Chapter 18 Hirsutism
INTRODUCTION
Hirsutism is a cosmetic concern from the patient’s perspective. Various studies have shown an increased prevalence of social fear and depression among hirsute women.1,2 Whether or not these psychological symptoms have a neuroendocrine basis remains to be elucidated.3 The old adage that “Even a single hair casts its own shadow” may help the clinician deal with the problem objectively. Indeed, even minor degrees of hirsutism may have major consequences on self-image in certain individuals.
Hirsutism needs to be differentiated from hypertrichosis. Hypertrichosis, referring to a diffuse increase in fine (vellus) hair that is not androgen dependent, may be congenital or associated with other medical conditions such as hypothyroidism or porphyria or may be associated with certain medications4,5 (Table 18-1).
| Disorders | Drugs |
|---|---|
| Thyroid disorders | Diphenylhydantoin |
| Anorexia nervosa | Diazoxide |
| Dermatologic disorders | Minoxidil |
| Cyclosporine | |
| Streptomycin | |
| Prolonged administration of cortisone | |
| Penicillamine | |
| Psoralens |
PREVALENCE
It has been estimated that between 5% and 15% of women surveyed are affected with hirsutism, although the exact prevalence remains uncertain.6,7 These estimates depend on the criteria used and the population studied. There is a wide degree of variation of what is considered normal hair growth. It is influenced heavily by the woman’s race and cultural background. It is generally accepted that women of Mediterranean origin have more body hair per unit area than Asians of the mongolian race.
Traditionally, hirsutism has been quantified according to criteria established by Ferriman and Gallwey.8 In this system, nine body areas sensitive to androgen are graded from 0 (absent), through 1 (minimal terminal hair) to 4 (frank virilization). In this scoring system, the minimum score is 0 and the maximum is 36. In their study of 161 women, Ferriman and Gallwey noted that 9.9% had a score of greater than 5, 4.3% had a score above 7, and 1.2% obtained a score above 10. In general, a score of 8 or more has been considered to represent hirsutism. Various investigators have modified this scoring system over the years.9,10
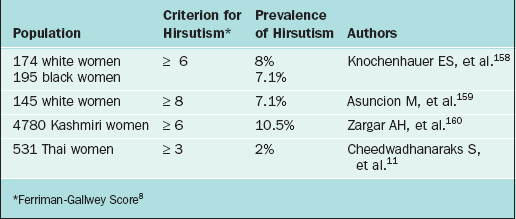
Hirsutism scores vary widely among women of different ethnic origins (Table 18-2). Consequently, a numeric cutoff point for hirsutism needs to be defined in the context of the population of interest. In clinical practice, the use of a standardized scoring system is impractical. It is limited by subjective variability as well as varying cutoff points depending on ethnicity.5,11 From a practical point of view, hirsutism may be classified simply as mild, moderate, or severe. Specifying the affected areas is helpful in following the response to therapy.
THE HAIR FOLLICLE AND HAIR GROWTH CYCLE
The hair follicle is composed of dermal and epidermal components.12 Together with the arrector pili muscles and sebaceous glands, it forms the pilosebaceous unit. In androgen-sensitive areas, each pilosebaceous unit has the capacity to differentiate into a terminal hair or into a sebaceous follicle.
There are three main types of human hair follicles. These have been classified according to the size and depth of the follicle13:
The hair follicle undergoes repetitive cycles of growth postnatally.14 In fact, the hair growth cycle has been described as a recapitulation of embryogenesis.15 The hair cycle includes three phases: telogen (resting), anagen (growth), and catagen (shortening). In humans, hair growth occurs in a mosaic pattern, where the activity of each follicle is independent of its neighbors.15 In human scalp hair, 85% to 90% of the hair follicles are in anagen, 13% are in telogen, and less than 1% are in catagen at any given time.16
Factors mediating the close relationship between the epidermal and dermal components of the hair follicle are the subject of increasing studies. Different growth factors have been implicated in the control of hair development—epidermal growth factor, fibroblast growth factor, tumor necrosis factor-β, and insulin-like growth factors.17–20 Hair growth is therefore the culmination of interactions between an organism’s genetic make-up influenced by environmental signals from the endocrine and paracrine environment. Although the morphologic changes in the hair growth cycle are well documented, the molecular mechanisms underlying the regulation of the phases of hair growth are still not well understood.
ANDROGENS AND HAIR GROWTH
Hirsutism is caused by high circulating androgen levels and/or by an increased sensitivity of the hair follicle to androgen exposure. Androgen excess has been described as the most frequent endocrine disorder in women of reproductive age.21 It is believed to occur in 75% to 85% of women with hirsutism.7 Hirsutism is only one of the clinical signs of androgen excess, which also includes androgenic alopecia, acne, and infertility.22
Androgen Production and Metabolism
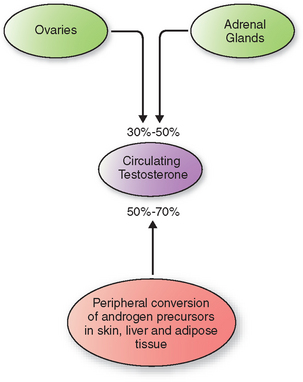
In women, direct secretion from the ovaries and the adrenal glands accounts for 30% to 50% of circulating testosterone (Fig. 18-1). The rest arises from peripheral conversion of androgen precursors to testosterone, mainly in the skin, liver, and adipose tissue.23 Androgen precursors include androstenedione, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS).
Testosterone is the most important circulating androgen. It is present either protein-bound or free in the circulation. Approximately 98% to 99% of plasma testosterone is protein bound—with higher affinity to sex hormone-binding globulin (SHBG) and more loosely to the larger pool of albumin and other proteins. Various factors affect the hepatic production of SHBG. Androgens, insulin, and growth hormone lower SHBG levels, whereas estrogens and thyroid hormone excess result in increased SHBG levels.24
Because of the greater affinity of SHBG to testosterone compared to estradiol, changes in SHBG concentration produce a much greater alteration in the percentage of unbound testosterone than of unbound estradiol. This relationship has been characterized as a see-saw, with SHBG as the fulcrum whose position moves in response to estrogens and androgens (Table 18-3).25 In this way the balance between the levels of free estrogen and free testosterone is determined by the amount of SHBG, whose concentration is determined by these hormones. Accordingly, SHBG acts to amplify the tissue exposure and response to androgen/estrogen balance.
Table 18-3 Role of SHBG in the Regulation of Free Estrogen and Testosterone
| High estrogen state: free estrogen increases and free testosterone decreases |
| Normal estrogenic state: balance |
| High androgen state: free estrogen decreases and free testosterone increases |
The free fraction of plasma testosterone is the biologically active portion. It is believed to enter the target cell by passive diffusion and then binds to the nuclear androgen receptor. Androgen binding leads to an allosteric conformational change, which in turn leads to receptor dimerization, nuclear transport, and target DNA interaction.26–28 These events culminate in target gene transcription.
