Hirsutism and virilization
Hirsutism is the excessive growth of terminal hair in androgen-dependent areas: upper lip, chin, sideburns, earlobes, tip of the nose, back, chest, areolae, axillae, lower abdomen, pubic triangle, and anterior thighs. Hirsutism is frequently associated with irregular menses and acne. Hirsutism should be distinguished from hypertrichosis, which is a nonandrogen-dependent increase in vellus hair. Hirsutism affects 5% to 10% of females.
Virilization consists of hirsutism, acne, and irregular menses along with signs of masculinization: deepening of the voice, increased muscle mass, temporal balding, clitoromegaly, and increased libido. Virilization results from high circulating levels of androgens, close to or in the male range, and is usually caused by an androgen-secreting tumor.
3. Where are androgens produced?
Twenty-five percent of testosterone comes from the ovaries, 25% from the adrenal glands, and 50% from peripheral conversion of androstenedione, which is produced by both the ovaries and adrenals. Testosterone is converted into dihydrotestosterone (DHT) by the enzyme 5-alpha-reductase, which is present in hair follicles, or to estradiol by the aromatase enzyme present in adipose tissue (Fig. 48-1). DHT binds to androgen receptors and is responsible for the transformation of vellus into terminal hair. Hair follicles also contain the enzymes that convert dehydroepiandrosterone (DHEA), which is produced by the adrenals, and androstenedione into testosterone.
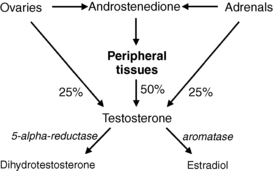
Figure 48-1. Testosterone production and metabolism.
Hirsutism is caused by hyperandrogenism. Androgens transform the fine, downy, minimally pigmented vellus hair in androgen-sensitive areas into coarse, pigmented, terminal hair. An increase in any of the androgenic steroids may cause high levels of DHT in the hair follicle and result in hirsutism.
Low levels of sex hormone–binding globulin (SHBG), which is produced by the liver, may promote hirsutism. Eighty percent of circulating testosterone is bound to SHBG, 19% is bound to albumin, and 1% is free. Decreases in SHBG increase the free fraction of hormone available to androgen-sensitive hair.
Increased activity of 5-alpha-reductase, even with normal circulating androgen levels, also may cause hirsutism by the excessive conversion of testosterone into DHT.
5. List the conditions that result in hirsutism
 Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome (PCOS)
 Congenital adrenal hyperplasia (CAH)
Congenital adrenal hyperplasia (CAH)
6. Describe the pathophysiology of PCOS.
The exact cause of PCOS is unknown, but affected patients have been shown to have an accelerated rate of pulsatile gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus. The gonadotropin secretory profile is highly dependent on the rate of GnRH pulsatility. Rapid GnRH pulses stimulate the secretion of luteinizing hormone (LH), but not follicle-stimulating hormone (FSH), from the pituitary gland. The increased LH/FSH secretory ratio results in arrested ovarian follicle development with cyst formation and hypertrophy of theca cells (hyperthecosis), thus leading to constant estrogen and increased androgen production with chronic anovulation.
PCOS affects 5% to 10% of premenopausal women and is the most common cause of hirsutism. The hirsutism is gradually progressive, usually beginning at puberty, and most patients have irregular menses from the onset of menarche. However, in a study of hirsute patients with regular menses, 50% had polycystic ovaries. PCOS patients also frequently have insulin resistance and hyperinsulinemia. Because insulin decreases SHBG and increases the ovarian androgen response to LH stimulation, the hyperinsulinemia contributes to the elevated free androgen levels in PCOS. Thus, PCOS presents as a spectrum: some patients have minimal findings, whereas others have the entire constellation of hirsutism, acne, obesity, infertility, amenorrhea or oligomenorrhea, male pattern alopecia, acanthosis nigricans, hyperinsulinemia, and hyperlipidemia. The hyperandrogenism-insulin resistance-acanthosis nigricans (HAIR-AN) syndrome is a subtype of PCOS with marked hyperinsulinemia and androgen excess frequently associated with insulin receptor defects.
