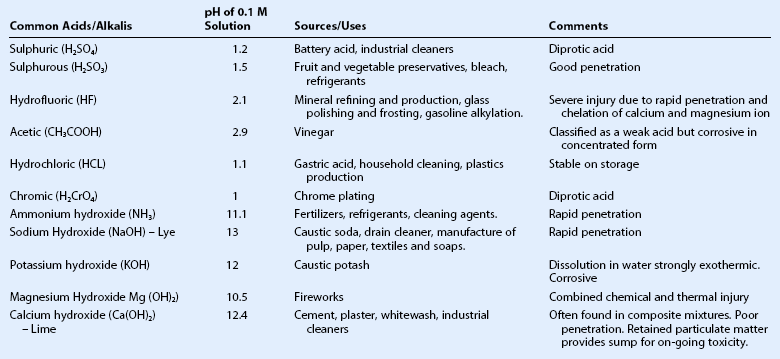
(Adapted from Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol 1997;41:275–313.)
Acids
The most common causes of acid burns are sulphuric, sulphurous, hydrofluoric, acetic, chromic, and hydrochloric acids.2 The strength of an acid depends on its ability to lose a proton; strong acids ionize completely in an aqueous solution. However, the most severe acid injuries are caused by hydrofluoric acid, as a result of its unique properties. In addition to the action of the dissociated proton, which is the primary mechanism of damage by other acids, hydrofluoric acid has a unique dissolving action which allows it to quickly penetrate into deeper tissues.2 Moreover, hydrofluoric acid chelates all calcium and magnesium from cells, thereby halting cellular biochemical activity.
Although alkalis typically cause the most serious chemical injuries, the presence of an acid injury does not preclude an equally devastating ocular injury as very strong acids penetrate just as rapidly as alkalis. Indeed, studies have shown no clinically significant differences in clinical course and prognosis between severe acid and alkali burns.9
Pathophysiology
Structural and Biochemical Alterations
The severity of an ocular chemical injury is influenced by the ability of the chemical to penetrate the eye. Alkalis characteristically penetrate the eye more rapidly than acids.2,3,10 The hydroxyl ion (OH) saponifies plasma membranes, resulting in cell disruption and death, while the cation is responsible for the penetration of the specific alkali.2,3,10 Stronger alkalis are associated with more rapid penetration and the penetration rate increases in ascending order from calcium hydroxide, potassium hydroxide, sodium hydroxide to ammonium hydroxide.10 Changes in aqueous humor pH are observed within a few seconds of contact with ammonium hydroxide, and within 3–5 minutes after sodium hydroxide injury.10,11 Ultimately, irreversible tissue damage occurs when the pH rises above 11.5.2,10
In alkali injuries, cations react with the carboxyl (COOH) groups of stromal collagen and glycosaminoglycans.2,10 Hydration of glycosaminoglycans results in loss of clarity of the stroma, whereas, hydration of collagen fibrils causes distortion of the trabecular meshwork and the release of prostaglandins, these sequelae combine to produce elevations in intraocular pressure.10
In general, as previously noted, acids penetrate the corneal stroma much less readily than alkalis.2,3,10,12 The hydrogen ion mediates damage due to pH alteration, while the anion causes precipitation and denaturation of proteins in the corneal epithelium and anterior stroma.10 Precipitation of the epithelial proteins affords a degree of protection by providing a physical barrier against further ingress.2,3,10,12 However, in the event that an acid succeeds in penetrating the stroma, the damage to ocular structures is similar to that observed in alkali injury.2 Alterations include precipitation of extracellular glycosaminoglycans and corneal opacification, distortion of the trabecular meshwork, changes in anterior chamber pH, damage to anterior chamber structures, and reduced aqueous ascorbate levels.2,10 Vascular damage results in ischemic injury.
Both acids and bases may mediate osmolar damage to the delicate physiology of the cornea.2 Chemical insults to the eye may initiate large changes in osmolarity, and the resultant osmolar stress gives rise to cellular dysfunction and destruction. The limited buffering capacity of the cornea affords little protection against a variety of chemical and toxic insults. In the event that the buffering capacity is overwhelmed, there is an immediate cessation of biochemical activity, such as protein synthesis.13
Injury, Repair and Differentiation
Ocular Surface
Following corneal epithelial injury, recovery is dependent on the centripetal migration of cells from the most proximal region of viable epithelium.14 The extent of the injury dictates the source of regenerating epithelium; epithelial defects involving a portion, or the entirety of the cornea are replenished by adjacent corneal epithelium and limbus, respectively. However, in the event of complete corneal and limbal epithelial loss, the conjunctiva is the only source of regenerating epithelium. The source of regenerating epithelium influences the rate of re-epithelialisation and the ultimate phenotype of the restored epithelium.2
A variety of factors may retard the rate of re-epithelisation following chemical injury, including a robust and persistent inflammatory response, and structural damage to the epithelial basement membrane.2 Non-healing corneal epithelial wounds pose a significant risk as they expose the cornea to potential microbial infection.