Both testosterone and dihydrotestosterone bind to the same receptor but with different affinities, that for dihydrotestosterone being much greater than that for testosterone.29 The affinity for DHEA and androstenedione is markedly less.30
Site of Androgen Action in the Skin
The skin is a major site for the peripheral conversion of weak androgen precursors to active androgens.30 DHEA and DHEAS have little androgenic activity. However, these may be converted to androstenedione and subsequently to testosterone in the adrenal glands and in the peripheral tissues.
The active androgen in the skin is dihydrotestosterone.23 Conversion from the testosterone precursor is catalyzed by the enzyme 5α-reductase. Two forms of 5α-reductase are known. Type 1 is found mainly in nongenital skin; the gene responsible is found on chromosome 5. Type 2, encoded on chromosome 2, is primarily found in androgen target tissue such as the prostate, epididymis, seminal vesicle, and genital skin.31 The two types share 50% homology.
Dermal papilla cells taken from androgen-dependent areas such as the beard have been demonstrated to express a greater number of androgen receptors as compared to nonbalding scalp cells.15 Higher levels of 5α-reductase activity have also been demonstrated in beard cells.32 The varying sites of expression of the androgen receptor in the different cell types in the skin likely account for the diverse effects of androgens in the skin. These likely account for the disparity in hair distribution and thickness observed among hirsute individuals with similar androgen levels. The increased sensitivity of the pilosebaceous unit to normal levels of androgen is believed to be due to enhanced peripheral 5α-reductase activity, polymorphisms of the androgen receptor, or altered androgen metabolism.
ETIOLOGY OF HIRSUTISM
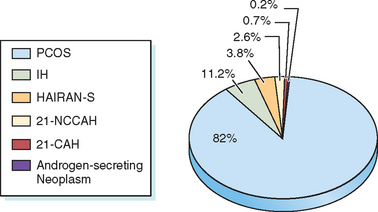
Hirsutism is a sign of increased androgen level or increased sensitivity of the hair follicles to androgen. Hyperandrogenemia is seen in 85% of patients with moderately severe hirsutism.6 Hirsutism may be idiopathic or due to androgen excess from either the ovary or the adrenal gland. Conditions associated with androgen excess from the ovaries include polycystic ovary syndrome (PCOS), hyperthecosis, and androgen-secreting ovarian tumors. Adrenal causes of androgen excess include Cushing’s syndrome, congenital adrenal hyperplasia (CAH), and androgen-secreting tumors. Most hirsute women will have PCOS33,34 (Fig. 18-2).
Idiopathic Hirsutism
The most widely accepted definition of idiopathic hirsutism includes normal androgen levels and normal ovulatory function in a hirsute patient.35 Using these criteria, the prevalence of idiopathic hirsutism has been estimated to be between 5% and 15%.36,37 Among 64 hirsute women claiming regular menses at intervals shorter than 35 days, 25 (39%) had an anovulatory cycle documented by a day 22 to 24 serum progesterone level of less than 4 ng/mL.36
Polycystic Ovary Syndrome
Although initially described in 1935 by Stein and Leventhal, a uniform definition for PCOS still does not exist.38 At the recent joint meeting of the European Society for Human Reproduction and the American Society of Reproductive Medicine, it was suggested that the diagnosis of PCOS is met when at least two of the three following elements are present: hyperandrogenism, chronic anovulation, and polycystic ovaries.39 The different diagnostic criteria for PCOS reflect the heterogeneous nature of this disorder. PCOS is still a diagnosis of exclusion. The prevalence of PCOS is estimated to be at 5% to 7% of reproductive age women.40 It is the most common cause of anovulatory infertility.
Polycystic ovary syndrome needs to be distinguished from polycystic ovaries alone. Although most women with PCOS have polycystic ovaries, a substantial population of normal women does as well. Polycystic ovarian morphology may be found in 20% or more of women of reproductive age.41,42 The significance of polycystic ovaries alone is unclear given that it seems to have no impact on fertility.43
Women with PCOS present with some or all of the following clinical features: oligomenorrhea, infertility, hirsutism, acne, alopecia, or obesity. It is important to remember that the syndrome has multiple components—reproductive, metabolic, and cardiovascular—that have health implications throughout the woman’s life.44
Insulin resistance, which refers to the diminished ability of insulin to exert its metabolic effects, is now thought to play a central role in PCOS.45,46 Beta-cell dysfunction, independent of insulin resistance, is also thought to contribute to the pathogenesis of the complications of PCOS.47 Women with PCOS have higher basal insulin concentrations and an increased prevalence of central obesity, type 2 diabetes mellitus, and hypertension.48 An estimated 50% to 70% of women with PCOS have insulin resistance.49 Not surprisingly, insulin resistance in women with PCOS is associated with obesity but may also be present in lean women with PCOS.50–52
The consequent hyperinsulinemia is believed to lead to androgen excess through several mechanisms: (1) stimulation of ovarian androgen production, (2) stimulation of adrenal androgen biosynthesis, (3) stimulation of luteinizing hormone (LH) release, and (4) decreasing hepatic SHBG production.53–56 Differentiating those women with PCOS with insulin resistance from those without insulin resistance therefore becomes important given the metabolic consequences and required surveillance for these women.57–59 Considering the paucity of data on the use of simple laboratory markers of insulin resistance in predicting risks and therapeutic response in women with PCOS, it is recommended that the best clinical approach may be the heightened surveillance in all patients for the clinical sequelae of insulin resistance, such as glucose intolerance, dyslipidemia, and hypertension.60
Congenital Adrenal Hyperplasia
CAH is a family of autosomal recessive disorders resulting from deficiency in one of the five enzymes necessary for the biosynthesis of cortisol and aldosterone.61 Clinical features depend on the degree of the enzyme defect. The classic forms of neonatal virilization result from a severe enzyme deficiency. Nonclassic forms, due to a less severe enzyme deficiency, may result in delayed signs of androgen excess. In this chapter, the three enzyme deficiencies that result in features of postnatal androgen excess are discussed (see Chapter 2 for details on steroidogenesis).
21-Hydroxylase Deficiency
This enzyme deficiency accounts for the majority (90% to 95%) of CAH cases.61,62 There is a wide variability in phenotypic expression, depending on the extent of the enzyme impairment. All the various forms of 21-hydroxylase deficiency are caused by either homozygous or compound heterozygous mutations in the CYP21A2 gene. The incidence is estimated to be 1:15,000 live births for the severe classic form of CAH.63 The prevalence is higher in certain ethnic groups, such as the Ashkenazi Jews.
Nonclassic 21-hydroxylase deficiency (21-OHD) has been reported to occur in from 1% to 10% of hyperandrogenic women.64 Affected individuals produce normal amounts of cortisol and aldosterone at the expense of elevated sex hormone precursors. Onset of symptoms is variable. Some may present with premature pubarche; others may be diagnosed after puberty when they present with symptoms resulting from androgen excess—menstrual irregularity, hirsutism, acne, or infertility. They are clinically indistinguishable from women with PCOS. Up to 50% of affected women (with nonclassic 21-OHD) have been reported to have polycystic ovaries on ultrasound examination.65 Serum androgen levels are comparable with those seen in PCOS as well.66 Measurement of 17-hydroxyprogesterone (basal or stimulated) currently is the main test used to diagnose nonclassic 21-OHD.