8. Describe the pathophysiology of the hyperandrogenism in CAH.
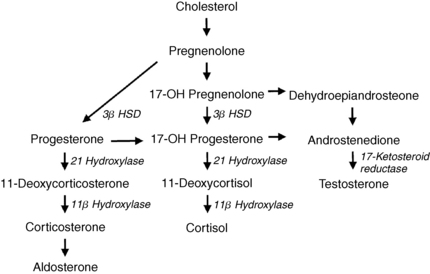
CAH results from a deficiency of one of the key enzymes in the cortisol biosynthesis pathway; it often manifests with precocious puberty and childhood hirsutism. Partial or late-onset CAH, resulting from milder deficiencies of the same enzymes, may cause postpubertal hirsutism. Ninety percent of CAH is secondary to 21-hydroxylase deficiency, which causes a defect in the conversion of 17-hydroxyprogesterone (17-OHP) to 11-deoxycortisol and of progesterone to desoxycorticosterone (DOC). The resulting low cortisol production rate leads to hypersecretion of pituitary adrenocorticotropic hormone (ACTH), which stimulates overproduction of 17-OHP and progesterone, as well as adrenal androgens, particularly androstenedione (Fig. 48-2). Hirsutism results from the androgen excess.
9. Do any other causes of CAH result in hirsutism?
Deficiency of 11-beta-hydroxylase decreases the conversion of 11-deoxycortisol to cortisol and of DOC to corticosterone. This stimulates hypersecretion of ACTH, with consequent overproduction of 11-deoxycortisol, DOC, and androstenedione. Patients also frequently develop hypertension from the mineralocorticoid DOC. Deficiency of 3-beta-hydroxysteroid dehydrogenase (3β HSD) decreases the conversion of pregnenolone to progesterone and 17-hydroxypregnenolone to 17-OHP. This defect increases pregnenolone, 17-hydroxypregnenolone, and the androgens DHEA, DHEA sulfate (DHEAS), and androstenediol, which promote the development of hirsutism. Deficiency of 17-ketosteroid reductase decreases the conversion of androstenedione to testosterone, DHEA to androstenediol, and estrone to estradiol. Affected patients have elevated basal levels of androstenedione, DHEA, and estrone (see Fig. 48-2).
10. Describe the pathophysiology of idiopathic and familial hirsutism.
Idiopathic hirsutism is believed to be caused by increased cutaneous activity of 5-alpha-reductase or enhanced skin sensitivity to androgens. Familial hirsutism is an ethnic tendency to have a higher density of hair follicles per unit area of skin. Mediterraneans and Hispanics have increased hair density, whereas Asians have lower density. Patients with idiopathic or familial hirsutism usually have the onset of hirsutism shortly after puberty with a slow subsequent progression. They have normal menses and fertility, as well as a normal hormonal profile.
11. How do Cushing syndrome, prolactinomas, hypothyroidism, and acromegaly cause hirsutism?
All causes of Cushing syndrome may result in hypertrichosis because of increased vellus hair on the face, forehead, limbs, and trunk secondary to cortisol hypersecretion. Cushing syndrome resulting from an adrenal tumor also may produce hirsutism and virilization from increased secretion of androgens with cortisol.
Hyperprolactinemia suppresses GnRH activity, which diminishes pulsatile LH secretion from the pituitary gland and results in decreased ovarian estrogen production and amenorrhea. Prolactin also increases the adrenal androgens, DHEA and DHEAS. Hypothyroidism decreases SHBG and thereby leads to an increase in free testosterone. Acromegaly is frequently associated with PCOS, and the hirsutism may result from the PCOS in conjunction with excessive insulin-like growth factor-I (IGF-I), growth hormone, and insulin resistance.
12. Which medications can cause hirsutism?
Danazol, testosterone, glucocorticoids, metyrapone, phenothiazines, anabolic steroids, valproic acid, and oral contraceptive pills (OCPs) containing levonorgestrel, norgestrel, and norethindrone can cause hirsutism. Phenytoin, cyclosporin, diazoxide, minoxidil, glucocorticoids, streptomycin, penicillamine, and psoralens can cause hypertrichosis.