Stroma
Severe chemical injuries deplete stromal keratocyte populations, and initiate collagenolytic processes which degrade collagen fibrils.2 These processes undermine the structural integrity of the corneal stroma, and may culminate in corneal ulceration and perforation. Keratocytes play a critical role in the maintenance and regeneration of the corneal stroma. Following corneal injury, keratocytes migrate into areas of damaged stroma from adjacent tissue. Keratocytes are responsible for collagen synthesis, and collagen production is maximal between days 7 and 56, with a peak at day 21 post injury.2 Collagen synthesis requires ascorbate and thus, may be significantly impaired by the scorbutic state induced in the cornea following severe chemical injury.15,16
Inflammation
Severe chemical injuries are characterized by two waves of inflammation; the first wave occurs in the first 24 hours and the second wave begins at approximately 7 days and peaks 2 to 3 weeks post injury. The intensity of the first wave may be critical for the recruitment of the second wave.2 The second wave of inflammation coincides with the period of maximal corneal degradation and repair, and may facilitate the sterile enzymatic digestion of the corneal stroma. Sterile ulceration is associated with the infiltration of polymorphonuclear leukocytes, and conversely, the exclusion of inflammatory cells from the corneal stroma is associated with cessation of sterile ulceration.2
Emergency Treatment
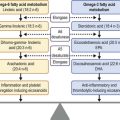
Emergency management is oriented towards prompt irrigation and the removal of residual chemical debris from the eye. The objectives are to minimize the ingress of the chemical agent into the anterior chamber, and to remove a potential reservoir for ongoing injury. The most important intervention is immediate copious irrigation at the scene of the incident.17,18 Irrigation should be continued until pH neutralization is achieved. Animal models have demonstrated that external irrigation for 90 minutes reduces the pH by 1.5 units.11 In a non-controlled human study involving 66 eyes, immediate copious irrigation resulted in less severe injury, compared with eyes which were not irrigated.17 Although there may be an advantage in the use of amphoteric buffering solutions,10 urgency may necessitate the use of any available neutral irrigation fluid.
Aqueous Humor Replacement
External irrigation is of limited value in eliminating chemicals once they have reached the intraocular chambers. Animal models of alkali injuries have shown that paracentesis lowers the aqueous humor pH by 1.5 pH units. Subsequent anterior chamber reformation, with buffered phosphate solution, lowers the aqueous humor pH by a further 1.5 pH units.11 However, the value of paracentesis and irrigation of the anterior chamber following a severe chemical injury remains controversial.2 Nonetheless, it may be reasonable to consider aqueous humor replacement in patients with severe injuries presenting within the first 2 hours post exposure.
Classification
Classification schemes for grading the severity of the initial injury are useful in guiding treatment and provide an estimation of prognosis. The Roper–Hall classification system19 (Table 29.3) was introduced in the mid-1960s and is the most established and commonly applied system. It provides prognostic guidelines based on the degree of corneal haze and the amount of perilimbal ischemia. However, the years following the introduction of the Roper–Hall classifications have seen changes in the understanding and management of ocular surface burns. An enhanced understanding of the role of the limbus in wound healing has been of particular importance. In order to reflect these changes, Dua proposed a new classification scheme in 2001 (Table 29.4) based upon clock hours of limbal involvement (as opposed to ischemia), as well as the percentage of conjunctival involvement. In a recent study, Gupta et al.20 concluded that the Dua classification had superior prognostic value over the Roper–Hall classification scheme in the context of severe ocular burns.
Table 29.3
Roper–Hall Classification 196519
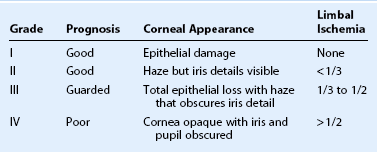
(From Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc UK 1965;85:631–53.)
Table 29.4
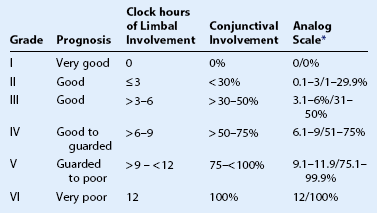
*The analog scale records accurately the limbal involvement in clock hours of affected limbus/percentage of conjunctival involvement. While calculating percentage of conjunctival involvement, only involvement of bulbar conjunctiva, up to and including the conjunctival fornices is considered.
(From Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. Br J Ophthalmol 2001;85:1379–83.)