11β-Hydroxylase Deficiency
This is the second most common cause of CAH, seen in about 5% to 8% of cases.63 Clinical features are similar to the virilizing form of CAH. However, hypertension is a clinical feature believed to be due to the excess production of the mineralocorticoid deoxycorticosterone. Hypokalemia may be seen as well. Mild, late-onset presentations have been reported. Diagnosis is based on the response to a corticotropin stimulation test similar to 21-OHD; however, in this condition, deoxycorticosterone and 11-deoxycortisol are elevated.
Androgen-secreting Tumors
Adrenal Tumors
A purely androgen-secreting adrenal tumor is rare. More frequently, a mixed picture of Cushing’s syndrome with virilization is seen in adults.67 In a review of cases of androgen excess seen in the Mayo Clinic from 1946 to 2002, 11 female patients were identified to have pure androgen-secreting adrenal tumors. Five of these were malignant.68 Malignant tumors were bigger (9.8 cm vs. 4.2 cm) and heavier (232 g vs. 44 g). Surprisingly, mortality was reported in only one patient, a finding in marked contrast to the lower survival rates observed in other forms of adrenocortical carcinoma.69
Ovarian Tumors
Androgen-secreting ovarian tumors are also rare.70 Sertoli-Leydig cell tumors are the most common virilizing ovarian tumor and account for 0.5% of all ovarian neoplasms; however, even nonfunctional ovarian tumors may cause hyperandrogenism, probably mediated by stimulation of stromal cells adjacent to the tumor.71,72
Ovarian Hyperthecosis
Hyperthecosis refers to the nests of luteinized theca in the ovarian stroma. There is a considerable clinical overlap between patients with PCOS and those with ovarian hyperthecosis. The latter, however, often present with a more severe form of hyperandrogenism with associated virilization. Laboratory evaluation typically shows a normal serum DHEAS level with extremely elevated testosterone levels, often greater than 200 ng/dL.71
Hyperandrogenism, Insulin Resistance, and Acanthosis Nigricans (HAIRAN) Syndrome
A unique disorder of severe insulin resistance associated with hyperandrogenism, HAIRAN syndrome is becoming more widely recognized. It is characterized as follows:73 (1) early age of onset of hyperandrogenism, (2) acanthosis nigricans, (3) marked insulin resistance, (4) positive correlation of the severity of the insulin resistance with the severity of androgen excess, (5) ovarian source for the androgen overproduction, and (6) ovarian hyperthecosis. Most affected women will have normal glucose concentration at the expense of markedly elevated levels of circulating insulin (<80 μU/mL fasting insulin and/or <500 μU/mL after an oral glucose challenge).7 The molecular cause of the insulin resistance in patients with HAIRAN syndrome is still not well understood.
Cushing’s Syndrome
The reported incidence of hirsutism in patients with Cushing’s syndrome is 64% to 81%.74 However, Cushing’s syndrome is a rare cause of hirsutism. Increased adrenal androgen production may accompany the increased production of cortisol. The onset of hirsutism is often distinct from menarche. There may also be increased growth of lanugo hair as a result of the hypercortisolism.75
Hyperprolactinemia
Elevated prolactin levels have been noted in some hirsute women. In a study of 158 women with hirsutism, elevated prolactin was noted in 6% of the women.76 The exact relationship between hyperprolactinemia and hirsutism is still unclear. It is postulated that elevated prolactin level may increase androgen production via an effect on the adrenal cortex. Use of dopamine agonists in hirsute, hyperprolactinemic women has been shown not only to reduce serum androgen levels, but also to improve hirsutism scores.77 On the other hand, the use of bromocriptine in women with PCOS but without hyperprolactinemia has not been demonstrated to alter serum androgen levels.78
Drug-Induced Hirsutism
Multiple medications have been implicated in hirsutism. Anabolic steroids such as danazol, oral contraceptives containing progestins that are 19-nortestosterone derivatives, and medications that can increase serum prolactin have been implicated.4 Among 19-nortestosterone derivatives, first-generation (norethynodrel) and second-generation progestins (norethindrone and its metabolites; levonorgestrel and its derivatives) are more androgenic than the third-generation progestins (desogestrel and norgestimate).79 With the increasing use of testosterone replacement in postmenopausal women, iatrogenic hirsutism will likely occur more frequently.80
APPROACH TO THE PATIENT WITH HIRSUTISM
History
A thorough history is necessary in the evaluation of a woman with hirsutism. Recognizing the patient’s concerns and goals for the visit will allow the physician to tailor the eventual therapy to the patient’s benefit and satisfaction. Most underlying etiologies for hirsutism are benign, considering that it may take months to even years of androgen exposure to transform vellus hair to terminal hair.81
Onset of Symptoms
Increased androgen exposure of the pilosebaceous unit occurs at puberty. Hence, hirsutism often starts at around this time or a few years thereafter. On occasion, it may accompany an early adrenarche. Not infrequently, increased facial hair development in women occurs postmenopausally. It is thought to result from the altered estrogen/androgen balance.82 Rapid hair growth of recent onset should be viewed with suspicion.
Associated Symptoms
Menstrual History
Oligomenorrhea is one of the features of PCOS. In adolescents, oligomenorrhea that persists after the first few years from the menarche may be an early sign of PCOS.30 Regular menses, however, do not predict normal androgen status in hirsute women. Although 40% of hirsute women will have regular menses, on evaluation, half of these women will be found to have elevated levels of one or more androgens.84
Regular menses do not prove normal androgen status or reliably normal ovulatory/luteal function. In one study, 39% of women reporting normal menstrual cycles actually had oligo-ovulation/anovulation when basal body temperature and day 22 to 24 progesterone levels were measured.36
Weight Changes
Weight gain and distribution need to be assessed. Central obesity is seen in patients with Cushing’s syndrome. Obese women with PCOS have a greater prevalence (73% vs. 56%) of hirsutism compared to nonobese women with PCOS.85 Obesity tends to enhance the androgenic state by two mechanisms:29 (1) the resultant insulin resistance and hyperinsulinemia leads to lowered hepatic SHBG production, in turn resulting in increased levels of free testosterone, and (2) the activation of androgenic precursors in the peripheral tissues is enhanced.
Family History
An increased prevalence of hirsutism, acne, and male-pattern balding has been observed in relatives of hirsute women.10 Among families of women diagnosed with PCOS, an autosomal dominant pattern of inheritance has been reported for the inheritance of polycystic ovaries and premature male-pattern balding.86 Whether this familial clustering is due to the genetic pattern of the underlying disorder or to some other factor is still unclear.
Ethnicity
Ethnic differences in hair quantity and distribution need to be kept in mind. Clearly, the criteria for assessing hirsutism among women of different racial background need to be adjusted and individualized (see Table 18-2).
DIAGNOSTIC TESTS
Laboratory Tests
Testosterone
Measurement of total and/or free testosterone is useful in the evaluation of any state of androgen excess. Although there have been considerable improvements in the commercially available assays for measuring total serum testosterone, there is still a wide degree of between-kit variability, especially in samples from women.88 It is important to keep in mind that an accurate measurement of free testosterone is highly dependent on an accurate assay for total testosterone.89,90 Consequently, it is important for a clinician to be familiar with the assay used and the normal ranges in the laboratory.