13. What conditions cause virilization?
| Ovarian Tumors | Adrenal Disorders |
| Thecoma | Congenital adrenal hyperplasia |
| Fibrothecoma | Adenoma |
| Granulosa and granulosa-theca cell tumors | Carcinoma |
| Arrhenoblastoma (Sertoli-Leydig cell tumors) | |
| Hilus cell tumors | |
| Adrenal rest tumors of the ovary | |
| Luteoma of pregnancy |
14. When should a patient be evaluated for hirsutism?
Any patient with moderate to severe hirsutism, rapid development of hirsutism, or coexistence of amenorrhea, irregular menses, infertility, acanthosis, or virilization should be evaluated. A patient with regular menses who shows significant concern about her hirsutism also may warrant a workup.
15. What information is important in the history?
 Age of onset, progression, and extent of hair growth
Age of onset, progression, and extent of hair growth
 Current measures of hair removal and frequency of use
Current measures of hair removal and frequency of use
 Age at menarche, regularity of menses, and fertility
Age at menarche, regularity of menses, and fertility
 Change in libido or change in voice
Change in libido or change in voice
 Symptoms of Cushing’s disease, prolactinoma, acromegaly, or hypothyroidism
Symptoms of Cushing’s disease, prolactinoma, acromegaly, or hypothyroidism
16. What findings are important on physical examination?
 Distribution and degree of hirsutism
Distribution and degree of hirsutism
 Increased muscle mass, temporal balding, clitoromegaly, or acne
Increased muscle mass, temporal balding, clitoromegaly, or acne
 Moon facies, plethora, buffalo hump, supraclavicular fat pads, striae, or thin skin
Moon facies, plethora, buffalo hump, supraclavicular fat pads, striae, or thin skin
 Goiter, loss of lateral eyebrows, periorbital edema, dry skin, or delayed reflexes
Goiter, loss of lateral eyebrows, periorbital edema, dry skin, or delayed reflexes
17. What laboratory tests should be ordered for a patient with hirsutism?
Laboratory testing should be guided by the results of the history and physical examination. Many authors advocate against testing in patients with regular menses and only gradual progression of mild hirsutism. However, serum levels of total and free testosterone, DHEAS, and 17-OHP can be useful tests, depending on the individual patient. Patients with signs or symptoms of hypothyroidism, hyperprolactinemia, acromegaly, or Cushing syndrome also should be evaluated with serum thyroid-stimulating hormone (TSH), prolactin, IGF-I, or 24-hour urine cortisol testing, respectively. Otherwise, these tests need not be obtained for every patient.
18. How are the results of these laboratory tests interpreted?
For a patient without signs of virilization, it is important to differentiate idiopathic hirsutism, PCOS, and CAH because each is treated differently. Total and free testosterone, DHEAS, and 17-OHP help in the differentiation. Idiopathic hirsutism has normal levels on all four tests. PCOS has mildly increased testosterone, normal or slightly increased DHEAS, and normal 17-OHP. CAH has elevated testosterone and DHEAS and mild to marked elevation of 17-OHP. An early morning follicular phase level of 17-OHP greater than 500 ng/dL (normal ≤200 ng/dL) is diagnostic.
19. What do you do if a patient has borderline (200–500 ng/dL) elevations of 17-OHP?
A borderline elevated level requires an ACTH stimulation test with assessment of 17-OHP levels at baseline and 60 minutes after stimulation with ACTH. The levels are then plotted on a nomogram to determine normals, heterozygous carriers of the abnormal 21-OH gene, and patients with late-onset 21-OH deficiency. Some patients with late-onset 21-OH deficiency have normal baseline 17-OHP levels; however, the ACTH-stimulated levels are usually diagnostic. Results can be confirmed with a molecular genetic test of CYP21A2.
20. What laboratory tests should be ordered in a patient with virilization?
A patient with virilization should be evaluated to determine whether she has an ovarian tumor, an adrenal tumor, or CAH. As in patients without virilization, tests should include serum total testosterone, DHEAS, and 17-OHP. A markedly increased testosterone level (>200 ng/dL) with normal values on the other tests points to an ovarian tumor. High levels of DHEAS (>700 ng/mL) with or without high testosterone levels suggest an adrenal tumor. Increased levels of 17-OHP with modest elevations of DHEAS and testosterone are more consistent with CAH. Laboratory values suggesting tumors should be followed with a transvaginal ultrasound scan of the ovaries or computed tomography (CT) of the adrenals or ovaries. If no mass is found, iodocholesterol scanning of the adrenals or venous sampling of the ovaries or adrenals can be performed for localization before surgical removal.