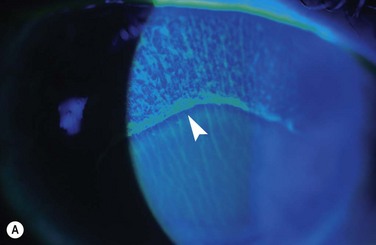
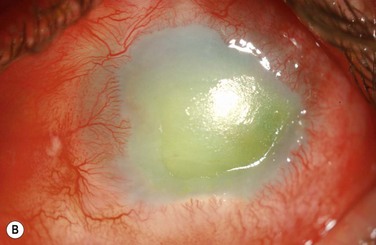
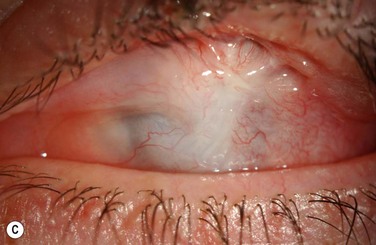
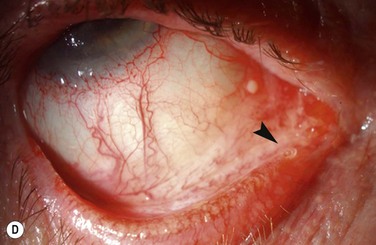
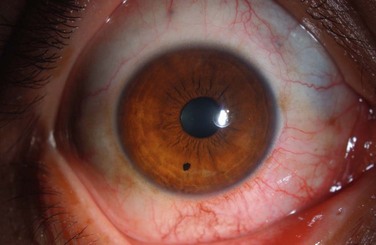
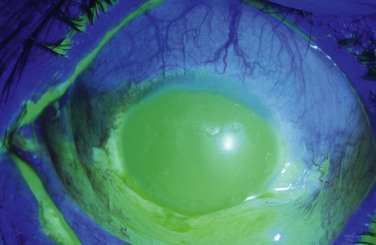
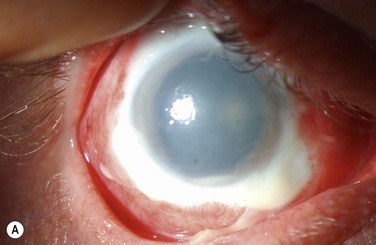
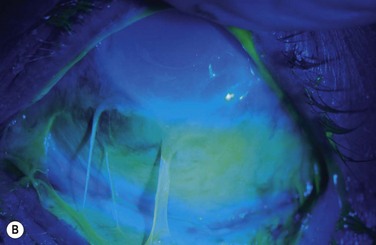
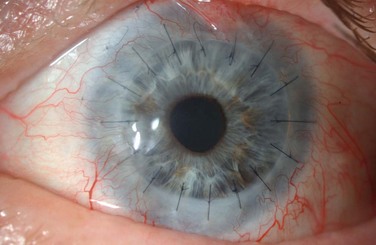
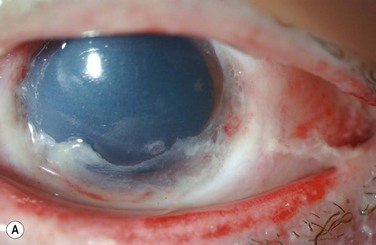
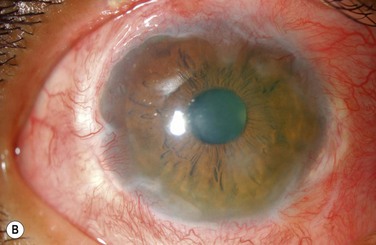
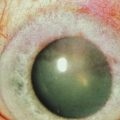
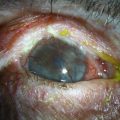
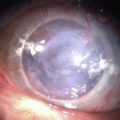
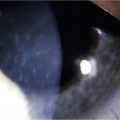
Examples of mild, moderate and severe chemical burns are highlighted in Figures 29.2, 29.3, and 29.4, respectively.
Medical Treatment
A retrospective study of patients with alkali burns revealed that intensive therapy with a combination of topical corticosteroids, antibiotics, ascorbate and citrate, atropine, and oral vitamin C, was most effective in the treatment of patients with Roper–Hall grade III injuries, with reference to time to re-epithelialization and visual acuity.21 Conversely, intensive therapy delayed re-epithelialization in the context of grade I and II injuries, presumably as a result of drug toxicity and inhibition of re-epithelialization by corticosteroid.
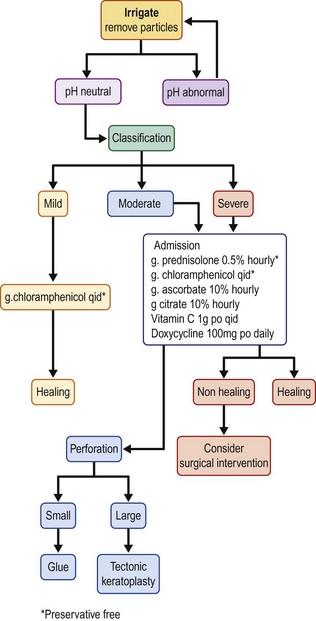
A suggested treatment flow chart is provided in Figure 29.5.
Preservative-Free Antibiotics
Epithelial defects warrant the use of a topical antibiotic for antimicrobial prophylaxis. A variety of topical antibiotics have been employed or advocated in the literature in drop or ointment form, including: chloramphenicol,6,21,22 tetracycline2 and ofloxacin.23 The choice of antibiotic must be considered with reference to the likelihood of microbial contamination at the time of injury, balanced with the potential for antibiotic or preservative-induced epithelial toxicity, which may hinder epithelial repair. Commercially available, non-preserved, antibiotic eye drops include chloramphenicol, moxifloxacin and ofloxacin. The presence of an unclean injury or microbial keratitis may necessitate the use of fortified antibiotics, such as tobramycin and cefazolin. However, these antibiotics must be used judiciously as they have a low therapeutic : toxic ratio.