Total testosterone value above 200 ng/dL has been suggested to signify an androgen-secreting neoplasm.91 However, in a more recent review, Friedman and colleagues92 followed 18 women with serum testosterone level greater than 200 ng/dL (7 nmol/L) for 5 years. Only two women were diagnosed with an androgen-secreting neoplasm. Most of the women were overweight. Conversely, if virilization is present even in the setting of only a modest elevation in serum testosterone, then further workup is warranted. It remains possible that small neoplasms are not being detected by conventional means (i.e., computed tomography [CT] scan of adrenal glands, transvaginal ultrasound of the ovaries).
Dehydroepiandrosterone Sulfate
Dehyroepiandrosterone sulfate is primarily produced by the adrenal glands; hence, it is a good marker for adrenal androgen production. Although a weak androgen, its main importance in androgen excess states is to provide a pool of precursor for testosterone formation in the periphery. It is frequently elevated in anovulatory women.93 Levels decline with age but with marked individual variability.94 DHEAS values greater than 700 μg/dL (24.3 μmol/L) are suggestive of a virilizing adrenal tumor.4
Testing for Congenital Adrenal Hyperplasia
As previously mentioned, most cases of CAH are due to 21-hydroxylase deficiency. Nonclassic 21-OHD is often clinically indistinguishable from PCOS. An unstimulated early-morning follicular phase 17-hydroxyprogesterone determination has been recommended as a screening tool for this condition because a value of less than 2 ng/dL has been shown to have a negative predictive value of 100%.95 Patients who have 17-hydroxyprogesterone levels greater than or equal to 2 ng/dL should proceed to a corticotropin stimulation test. Measuring 17-hydroxyprogesterone level at baseline and 60 minutes after an intravenous injection of 250 μg of synthetic corticotropin is used to establish the diagnosis of late-onset 21-hydroxylase deficiency Post-stimulation values greater than 10 ng/mL (30.3 nmol/L) constitute a positive test.66
Testing for Cushing’s Syndrome
In those hirsute women with other clinical signs suggestive of hypercortisolism, screening for Cushing’s syndrome is warranted. A 24-hour urine free cortisol is widely used to establish the presence of Cushing’s syndrome. The concomitant measurement of creatinine in the urine sample ensures the adequacy of the collection. Sensitivity ranging from 95% to 100% and specificity from 94% to 98% has been cited by other authors.97 Other screening tests include low-dose (1 mg) overnight dexamethasone suppression testing, midnight serum cortisol, and midnight salivary cortisol. To date, there is no specific protocol advocated or one test shown as being clearly superior to the others in confirming the diagnosis of Cushing’s syndrome. Most clinicians will use a combination of these tests. Once the diagnosis is confirmed, additional tests are required to establish the specific etiology of Cushing’s syndrome. Referral to a center that deals with such patients is usually recommended.
Serum Luteinizing Hormone and Follicle-Stimulating Hormone
Measurements of serum LH and follicle-stimulating hormone (FSH) are of little clinical utility in most cases of hirsutism. A disproportionate increase in serum LH as compared to FSH has been observed frequently in women with PCOS.97 A LH:FSH ratio of greater than 3 has been used by some to indicate PCOS; however, a ratio lesss than 3 does not exclude the diagnosis.
Imaging Studies
Ultrasonography
The diagnosis of PCOS is often established on clinical grounds with minimal supportive laboratory data. Polycystic ovaries using ultrasonography are not found in all women with PCOS; conversely, as many as 20% to 25% of normal women may have polycystic ovaries on ultrasound. Some women with CAH and adrenal tumors may also have polycystic ovaries. Hence, ultrasound is most often utilized when an ovarian neoplasm is suspected. The positive predictive value of ultrasound for adnexal malignancy is cited to be 73%, with a negative predictive value of 91%.98 Transvaginal ultrasonography is the preferred method of imaging the ovary with the principal aim of lateralizing the lesion.
Adrenal Imaging
Due to the rarity of androgen-secreting adrenal tumors, adrenal imaging is not indicated unless there is a strong suspicion for an adrenal neoplasm. Incidental adrenal masses (“incidentalomas”) are found in 1% to 4% of abdominal imaging studies of patients without suspected adrenal pathology.99 In postmortem autopsies, incidental adrenal masses are seen in up to 8.7% of unselected populations.100 If clinically indicated CT or MRI are quite sensitive for detecting adrenal tumors.
Ovarian and Adrenal Venous Sampling
In cases in which adrenal and ovarian imaging fail to identify a source for excessive androgen levels, biochemical analysis of blood samples from the adrenal and ovarian veins have been advocated by some authors.101 However, use of this technique should be restricted to centers experienced in its use.102
MANAGEMENT
Hair Removal
Nonpharmacologic measures of hair removal include waxing, shaving, plucking, bleaching, and use of depilatory creams as well as electrosurgical methods such as electrolysis and laser therapy. Most of these methods are effective transiently; none treats the underlying etiology. Shaving, epilation (plucking and waxing), chemical depilation, and bleaching are all safe, easy, and inexpensive. Shaving is not popular among women for facial hair removal because of the unsightly stubble that occurs once regrowth occurs. Mild skin irritation either due to local trauma or to chemical reaction may occur with any of these methods.103,104
Hair removal is achieved in electrolysis by delivering an electric current to the hair follicle using a probe. This is a slow, expensive, and often painful process. Scarring, as well as hypopigmetnation and hyperpigmentation, have been reported to occur.105
Laser Hair Removal
Laser hair removal targets the melanin in the hair bulb.103 It is based on the principle that selective thermal injury is achieved only in the target capable of absorbing the specific wavelength used for a given amount of time.106 Only hair in the anagen phase is susceptible to injury from the lasers. Different lasers are available, such as the ruby, alexandrite, diodide, and neodymium;yttrium-aluminum-garnet (Nd:YAG) lasers. These appear to be equally efficacious.105 More studies with longer follow-up are needed to be able to define their role conclusively.
Topical Agents
Eflornithine
Eflornithine (Vaniqa) is a topical agent that inhibits ornithine decarboxylase—a rate-limiting enzyme in the biosynthesis of polyamines.104 Reduction in hair growth rates have been reported in 32% of patients who used this product as compared to placebo.4 Its effects may last up to 8 weeks after the therapy is discontinued.103 Side effects reported include stinging, tingling, and rash.
Androgen Suppression
Oral Contraceptive Pills
Like gonadotropin-releasing hormone (GnRH) analogs, exogenous estrogen and progesterone in oral contraceptives inhibit gonadotropin secretion, leading to decreased ovarian androgen production. In addition, oral contraceptives have also been shown to increase SHBG levels and to decrease adrenal production of androgens and their precursors.107–109 All of these make oral contraceptives ideal for use in hirsute women with hyperandrogenism. In general, 60% to 100% of hirsute women show improvement with oral contraceptive use.