21. How is PCOS treated in a patient desiring pregnancy?
If the patient’s primary concern is fertility, clomiphene is the usual drug of choice. If clomiphene fails to induce ovulation, cyclic gonadotropin administration is often useful. Pulsatile GnRH also has been used with some success. In obese patients, weight reduction alone has been shown to increase the spontaneous ovulation rate. If a component of adrenal androgen (DHEAS) hypersecretion appears to be present, low-dose dexamethasone, 0.125 to 0.5 mg, or prednisone, 5 to 7.5 mg, can be added at night. Steroids may improve the ovulation rate, as well as decrease hirsutism. In patients resistant to medical management, surgical destruction of small sections of the ovaries induces ovulation in some patients. Wedge resection of the ovaries has been replaced by laparoscopic ovarian diathermy, in which laser or electrocautery is used to destroy portions of the ovaries.
22. How is PCOS treated in a patient not desiring pregnancy?
If fertility is not the issue, OCPs or cyclic progestins are used to induce regular menses and thereby decrease the risk of endometrial cancer. Preparations containing androgenic progestins, such as norgestrel and norethindrone, should be avoided. Weight reduction should be encouraged. As noted earlier, steroids may be added in patients with an elevated DHEAS level; however, this may increase glucose in an already glucose-intolerant patient. If hirsutism does not improve with these measures, the agents listed in questions 24 and 27 through 30 may be necessary.
23. What can be done about the hyperinsulinemia of PCOS?
Patients with PCOS should be evaluated with a fasting blood glucose or an oral glucose tolerance test and a lipid profile because of the high prevalence of glucose intolerance, diabetes, and hyperlipidemia in this disorder. These problems should be addressed separately because they are not resolved by treating the hyperandrogenism alone. The insulin sensitizers metformin and thiazolidinediones have been used in patients with PCOS with and without increased glucose levels. Metformin, rosiglitazone, and pioglitazone improve insulin resistance, decrease androgens, increase SHBG, improve regularity of menses, and increase fertility. In patients whose condition is not controlled on metformin alone, there is some added benefit in combination with pioglitazone, thus resulting in further increases in SHBG, insulin sensitivity, and improved menstrual regularity. Thiazolidinediones have several side effects, including fluid retention, exacerbation of heart failure, increased osteoporotic fractures, and possible increased risk of cardiovascular events and bladder cancer, and therefore these drugs should be used with caution.
24. What is the treatment for CAH?
Glucocorticoid replacement decreases ACTH secretion and thereby reduces excessive adrenal androgen production. Mineralocorticoid replacement is also required for some causes of CAH. Treatment with the regimens listed in questions 26 through 30 can hasten improvement of the hirsutism.
25. Describe how OCPs are used for the treatment of hirsutism.
OCPs are the most commonly used therapy. They increase serum estrogens and SHBG, which decreases free testosterone levels. Monophasic and triphasic preparations work equally well. Preparations containing the progestins desogestrel, norgestimate, drospirenone, and gestodene are believed to be the best because they are the least androgenic. Potential side effects include weight gain, bloating, nausea, emotional lability, breast pain, and deep venous thrombosis.
26. Describe how antiandrogens are used for the treatment of hirsutism.
Spironolactone is an androgen receptor blocker and inhibits 5-alpha-reductase. Side effects include diuresis, fatigue, and dysfunctional uterine bleeding. Initial doses are 50 to 100 mg twice daily, tapered to 25 to 50 mg/day after an effect has been seen. Flutamide, another androgen receptor blocker, is dosed at 62.5 to 500 mg daily. Side effects include increased liver function tests (LFTs) and rare fatal hepatotoxicity. Finasteride, a 5-alpha-reductase inhibitor, effectively decreases hirsutism. Side effects include headache and depression. Dosage is 2.5 to 7.5 mg/day. The antiandrogens are usually used in combination with OCPs for additive effects and to give adequate birth control because antiandrogens can feminize a male fetus.
27. Describe how GnRH agonists are used for the treatment of hirsutism.
By providing constant rather than pulsatile GnRH levels to the pituitary, GnRH agonists reduce gonadotropin secretion and thereby decrease ovarian production of both estrogen and androgen. Estrogen replacement must be given to avoid hot flashes, vaginal dryness, and bone density loss. Leuprolide (3.75 mg/month intramuscularly), buserelin or nafarelin nasal spray, and goserelin subcutaneous implants effectively reduce hirsutism. Some studies demonstrated an increased effect over OCPs alone, whereas others showed similar effects. The preparations are expensive and thus are usually reserved for severe PCOS unresponsive to other therapies.