Topical Corticosteroids
There is continuing controversy regarding the use and timing of topical corticosteroids in the treatment of chemical burns. While corticosteroids have the advantageous effects of inflammatory cell suppression and collagenase inhibition, they also suppress keratocyte migration and collagen production, and therefore, may cause corneal thinning. Indeed, an early animal study by Donshik et al.24 observed that the prolonged use of topical corticosteroids was associated with an increase in the incidence and severity of corneal ulceration.
Sterile ulceration occurs when there is an imbalance between collagen synthesis and proteolytic degradation. Consequently, the risk of sterile ulceration in the first week following a chemical injury is relatively modest but increases as the corneal repair process becomes established around day 14 post injury.2 Experimental models have demonstrated that the use of corticosteroids in the first 10 days post injury does not appear to have an adverse effect on outcome.24 Interestingly, more recent studies have suggested that the corneal ulceration observed in the Donshik et al. study with longer duration topical corticosteroid,24 may have actually been a product of the prolonged scorbutic state of the aqueous humor, rather than a direct action of topical steroids per se.22 Consequently, Davis et al.22 and Brodovsky et al.21 concluded that the prolonged use of topical steroids is not associated with corneoscleral melting, when used in conjunction with topical ascorbate.
Ascorbate
Following a chemical injury, ascorbate levels in the aqueous humor may fall as a result of damage to the ciliary body epithelium.2,15 This scorbutic state has the potential to compromise stromal repair because ascorbate is a key co-factor in collagen synthesis.15 Topical or systemic supplementation of depleted aqueous ascorbate levels has been demonstrated to reduce the incidence of corneal thinning and ulceration, following experimental alkali injuries if an aqueous humor concentration of 15 mg/dL can be achieved.25 Supplementation can be achieved through the hourly topical application of 10% sodium citrate eye drops and/or 1000 mg of oral ascorbic acid, given four times a day.
In severe injuries, topical administration is superior to systemic administration, presumably as a result of the reduced capacity of the ciliary body epithelium to concentrate ascorbate into the aqueous humor.25 Early supplementation is absolutely critical as ascorbate has no demonstrable effect on the progression of established ulceration.2
Citrate
Citrate chelates extracellular calcium and diminishes the activity of polymorphonuclear leukocytes by reducing membrane and intracellular calcium levels.2 In the early and late phases following chemical injury, it has been shown to reduce neutrophil infiltration by 63% and 92%, respectively.26 Citrate also has an inhibitory effect on collagenase.
In contrast to ascorbate, citrate is effective in both preventing and retarding the progression of corneal ulcers.16 The topical route is superior to the systemic route and a 10% solution of citrate eye drops may be administered hourly.
Both citrate and ascorbate have been demonstrated to reduce corneal ulceration, albeit by different mechanisms. The combined effect of citrate and ascorbate has a therapeutic advantage over citrate treatment alone.16 Citrate has a greater effect than ascorbate on more severely injured eyes because of its inhibitory action on the inflammatory response.16 Severe chemical injuries may deplete collagen-producing fibroblast populations in the corneal stroma, thereby limiting the beneficial effect of ascorbate.16
Preservative-Free Lubricants
Chemical burns may result in tear film abnormalities, resulting from damage to conjunctival goblet cells. Severe injuries may also directly damage the lacrimal system. Therefore, frequent preservative-free tear substitutes may assist in promoting healing and re-epithelialization, both by washing out debris and inflammatory cells, and by hydrating the ocular surface.3 Temporary or permanent punctal occlusion may also augment the function of tear substitutes.
Antihypertensives
Alterations of the trabecular meshwork and the release of inflammatory mediators may impair aqueous outflow, resulting in elevated intraocular pressure.2 In this context, suppression of aqueous humor production is the treatment of choice. In the absence of contraindications, short-term oral administration of acetazolamide may be preferable to minimize epithelial toxicity from preservatives in topical agents.27
Tetracyclines
The efficacy of tetracyclines in reducing collagenase activity and corneal ulceration has been demonstrated in experimental alkali injuries.2,3 This action is independent of their antimicrobial properties and is thought to occur through the chelation of zinc; an element indispensable to the activity of matrix metalloproteinases.2,3 Tetracyclines also inhibit the activity of polymorphonuclear leucocytes.2,3
Antivascular Endothelial Growth Factor (VEGF)
In murine models of ocular chemical burn, local bevacizumab has been shown to exhibit both anti-inflammatory and antineovascular properties.28 Experimental studies have demonstrated that the neovascularization cascade develops early, with significantly increased levels of VEGF expression, detectable as little as 6 hours after an alkali burn.28 Consequently, early initiation of treatment may be superior to delayed treatment. However, the use of anti-VEGF on corneal neovascularization in chemical burns has not been evaluated in humans. Theoretically, anti-VEGF agents could actually aggravate scleral ischemia and necrosis, and, therefore, anti-VEGF therapy in acute chemical burns must currently be viewed with caution.28
Serum Eye Drops
Both peripheral blood serum and umbilical cord serum have been shown to be effective in the treatment of a variety of ocular surface diseases owing to the presence of various growth factors. A recent study revealed that umbilical cord serum therapy is more effective than autologous serum eye drops or artificial tears in ocular surface restoration after chemical burns in patients with Dua classification grade III, IV and V injuries.23 Presumably this reflects the higher concentration of growth factors in umbilical cord serum, compared with peripheral blood serum.