Currently, low-dose estrogen formulations in almost all the oral contraceptives available in the United States have circumvented the adverse effects associated with high estrogen content while demonstrating comparable reduction in androgen level and improvement in hair growth.110 However, there are differences in the oral progestins available. Not all oral progestins are purely progestational, and most of these have been shown to have androgenic activity.79
In the older oral contraceptive pills, lowering the progestin dose minimized its androgenic side effects. Currently, newer progestins with improved progesterone-receptor selectivity are available. These include norgestimate, desogestrel, and gestodene.111 Oral contraceptives formulations containing these newer progestins have been shown to be efficacious in the treatment of hirsutism.112,113
Drosperinone is a new progestin contained in one oral contraceptive pill as well as in an agent used for hormone replacement therapy. It is an analog of spironolactone that has both anti-mineralocorticoid and progestogenic properties. In most hirsute women treated with 30 μg ethinyl estradiol plus 3mg drosperinone for 12 cycles, hirsutism scores improved from the sixth cycle onward.114 However, no direct comparative trials with other oral contraceptive pills have been reported.
Gonadotropin-releasing Hormone Agonists
GnRH is a hypothalamic decapeptide hormone released in a pulsatile fashion and regulates the production and release of both LH and FSH from the anterior pituitary gland.115 GnRH and its agonists have been used either to stimulate the pituitary-gonadal axis (an acute event) or to down-regulate the GnRH receptor by continuous exposure, leading to gonadotropin/gonadal suppression (chemical castration).116,117 Several studies have shown the efficacy of using GnRH agonists in the treatment of hirsutism.118 Treatment of hirsute women with nafarelin for 6 months results in significant decreases in serum gonadotropin, testosterone, free testosterone, and androstenedione levels, as well as significant decreases in hirsutism scores.119 However, cost of therapy and clinical consequences of hypoestrogenism—such as hot flashes and decreased bone mineral density—limit its usefulness. Currently, their use is not recommended for first-line therapy of hirsutism.
Combination of GnRH agonists with estrogen therapy has ameliorated the side effects from hypoestrogenism. In a comparative study of leuprolide plus estrogen versus an oral contraceptive pill for the treatment of hirsutism, the combination of leuprolide plus estrogen demonstrated a more rapid improvement in hirsutism.120
Glucocorticoids
Glucocorticoid treatment lowers adrenal androgen production. However, its efficacy in treating hirsutism is limited. Low doses need to be used to avoid side effects such as weight gain, osteoporosis, glucose intolerance, and adrenal suppression. Even in women with late-onset CAH, the results of treating hirsutism with glucocorticoids are often disappointing. In a comparative study of cyproterone acetate versus hydrocortisone in treating hirsutism in women with this condition,121 a 54% reduction in hirsutism score was noted in cyproterone acetate-treated patients versus a 26% reduction noted in the hydrocortisone group after 1 year. However, hydrocortisone has a shorter half-life as compared to prednisone or dexamethasone, which may have accounted for these results. The latter two agents are preferred in this condition. The commonly used dosage for prednisone is 2.5 mg twice a day, whereas for dexamethasone, it is 0.125–0.25mg at bedtime. Higher doses are not recommended.
Insulin Sensitizers
Insulin resistance, with its consequent hyperinsulinemia, plays an important role in the pathogenesis of PCOS. Hyperinsulinemia has been shown to increase ovarian androgen production122,123 and to decrease the production of SHBG.56 Dietary weight loss in obese women with PCOS has been shown to improve insulin resistance and reduce hyperandrogenism with consequent decreased hirsutism and improved ovulation, and fertility rates.124,125
On the other hand, metformin is an effective treatment for anovulation in women with PCOS.126 Metformin is a biguanide that lowers glucose in the presence of insulin by reducing hepatic gluconeogenesis and increasing peripheral cellular glucose uptake.127 Metformin also reduces levels of free testosterone, which has been attributed to increased SHBG levels rather than decreased total serum testosterone levels.128–130 There is also the question of whether it is the weight loss from the anorectic effects of metformin rather than an independent effect of metformin that leads to improvement in free testosterone levels.131
Metformin has been shown to improve hirsutism in several but not all studies.131–134 In a 12-month study comparing metformin with ethinyl estradiol/cyproterone acetate, metformin resulted in a greater reduction in the hirsutism score.135 In another study, the opposite was observed.136 Currently, the use of metformin in doses between 1.5 and 2.0 g/day is recommended to ameliorate hirsutism in the PCOS population.135,137
Thiazolidinedione compounds comprise another class of insulin-sensitizing drugs, which are selective ligands of the nuclear transcription factor peroxisome-proliferator-activated-receptor γ (PPARγ). These enhance glucose uptake mainly in adipose and muscle tissues. Two drugs are currently available—rosiglitazone and pioglitazone. The Food and Drug Administration (FDA) withdrew troglitazone, the first available thiazolidinedione, from the market in 1999 because of hepatotoxicity. Earlier studies done using troglitazone in PCOS demonstrated significant reductions in mean serum free testosterone levels, improvement in ovulatory frequency, and improvement in hirsutism scores.138–140 Similarly, small studies using rosiglitazone or pioglitazone showed improved ovulatory rates and insulin sensitivity.141–144 Results of hormonal assessments were variable but only two studies using pioglitazone alone and in combination with metformin showed an increase in SHBG levels.143,145
Peripheral Androgen Blockade
Spironolactone
The aldosterone antagonist spironolactone also competes with testosterone and dihydrotestosterone for the androgen receptor. Spironolactone (50 to 200 mg/day) will reduce the quantity and quality of facial hair growth in the majority of women with moderate to severe hirsutism.146 The maximal effect is noted by 6 months and maintained at 12 months of treatment. It was equally effective in treating hirsutism in women with either idiopathic or PCOS-related hirsutism. Reduction in ovarian androgen production was observed, whereas it had no effect on adrenal androgen or cortisol levels. Diuresis was limited to the first few days of treatment.
A similar reduction in total testosterone has been demonstrated in doses of 100 mg/day to 200 mg/day of spironolactone.147 Anagen hair shaft diameters decreased in both groups (19% and 30%, respectively). Although no direct comparative trial with an oral contraceptive and spironolactone is available, combination therapy has been shown to significantly improve hirsutism and reduce serum total and free testosterone levels.148 Furthermore, the addition of an oral contraceptive will decrease the frequency one of the side effects of spironolactone that could decrease compliance: irregular menstrual cycles.
Side effects commonly associated with spironolactone include gastrointestinal discomfort, polyuria, nocturia, fatigue, headaches, irregular menstrual bleeding, decreased libido, and atopic reactions.24 Hyperkalemia is uncommon in women with normal renal function.
Flutamide
An agent used for the hormonal treatment of prostate cancer, flutamide is effective in the treatment of hirsutism.149 In a study of 18 hirsute women treated with flutamide 125 mg three times a day for 12 months,150 hirsutism scores were markedly improved in all women, with concomitant reduction in serum androgen levels. It is also more effective than spironolactone in treating hirsutism, with reduction in hirsutism scores to almost normal after 6 months of therapy with flutamide versus only a 30% reduction in women treated with spironolactone.151 Major concerns include the expense and rare hepatotoxicity, which can sometimes be fatal.152 Consequently, its use for hirsutism is currently off-label; it is not approved for this use by the FDA.