28. What topical agent is approved for the treatment of hirsutism?
Eflornithine HCl 13.9% cream is the newest agent for the treatment of facial hirsutism. Eflornithine HCl irreversibly inhibits ornithine decarboxylase, an enzyme necessary for hair follicle cell division. Inhibition of ornithine decarboxylase results in a decreased rate of hair growth. In clinical trials, 58% of patients had marked improvement or some improvement as compared with 34% of controls after 24 weeks of treatment. The most common side effects are acne, pseudofolliculitis barbae, burning, tingling, erythema, or rash over the applied area. Generally, side effects resolve without treatment and rarely require discontinuation of the medication. The cream is applied to the face twice daily. The patients’ hirsutism will return to baseline by 8 weeks following discontinuation of the medication.
29. What cosmetic measures can be used for the treatment of hirsutism?
Bleaching, shaving, plucking, waxing, depilating, and electrolysis are effective measures that can be used alone or in combination with the previously described treatments. They remove terminal hair that is already present while the patient waits for medications to decrease new growth and rate of transformation to terminal hair.
Laser-assisted hair removal is an effective treatment for hirsutism. It is an outpatient procedure that uses ruby, alexandrite, diode, or yttrium aluminum garnet lasers, or intense pulsed light therapy, all of which cause thermal injury to the hair follicle. At least three to six treatments about 2 to 2.5 months apart are required. The techniques result in removal of hair, and a period of 2 to 6 months before the regrowth of hair, which is thinner and lighter. Alexandrite and diode lasers appear to be the most effective. Patients with light skin and dark hair have the best results with the fewest side effects. The side effects include minimal discomfort, local edema and erythema lasting 24 to 48 hours, rare petechiae, and infrequent hyperpigmentation lasting less than 6 months.
30. How do you choose the appropriate therapy for the patient’s hirsutism?
Most patients are given a trial of OCPs, with or without spironolactone, and are advised to use cosmetic measures while waiting for the medications to work. The topical cream eflornithine HCl may be used alone or in combination with other measures. Because of their more serious side effects and higher cost, the other medications are reserved for the most severe cases in which OCPs and spironolactone fail. No matter what therapy is chosen, the patient must be made aware that results will not be seen for at least 3 to 6 months. Although many medications and combinations have been used, only topical eflornithine HCl is currently approved by the Food and Drug Administration for treatment of hirsutism. Unfortunately, most patients have a relapse of hirsutism approximately 12 months after discontinuation of medical therapy.
Blank, S, McCartney, C, Marshall, J. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361.
Cosma, M, Swiglo, B, Flynn, D, et al, Clinical review. insulin sensitizers for the treatment of hirsutisma systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008;93:1135–1142.
Lapidoth, M, Dierickx, C, Lanigan, S, et al, Best practice options for hair removal in patients with unwanted facial hair using combination therapy with laser. guidelines drawn up by an expert working group. Dermatology 2010;221:34–42.
Martin, K, Chang, R, Ehrmann, D, et al, Evaluation and treatment of hirsutism in premenopausal women. an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2008;93:1105–1120.
Moghetti, P, Tosi, F, Tosti, A, et al, Comparison of spironolactone, flutamide, and finasteride efficacy in the treatment of hirsutism. a randomized, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2000;85:89–94.
, Practice Committee of the American Society for Reproductive Medicine. The evaluation and treatment of androgen excess. Fertil Steril 2006;86:S241–S247.
Rothman, M, Wierman, M. How should postmenopausal androgen excess be evaluated. Clin Endocrinol. 2011;75:160–164.
Smith, S, Piacquadio, D, Beger, B, et al, Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women. a randomized trial. Dermatol Surg 2006;32:1237–1243.
Unluhizarci, K, Kaltsas, G, Kelestimur, F. Nonpolycystic ovary syndrome–related endocrine disorders associated with hirsutism. Eur J Clin Invest. 2012;42:86–94.
Wolf, J, Jr., Shander, D, Huber, F, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46:94–98.