Early Surgical Intervention
The prevention of symblepharon formation should be considered in all burns involving the conjunctiva.29 Strategies to maintain the fornices include breaking symblepharon, by repeatedly using a glass rod, inserting a symblepharon ring and lining the eyelid and palpebral conjunctiva with amniotic membrane or a sutured plastic drape.3 However, the success of these techniques will be limited in the setting of a severe injury.
Tenon’s Advancement
The presence of a severe ocular burn with total loss of limbal vasculature places the eye at immediate risk of anterior segment necrosis. A Tenon’s advancement may be effective in reestablishing limbal circulation and curbing the progression towards necrosis and aseptic ulceration.3 This procedure consists of advancing a viable Tenon’s sheet from the orbital region to the level of the limbus in order to cover the ischemic or ulcerating sclera with healthy vascularized tissue.27 While it may not prevent subsequent limbal stem cell deficiency, this technique is useful in preventing scleral perforation.
Intermediate Surgical Intervention
Intermediate surgical intervention is directed toward encouraging re-epithelialization, controlling inflammation, and protecting and maintaining the ocular surface. Persistent epithelial defects, with the attendant risk of microbial and sterile ulceration, may develop if the corneal epithelium fails to regenerate. Various strategies may be employed to promote epithelialization, including botulinum toxin-induced ptosis, surgical tarsorrhaphy, or an amniotic membrane graft.29 These procedures may also be useful in reducing ocular pain. Bandage contact lenses are not commonly used as they are frequently not tolerated by patients and they may increase the risk of infection in inflamed or dry eyes.
Amniotic Membrane Transplant
Amniotic membrane transplantation (AMT) can be considered an early or intermediate surgical procedure to promote epithelialization and suppress inflammation in order to prevent or diminish scarring-induced sequelae in the late phase.30 AMT fosters epithelial healing by acting as a basement membrane rich in growth factors, such as transforming growth factors β1 (TGF-βß1) and β2 (TGF-β2), hepatocyte growth factor (HGF) and epithelial growth factor (EGF).27 The action of growth factors in concert with other cytokines is thought to stimulate epithelialization and inhibit fibrosis.27 Amniotic membrane also functions as a barrier against the influx of immune cells as it exhibits antiangiogenic properties and tempers the immune response.27
A study by Gupta et al.20 suggests that AMT in conjunction with medical therapy results in improved outcomes, compared with medical treatment alone, in patients with Dua classification grade IV injuries. However, there was no demonstrable improvement with AMT in patients with grade VI burns. These observations are in agreement with the earlier work of Meller and colleagues30 who concluded that AMT was ineffective in preventing the development of limbal stem cell deficiency in severe chemical burns (Roper–Hall grade IV). In such cases, AMT should be performed in conjunction with a limbal stem cell transplant (LSCT). However, LSCT should not be performed in eyes with significant inflammation, as the success rate is very low. The delay for LSCT is especially important when the source of the stem cells is the fellow eye or a living relative. AMT may help prevent corneal perforation in severe cases and may provide a more favorable platform for future restorative procedures.27 However, the presence of a robust inflammatory process may rapidly dissolve the AMT, necessitating a number of repeat surgeries. (Refer to Chapter 37 for further details regarding AMT.)
Tenonplasty Flaps
Tenonplasty is an alternative method of promoting corneal epithelialization and preventing ulceration following severe injuries. This technique involves rotating a vascularized pedicle of Tenon’s capsule over the cornea.29
Tissue Glue
In the event of corneal thinning with threatened or actual perforation, the application of tissue glue offers a means of preserving the integrity of the globe. Tissue glue is usually accompanied by the application of a soft bandage contact lens, which enhances comfort and reduces the risk of glue dislodgement.2 In addition to providing tectonic support, tissue glue can stop further melting by excluding inflammatory cells and their mediators.29
Tissue adhesives provide a means of delaying penetrating keratoplasty and, thereby, modifying the risk of graft rejection, which is extremely high in the acute phase. However, a tectonic keratoplasty may be unavoidable for large perforations. Neither tissue glue nor tectonic keratoplasty in isolation ameliorate underlying ocular surface deficiency and aberrant repair mechanisms – in particular, they do not address any avascular limbal zone. Consequently, these temporizing procedures do not prevent progressive vascularization, and scarring is, therefore, inevitable in the absence of adjunctive treatment.2
Intermediate/Late Surgical Interventions
Intermediate/late surgical interventions are targeted towards optimizing the ocular surface environment in order to provide more favorable conditions for subsequent ocular surface reconstruction.29 Strategies which enhance the prognosis of ocular surface reconstruction include reconstruction of the fornices and correction of eyelid malposition. Surgery may also be necessary to eliminate corneal exposure.
Conjunctival Transplantation
Conjunctival transplantation is a means of restoring the conjunctival fornices after they have been morphologically altered by cicatricial fibrosis.3 The advantage of a conjunctival transplant over other mucosal sources is that it provides compatible tissue with a basement membrane, in addition to mucus cells.3 However, conjunctival grafts are only available if the contralateral eye remains undamaged. The procedure involves taking a sample of upper bulbar conjunctiva from the contralateral eye but this must not compromise the limbus or conjunctiva, thus, limiting the size of donor graft.