Cyproterone Acetate
Cyproterone acetate is an antiandrogen as well as a progestin. It is used as a part of an oral contraceptive or added to an estrogen or an oral contraceptive pill.153 It reduces circulating testosterone and androstenedione levels by suppressing LH.7 In the periphery, it inhibits androgen binding to its receptor.121 In a prospective, randomized trial comparing low dose flutamide, finasteride, ketoconazole, and cyproterone acetate–estrogen in treating hirsutism, cyproterone acetate–estrogen and flutamide were noted to be the most efficacious.154 The cyproterone acetate–estrogen combination reduced hair growth most rapidly. However, cases of fatal hepatitis have also been reported in association with use of cyproterone acetate. Currently, it is not available in the United States.
Finasteride
Finasteride is an inhibitor of type 2 5α-reductase and is currently approved for the treatment of benign prostatic hyperplasia.155 It has also been approved for the treatment of androgenic alopecia in men at a dose of 1 mg/day. In a study of 27 women with idiopathic hirsutism,156 treatment with finasteride 5mg/day alone or in combination with an oral contraceptive for 6 months showed significant improvement in clinical hirsutism scores. No change in serum androgen levels was noted in the finasteride only group. However, the group that received combination therapy showed decreased dihydrotestosterone levels.
Finasteride treatment appears to be as effective as spironolactone in treating hirsutism.157 In a prospective study comparing low-dose flutamide, finasteride, ketoconazole, and cyproterone acetate–estrogen regimens, improvement in hirsutism scores was smaller in the group receiving finasteride.154,157 That group was also slowest in decreasing hair growth rate. However, it was the best tolerated among the four regimens. The FDA has not approved finasteride for use in hirsutism.
CONCLUSION
1 Sonino N, et al. Quality of life of hirsute women. Postgrad Med J. 1993;69:186-189.
2 Shulman LH, et al. Serum androgens and depression in women with facial hirsutism. J Am Acad Dermatol. 1992;27:178-181.
3 Derogatis LR, et al. Serum androgens and psychopathology in hirsute women. J Psychosom Obstet Gynaecol. 1993;14:269-282.
4 Hunter MH, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Phys. 2003;67:2565-2572.
5 Leung AK, Robson WL. Hirsutism. Int J Dermatol. 1993;32:773-777.
6 Ahmed B, Jaspan JB. Hirsutism: A brief review. Am J Med Sci. 1994;308:289-294.
7 Azziz R. The evaluation and management of hirsutism. Obstet Gynecol. 2003;101:995-1007.
8 Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440-1447.
9 Hatch R, et al. Hirsutism: Implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
10 Moncada E. Familial study of hirsutism. J Clin Endocrinol Metab. 1970;31:556-564.
11 Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C. Clinical diagnosis of hirsutism in Thai women. J Med Assoc Thai. 2004;87:459-463.
12 Messenger AG. The control of hair growth: An overview. J Invest Dermatol. 1993;101(1 Suppl):4S-9S.
13 Yen S. Chronic anovulation caused by peripheral endocrine disorders. In: Yen S, Jaffe R, editors. Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 3rd ed. Philadelphia: WB Saunders; 1991:1016.
14 Rosenfield RL. Pilosebaceous physiology in relation to hirsutism and acne. Clin Endocrinol Metab. 1986;15:341-362.
15 Randall VA, et al. Hormones and hair growth: Variations in androgen receptor content of dermal papilla cells cultured from human and red deer (Cervus elaphus) hair follicles. J Invest Dermatol. 1993;101(Suppl):114S-120S.
16 Oliver RF, Jahoda CA. Dermal–epidermal interactions. Clin Dermatol. 1988;6:74-82.
17 Moore GP, Panaretto BA, Robertson D. Epidermal growth factor delays the development of the epidermis and hair follicles of mice during growth of the first coat. Anat Rec. 1983;205:47-55.
18 Scott G, et al. Localization of basic fibroblast growth factor mRNA in melanocytic lesions by in situ hybridization. J Invest Dermatol. 1991;96:318-322.
19 Seiberg M, Marthinuss J, Stenn JN. Changes in expression of apoptosis-associated genes in skin mark early catagen. J Invest Dermatol. 1995;104:78-82.
20 Batch JA, Mercuri FA, Werther GA. Identification and localization of insulin-like growth factor-binding protein (IGFBP) messenger RNAs in human hair follicle dermal papilla. J Invest Dermatol. 1996;106:471-475.
21 Cibula D, Hill M, Starka L. The best correlation of the new index of hyperandrogenism with the grade of increased body hair. Eur J Endocrinol. 2000;143:405-408.
22 Thiboutot DM. Clinical review 74: Dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab. 1995;80:3082-3087.
23 Rittmaster RS. Clinical relevance of testosterone and dihydrotestosterone metabolism in women. Am J Med. 1995;98:17S-21S.
24 Shifren JL, Schiff I. The aging ovary. J Womens Health Gend Based Med. 2000;9(Suppl 1):S3-S7.
25 Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf). 1974;3:69-96.
26 Quigley CA, et al. Androgen receptor defects: Historical, clinical, and molecular perspectives. Endocrine Rev. 1995;16:271-321.
27 Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001-3015.
28 Lee DK, Chang C. Endocrine mechanisms of disease: Expression and degradation of androgen receptor: Mechanism and clinical implication. J Clin Endocrinol Metab. 2003;88:4043-4054.
29 Bernasconi D, et al. The impact of obesity on hormonal parameters in hirsute and nonhirsute women. Metabolism. 1996;45:72-75.
30 Rosenfield RL, Lucky AW. Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess. Endocrinol Metab Clin North Am. 1993;22:507-532.
31 Russell DW, Wilson JD. Steroid 5 α-reductase: Two genes/two enzymes. Annu Rev Biochem. 1994;63:25-61.
32 Hamada K, et al. The metabolism of testosterone by dermal papilla cells cultured from human pubic and axillary hair follicles concurs with hair growth in 5 α-reductase deficiency. J Invest Dermatol. 1996;106:1017-1022.
33 Azziz R, et al. Androgen excess in women: Experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
34 O’Driscoll JB, et al. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol (Oxf). 1994;41:231-236.
35 Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev. 2000;21:347-362.
36 Azziz R, et al. Idiopathic hirsutism: An uncommon cause of hirsutism in Alabama. Fertil Steril. 1998;70:274-278.
37 Carmina E. Prevalence of idiopathic hirsutism. Eur J Endocrinol. 1998;139:421-423.
38 Stein I, Leventhal M. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181-191.
39 Carmina E. Diagnosis of polycystic ovary syndrome: From NIH criteria to ESHRE–ASRM guidelines. Minerva Ginecologica. 2004;56:1-6.
40 Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): Arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897-1899.
41 Polson DW, et al. Polycystic ovaries—a common finding in normal women. Lancet. 1988;1:870-872.
42 Clayton RN, et al. How common are polycystic ovaries in normal women and what is their significance for the fertility of the population? Clin Endocrinol (Oxf). 1992;37:127-134.
43 Hassan MA, Killick SR. Ultrasound diagnosis of polycystic ovaries in women who have no symptoms of polycystic ovary syndrome is not associated with subfecundity or subfertility. Fertil Steril. 2003;80:966-975.
44 Erhmann D. Polycystic ovary syndrome. NEJM. 2005;352:1223-1295.
45 Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature. 2001;414:788-791.
46 Diamanti-Kandarakis E, et al. A modern medical quandary: Polycystic ovary syndrome, insulin resistance, and oral contraceptive pills. J Clin Endocrinol Metab. 2003;88:1927-1932.
47 Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:942-947.
48 Dahlgren E, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: A long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57:505-513.
49 Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002;77:1095-1105.
50 Mor E, et al. The insulin resistant subphenotype of polycystic ovary syndrome: Clinical parameters and pathogenesis. Am J Obstet Gynecol. 2004;190:1654-1660.
51 Cabrol C, et al. Current problems in cardiac transplantation. Biomed Pharmacother. 1989;43:87-92.
52 Baillargeon JP, et al. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril. 2004;82:893-902.
53 Rosenfield RL, et al. Dysregulation of cytochrome P450c 17 α as the cause of polycystic ovarian syndrome. Fertil Steril. 1990;53:785-791.
54 Moghetti P, et al. Insulin infusion amplifies 17 α-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: Apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab. 1996;81:881-886.
55 Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441-1449.
56 Nestler JE, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83-89.
57 Ehrmann DA, et al. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141-146.
58 Mor E, et al. The insulin resistant subphenotype of polycystic ovary syndrome: Clinical parameters and pathogenesis. Am J Obstet Gynecol. 2004;190:1654-1660.
59 Rajkhowa M, et al. Altered composition of high density lipoproteins in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:3389-3394.
60 Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: Purposes and pitfalls. Obstet Gynecol Surv. 2004;59:141-154.
61 Speiser PW, White PC. Congenital adrenal hyperplasia. NEJM. 2003;349:776-788.
62 Miller WL. Clinical review 54: Genetics, diagnosis, and management of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1994;78:241-246.
63 New MI. Diagnosis and management of congenital adrenal hyperplasia. Annu Rev Med. 1998;49:311-328.
64 McLaughlin B, et al. Late onset adrenal hyperplasia in a group of Irish females who presented with hirsutism, irregular menses and/or cystic acne. Clin Endocrinol. 1990;32:57-64.
65 Kuttenn F, et al. Late-onset adrenal hyperplasia in hirsutism. NEJM. 1985;313:224-231.
66 Azziz R, et al. Screening for 21-hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: A prospective study. Fertil Steril. 1999;72:915-925.
67 Latronico AC, Chrousos GP. Extensive personal experience: Adrenocortical tumors. J Clin Endocrinol Metab. 1997;82:1317-1324.
68 Cordera F, et al. Androgen-secreting adrenal tumors. Surgery. 2003;134:874-880.
69 Bodie B, et al. The Cleveland Clinic experience with adrenal cortical carcinoma. J Urol. 1989;141:257-260.
70 Young R, Sinclair R. Hirsutes. I: Diagnosis. Australas J Dermatol. 1998;39:24-28.
71 Lobo RA. Ovarian hyperandrogenism and androgen-producing tumors. Endocrinol Metab Clin North Am. 1991;20:773-805.
72 Aiman J. Virilizing ovarian tumors. Clin Obstet Gynecol. 1991;34:835-847.
73 Barbieri RL, Ryan KJ. Hyperandrogenism, insulin resistance, and acanthosis nigricans syndrome: A common endocrinopathy with distinct pathophysiologic features. Am J Obstet Gynecol. 1983;147:90-101.
74 Howlett TA, Rees LH, Besser GM. Cushing’s syndrome. Clin Endocrinol Metab. 1985;14:911-945.
75 Barnes RB. Adrenal dysfunction and hirsutism. Clin Obstet Gynecol. 1991;34:827-834.
76 Wu CH. Plasma androgens, progestins, and prolactin in hirsutism. Eur J Obstet Gynecol Reprod Biol. 1982;13:377-387.
77 Hagag P, et al. Androgen suppression and clinical improvement with dopamine agonists in hyperandrogenic-hyperprolactinemic women. J Reprod Med. 2001;46:678-684.
78 Buvat J, et al. A double blind controlled study of the hormonal and clinical effects of bromocriptine in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1986;63:119-124.
79 Collins DC. Sex hormone receptor binding, progestin selectivity, and the new oral contraceptives. Am J Obstet Gynecol. 1994;170:1508-1513.
80 Bates GW, Cornwell CE. Iatrogenic causes of hirsutism. Clin Obstet Gynecol. 1991;34:848-851.
81 Redmond G.P. Androgenic disorders of women: Diagnostic and therapeutic decision making. Am J Med. 1995;98:120S-129S.
82 Spark RF. Dehydroepiandrosterone: A springboard hormone for female sexuality. Fertil Steril. 2002;77(Suppl 4):S19-S25.
83 Cela E, et al. Prevalence of polycystic ovaries in women with androgenic alopecia. Eur J Endocrinol. 2003;149:439-442.
84 Mehta A, et al. Should androgen levels be measured in hirsute women with normal menstrual cycles? Int J Fertil. 1992;37:354-357.
85 Kiddy DS, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: An analysis of 263 consecutive cases. Clin Endocrinol (Oxf). 1990;32:213-220.
86 Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: Analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84:38-43.
87 Tafaro E, et al. Importance of serum androgens chromatography on the ACTH stimulated adrenal steroidogenesis evaluation in hirsute women. Boll Soc Ital Biol Sper. 1983;59:1877-1882.
88 Boots LR, et al. Measurement of total serum testosterone levels using commercially available kits: High degree of between-kit variability. Fertil Steril. 1998;69:286-292.
89 Miller KK, et al. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89:525-533.
90 Matsumoto AM, Bremner WJ. Serum testosterone assays—accuracy matters. J Clin Endocrinol Metab. 2004;89:520-524.
91 Meldrum DR, Abraham GE. Peripheral and ovarian venous concentrations of various steroid hormones in virilizing ovarian tumors. Obstet Gynecol. 1979;53:36-43.
92 Friedman CI, et al. Serum testosterone concentrations in the evaluation of androgen-producing tumors. Am J Obstet Gynecol. 1985;153:44-49.
93 Hoffman DI, Klove K, Lobo RA. The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women. Fertil Steril. 1984;42:76-81.
94 Tannenbaum C, et al. A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: The Rancho Bernardo Study. Eur J Endocrinol. 2004;151:717-725.
95 Azziz R, Zacur HA. 21-Hydroxylase deficiency in female hyperandrogenism: Screening and diagnosis. J Clin Endocrinol Metab. 1989;69:577-584.
96 Boscaro M, Barzon L, Sonino N. The diagnosis of Cushing’s syndrome: Atypical presentations and laboratory shortcomings. Arch Intern Med. 2000;160:3045-3053.
97 Waldstreicher J, et al. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: Indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165-172.
98 Benacerraf BR, et al. Sonographic accuracy in the diagnosis of ovarian masses. J Reprod Med. 1990;35:491-495.
99 Duh QY. Adrenal incidentalomas. Br J Surg. 2002;89:1347-1349.
100 Hamrahian AH, et al. Clinical utility of noncontrast computed tomography attenuation value (hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J Clin Endocrinol Metab. 2005;90:871-877.