Buccal and Nasal Mucosa Transplantation
Buccal mucosa grafts can be used to treat symblepharon, trichiasis, distichiasis, entropion or a keratinized zone of the conjunctiva of the palpebral margin.3 The graft is usually obtained from the posterior aspect of the upper or lower lip. The advantage of nasal mucosal grafts resides in the ability to obtain large-sized grafts, and the transplantation of intraepithelial mucus cells.3 The sample is taken from the septum and the lower or medium turbinates.
Late Surgical Interventions
Limbal compromise results in ocular surface abnormalities characterized by chronic epithelial defects, stromal inflammation, and corneal conjunctivalization and neovascularization.2,31 Surgical options to reconstruct the ocular surface pivot around limbal stem cell transplantation which offers the possibility of restoring the corneal epithelial phenotype.29 Limbal autografts are the treatment of choice as there is no risk of rejection. However, they are only available in unilateral limbal deficiencies where there is a healthy contralateral donor eye. Typically, two 60–90-degree segments encompassing peripheral cornea, conjunctiva and limbus are harvested from the superior and inferior cornea of the donor eye.29 The graft must not encircle more than 180 degrees in order to avoid limbal deficiency in the donor eye.3 The abnormal epithelium of the recipient eye is removed and the two donor segments are sutured to the superior and inferior cornea of the host eye.29 Additional protection is afforded by an amniotic membrane onlay graft, a therapeutic contact lens or temporary tarsorrhaphy.
Alternatively, in vitro amplification techniques can be used to minimize the size of the limbal biopsy taken from the healthy donor eye (Fig. 29.6).31 A small 2-mm biopsy is taken from the limbus of the unaffected eye and cultured in the laboratory on a carrier such as amniotic membrane or fibrin sheet. Once a confluent epithelial layer is obtained, the tissue is grafted onto the affected eye. The relative success of in vitro amplification techniques appears to be similar to that of conjunctival limbal autograft.29
Limbal allografts address bilateral limbal deficiencies and may be harvested from eye bank eyes or living donors. However, limbal allografts are accompanied by a significant risk of rejection and, therefore, require long-term systemic immunosuppression.3
Corneal Transplantation
Penetrating Keratoplasty
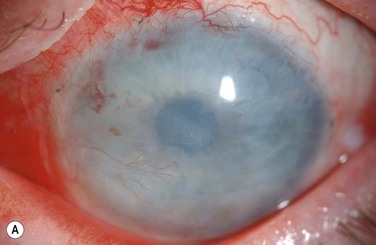
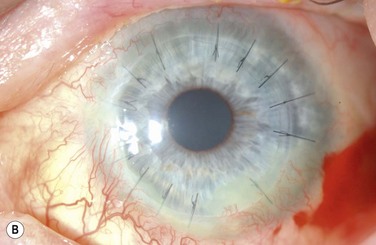
The choice of corneal transplantation procedures in chemical burns must always be considered in relation to the status of the adjacent limbus. Ultimately, long-term corneal graft clarity is untenable in the absence of a stable ocular surface. When severe, widespread corneal injury occurs, large diameter (11–12 mm) penetrating keratoplasties may confer two advantages: enhanced tectonic support, and the passage of corneal limbal stem cells from the donor globe.3 However, the success of large-diameter grafts is undermined by the significant risk of rejection. Consequently, a normal-diameter (≤8.00 mm) graft preceded, or accompanied by a limbal stem cell transplant, is the preferred treatment where extensive corneal and limbal damage coincide (Fig. 29.7).
In general, chemical burns are associated with a high risk of subsequent corneal graft rejection, largely due to the presence of corneal neovascularization. The prognosis for successful penetrating keratoplasty correlates with the severity and sequelae of the original chemical insult.2 The likelihood of success is remote in the setting of intraocular abnormalities, such as glaucoma, hypotony, anterior chamber membrane formation, and retinal detachment.2 Preliminary rehabilitation of the ocular surface is vital to the success of penetrating keratoplasty.
The End-Stage Eye
Artificial corneas represent the last treatment avenue in patients with severely damaged eyes who are unsuitable for penetrating keratoplasty. Keratoprosthesis offers the potential for visual recovery, and early studies suggest favorable retention rates.12 Complications of keratoprosthesis placement include infection, corneal melt, glaucoma and the formation of a retroprosthetic membrane.12 Keratoprosthesis is discussed in greater detail in Chapters 49 to 53.
Ocular Thermal Burns
Facial burns are a frequent component of thermal trauma and ocular involvement is estimated to affect between 7.5% and 27% of patients admitted to burn units.4 Fortunately, thermal burns are not commonly associated with severe ocular sequelae, courtesy of inherent protective mechanisms, such as the blink reflex, Bell’s phenomenon, and reflex shielding movements of the head and arms.3 The loss of an eye primarily from thermal trauma is uncommon and the risk of permanent visual impairment can be minimized with timely and effective treatment.4 Involvement of the eyelids and lid margins is the most frequent ophthalmic manifestation, and ocular complications are more commonly secondary to lid pathology than the result of direct thermal damage to the eye.4 However, ocular trauma may occur in the absence of eyelid injury and all patients who have been exposed to fumes, heat and smoke warrant comprehensive ophthalmic evaluation. Figure 29.8 shows two examples of a combined chemical and thermal injury from a firework.
Pathophysiology
The severity of a thermal ocular injury is a function of the thermal dose and the surface area of contact. A thermal dose can be defined by a time-temperature relationship. Goldblatt et al.32 explored the limits of thermal tolerance of rabbit corneas, by applying well-defined heat doses (temperature × time) and examining the gross and histological effects on the tissue. They determined that a heat dose of 45°C resulted in no perceptible damage to the cornea when applied for 15 minutes, and produced only mild transient stromal edema when applied for 45 minutes. Higher thermal doses produced a spectrum of damage, with total destruction of cellular elements, massive edema and stromal disorganization at a temperature of 59°C for 45 minutes. This degree of thermal insult resulted in severe degeneration of all structures and total necrosis at 1 week.
Clinical Evaluation
Patients with suspected ocular thermal trauma should undergo early ophthalmic evaluation to assess the extent of the injury and exclude the possibility of an intraocular or intraorbital foreign body.4,12 The frequency of ophthalmic review must balance the risk of ocular sequelae with the risk of contaminating a susceptible burns victim. Early assessment is preferable, as subsequent conjunctival and lid edema may preclude a comprehensive examination. A critical issue is to establish the integrity of the ocular surface. Most ocular thermal injuries result in superficial burns to the cornea or conjunctiva. Superficial burns may produce a spectrum of damage limited to the corneal epithelium, ranging from minor punctate changes, to widespread loss of epithelium. Deeper injuries generate a characteristic ground-glass appearance which may result in stromal scarring. The resultant eschar eventually sloughs off, leaving a thin corneal tissue that is susceptible to ectatic changes.4 Severe injuries may instigate a catastrophic process of corneal necrosis and eventual perforation.
The extent and depth of eyelid and facial burns should be assessed and carefully documented, as most ocular sequelae are secondary to the development of eyelid deformities, such as lagophthalmos and entropion.3,4,12,33 The loss of eyebrow hairs and eyelashes is usually associated with a deep partial-thickness or full-thickness burn.4 Initial assessment should note the presence or absence of Bell’s phenomenon and corneal sensation in order to appraise the risk of corneal ulceration from corneal exposure. An absent Bell’s phenomenon in the presence of lagophthalmos warrants daily ophthalmic review. Lagophthalmos from contracture of the eyelids may begin within 2 weeks post injury.
Patients with extensive burns are at risk of orbital compartment syndrome as a result of profound capillary leak into the enclosed space of the orbit.4 Elevated intraorbital pressure may result in ischemic optic neuropathy secondary to elevated intraocular pressure. In the presence of orbital compartment syndrome, an urgent lateral canthotomy and inferior cantholysis is indicated.
Clinical Management
Initial management involves careful removal of debris with irrigation and sterile cotton swabs. Early prophylactic ocular lubrication is recommended, as patients may have reduced tear production, blink reflex, and eyelid mobility or excursion.4 Ointments are considered to be more effective than drops. The presence of an epithelial defect necessitates the addition of prophylactic topical antibiotics to the lubrication regimen.3 Preservative-free chloramphenicol can be used in the presence of punctate staining, and a frank epithelial defect can be treated with preservative-free ofloxacin drops. In general, topical corticosteroids should be avoided due to the risk of secondary infection.
Singed or scorched lashes should be removed to prevent char from falling into the eye. The management of scorched lashes has largely been neglected in the literature and the relative merits of trimming eyelashes compared with epilating have not been evaluated. One study advocated trimming lashes with scissor blades coated with an ophthalmic ointment in order to prevent cut lashes from falling into the conjunctival sac.4 In the cases where cicatricial entropion may occur, epilation is the preferred option. Management of eyelid and facial burns should be conducted in close collaboration with oculoplastic or plastic surgery services.12 Tarsorrhaphy is recommended in the context of a non-healing epithelial defect, secondary to lagophthalmos and a poor Bell’s phenomenon.
In contrast to ocular chemical injuries, the efficacy of citrate and ascorbate in the context of ocular thermal injuries has not been scientifically assessed. Consequently, a lack of consensus is found in the literature. Some articles recommend the addition of citrate and ascorbate to treatment protocols,12,33 whereas others exclude their use.3,4
In the event of corneal necrosis and perforation, surgical interventions such as lamellar keratoplasty, penetrating keratoplasty or a limbal stem cell transplant procedure may be employed.12 The principles of surgical management are similar to those of ocular chemical burns.
Ocular Radiation Burns
Both ultraviolet (UV) and infrared rays have the ability to cause radiant injury to the eyes. Burns from UV rays are the most frequent.3 The sources of emission are various and include strongly reflected sunlight and welding lights. Ultraviolet rays are almost totally absorbed by the cornea, where they produce epithelial disturbances and stromal edema. Within 12 hours after exposure, UV burns manifest with pain, blepharospasm and tearing. Ocular examination reveals the presence of punctate epithelial erosions and conjunctival hyperemia. They typically heal within 48 hours and can be treated symptomatically with topical lubricants.
References
1. Pfister, RR. Chemical injuries of the eye. Ophthalmology. 1983;90:1246–1253.
2. Wagoner, MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275–313.
3. Merle, H, Gérard, M, Schrage, N. Ocular burns. J Fr Ophtalmol. 2008;31:723–734.
4. Malhotra, R, Sheikh, I, Dheansa, B. The management of eyelid burns. Surv Ophthalmol. 2009;54:356–371.
5. Morgan, SJ. Chemical burns of the eye: causes and management. Br J Ophthalmol. 1987;71:854–857.
6. Beare, JD. Eye injuries from assault with chemicals. Br J Ophthalmol. 1990;74:514–518.
7. Hong, J, Qiu, T, Wei, A, et al. Clinical characteristics and visual outcome of severe ocular chemical injuries in Shanghai. Ophthalmology. 2010;117:2268–2272.
8. Kuckelkorn, R, Makropoulos, W, Kottek, A, et al. Retrospective study of severe alkali burns of the eyes. Klin Monatsbl Augenheilkd. 1993;203:397–402.
9. Kuckelkorn, R, Kottek, A, Reim, M. Intraocular complications after severe chemical burns–incidence and surgical treatment. Klin Monatsbl Augenheilkd. 1994;205:86–92.
10. Kuckelkorn, R, Schrage, N, Keller, G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4–10.
11. Paterson, CA, Pfister, RR, Levinson, RA. Aqueous humor pH changes after experimental alkali burns. Am J Ophthalmol. 1975;79:414–419.
12. Fish, R, Davidson, RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Curr Opin Ophthalmol. 2010;21:317–321.
13. Schrage, N. Chemical ocular burns. New York: Springer; 2010.
14. Dua, HS, Azuara-Blanco, A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425.
15. Levinson, RA, Paterson, CA, Pfister, RR. Ascorbic acid prevents corneal ulceration and perforation following experimental alkali burns. Invest Ophthalmol. 1976;15:986–993.
16. Pfister, RR, Haddox, JL, Yuille-Barr, D. The combined effect of citrate/ascorbate treatment in alkali-injured rabbit eyes. Cornea. 1991;10:100–104.
17. Ikeda, N, Hayasaka, S, Hayasaka, Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225–228.
18. Burns, FR, Paterson, CA. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33–36.
19. Roper-Hall, MJ. Thermal and chemical burns. Trans Ophthalmol Soc UK. 1965;85:631–653.
20. Gupta, N, Kalaivani, M, Tandon, R. Comparison of prognostic value of Roper Hall and Dua classification systems in acute ocular burns. Br J Ophthalmol. 2011;95:194–198.
21. Brodovsky, SC, McCarty, CA, Snibson, G, et al. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835.
22. Davis, AR, Ali, QK, Aclimandos, WA, et al. Topical steroid use in the treatment of ocular alkali burns. Br J Ophthalmol. 1997;81:732–734.
23. Sharma, N, Goel, M, Velpandian, T, et al. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52:1087–1092.
24. Donshik, PC, Berman, MB, Dohlman, CH, et al. Effect of topical corticosteroids on ulceration in alkali-burned corneas. Arch Ophthalmol. 1978;96:2117–2120.
25. Pfister, RR, Paterson, CA, Spiers, JW, et al. The efficacy of ascorbate treatment after severe experimental alkali burns depends upon the route of administration. Invest Ophthalmol Vis Sci. 1980;19:1526–1529.
26. Pfister, RR, Nicolaro, ML, Paterson, CA. Sodium citrate reduces the incidence of corneal ulcerations and perforations in extreme alkali-burned eyes–acetylcysteine and ascorbate have no favorable effect. Invest Ophthalmol Vis Sci. 1981;21:486–490.
27. Gicquel, JJ. Management of ocular surface chemical burns. Br J Ophthalmol. 2011;95:159–161.
28. Hosseini, H, Nowroozzadeh, MH, Salouti, R, et al. Anti-VEGF Therapy with bevacizumab for anterior segment eye disease. Cornea. 2012;31:322–334.
29. Tuft, SJ, Shortt, AJ. Surgical rehabilitation following severe ocular burns. Eye. 2009;23:1966–1971.
30. Meller, D, Pires, RT, Mack, RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989.
31. Crawford, AZ, McGhee, CNJ. Management of limbal stem cell deficiency in severe ocular chemical burns. Clin Exp Ophthalmol. 2012;40:227–229.
32. Goldblatt, WS, Finger, PT, Perry, HD, et al. Hyperthermic treatment of rabbit corneas. Invest Ophthalmol Vis Sci. 1989;30:1778–1783.
33. Czyz, CN, Kalwerisky, K, Stacey, AW, et al. Initial treatment of ocular exposure and associated complications in severe periorbital thermal injuries. J Trauma. 2011;71:1455–1459.
34. Dua, HS, King, AJ, Joseph, A. A new classification of ocular surface burns. Br J Ophthalmol. 2001;85:1379–1383.