101 Moltz L, et al. Ovarian and adrenal vein steroids in seven patients with androgen-secreting ovarian neoplasms: Selective catheterization findings. Fertil Steril. 1984;42:585-593.
102 Kaltsas GA, et al. Is ovarian and adrenal venous catheterization and sampling helpful in the investigation of hyperandrogenic women? Clin Endocrinol. 2003;59:34-43.
103 Shenenberger DW, Utecht LM. Removal of unwanted facial hair. Am Fam Phys. 2002;66:1907-1911.
104 Ramos-e-Silva M, de Castro MC, Carneiro LVJr. Hair removal. Clin Dermatol. 2001;19:437-444.
105 Lanigan SW. Management of unwanted hair in females. Clin Exp Dermatol. 2001;26:644-647.
106 DiBernardo BE, et al. Laser hair removal: Where are we now? Plastic Reconstruct Surg. 1999;104:247-258.
107 Givens JR, et al. The effectiveness of two oral contraceptives in suppressing plasma androstenedione, testosterone, LH, and FSH, and in stimulating plasma testosterone-binding capacity in hirsute women. Am J Obstet Gynecol. 1976;124:333-339.
108 Fern M, Rose DP, Fern EB. Effect of oral contraceptives on plasma androgenic steroids and their precursors. Obstet Gynecol. 1978;51:541-544.
109 Madden JD, et al. The effect of oral contraceptive treatment on the serum concentration of dehydroisoandrosterone sulfate. Am J Obstet Gynecol. 1978;132:380-384.
110 Raj SG, et al. Normalization of testosterone levels using a low estrogen-containing oral contraceptive in women with polycystic ovary syndrome. Obstet Gynecol. 1982;60:15-19.
111 Bringer J. Norgestimate: A clinical overview of a new progestin. Am J Obstet Gynecol. 1992;166:1969-1977.
112 Dewis P, et al. The treatment of hirsutism with a combination of desogestrel and ethinyl oestradiol. Clin Endocrinol (Oxf). 1985;22:29-36.
113 Volpe A, et al. Efficacy on hyperandrogenism and safety of a new oral contraceptive biphasic formulation containing desogestrel. Eur J Obstet Gynecol Reprod Biol. 1994;53:205-209.
114 Guido M, et al. Drospirenone for the treatment of hirsute women with polycystic ovary syndrome: A clinical, endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817-2823.
115 Henzl MR. Gonadotropin-releasing hormone and its analogues: From laboratory to bedside. Clin Obstet Gynecol. 1993;36:617-635.
116 Rittmaster RS. Use of gonadotropin-releasing hormone agonists in the treatment of hyperandrogenism. Clin Obstet Gynecol. 1993;36:679-689.
117 Chang RJ, et al. Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab. 1983;56:897-903.
118 Rittmaster RS, Thompson DL. Effect of leuprolide and dexamethasone on hair growth and hormone levels in hirsute women: The relative importance of the ovary and the adrenal in the pathogenesis of hirsutism. J Clin Endocrinol Metab. 1990;70:1096-1102.
119 Andreyko JL, Monroe SE, Jaffe RB. Treatment of hirsutism with a gonadotropin-releasing hormone agonist (nafarelin). J Clin Endocrinol Metab. 1986;63:854-859.
120 Azziz R, et al. Leuprolide and estrogen versus oral contraceptive pills for the treatment of hirsutism: A prospective randomized study. J Clin Endocrinol Metab. 1995;80:3406-3411.
121 Spritzer P, et al. Cyproterone acetate versus hydrocortisone treatment in late-onset adrenal hyperplasia. J Clin Endocrinol Metab. 1990;70:642-646.
122 Barbieri RL, Makris A, Ryan KJ. Effects of insulin on steroidogenesis in cultured porcine ovarian theca. Fertil Steril. 1983;40:237-241.
123 Erickson GF, et al. The ovarian androgen producing cells: A review of structure/function relationships. Endocrine Rev. 1985;6:371-399.
124 Pasquali R, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab. 1989;68:173-179.
125 Jakubowicz DJ, Nestler JE. 17 α-Hydroxyprogesterone responses to leuprolide and serum androgens in obese women with and without polycystic ovary syndrome offer dietary weight loss. J Clin Endocrinol Metab. 1997;82:556-560.
126 McCarthy EA, et al. Metformin in obstetric and gynecologic practice: A review. Obstet Gynecol Surv. 2004;59:118-127.
127 Moghetti P, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139-146.
128 Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: Systematic review and meta-analysis. BMJ. 2003;327:951-953.
129 Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 α activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. NEJM. 1996;335:617-623.
130 Vandermolen DT, et al. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril. 2001;75:310-315.
131 Ehrmann DA, et al. Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:524-530.
132 Pasquali R, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:2767-2774.
133 Ibanez L, et al. Sensitization to insulin in adolescent girls to normalize hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism after precocious pubarche. J Clin Endocrinol Metab. 2000;85:3526-3530.
134 Sturrock ND, Lannon B, Fay TN. Metformin does not enhance ovulation induction in clomiphene resistant polycystic ovary syndrome in clinical practice. Br J Clin Pharmacol. 2002;53:469-473.
135 Harborne L, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116-4123.
136 Morin-Papunen LC, et al. Endocrine and metabolic effects of metformin versus ethinyl estradiol–cyproterone acetate in obese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab. 2000;85:3161-3168.
137 Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217-221.
138 Dunaif A, et al. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:3299-3306.
139 Ehrmann DA, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2108-2116.
140 Azziz R, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626-1632.
141 Ghazeeri G, et al. Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertil Steril. 2003;79:562-566.
142 Belli SH, et al. Effect of rosiglitazone on insulin resistance, growth factors, and reproductive disturbances in women with polycystic ovary syndrome. Fertil Steril. 2004;81:624-629.
143 Brettenthaler N, et al. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:3835-3840.
144 Romualdi D, et al. Selective effects of pioglitazone on insulin and androgen abnormalities in normo- and hyperinsulinaemic obese patients with polycystic ovary syndrome. Hum Reprod. 2003;18:1210-1218.
145 Glueck CJ, et al. Pioglitazone and metformin in obese women with polycystic ovary syndrome not optimally responsive to metformin. Hum Reprod. 2003;18:1618-1625.
146 Cumming DC, et al. Treatment of hirsutism with spironolactone. JAMA. 1982;247:1295-1298.
147 Lobo RA, et al. The effects of two doses of spironolactone on serum androgens and anagen hair in hirsute women. Fertil Steril. 1985;43:200-205.
148 Board JA, Rosenberg SM, Smeltzer JS. Spironolactone and estrogen–progestin therapy for hirsutism. South Med J. 1987;80:483-486.
149 Ciotta L, et al. Treatment of hirsutism with flutamide and a low-dosage oral contraceptive in polycystic ovarian disease patients. Fertil Steril. 1994;62:1129-1135.
150 Moghetti P, et al. Flutamide in the treatment of hirsutism: Long-term clinical effects, endocrine changes, and androgen receptor behavior. Fertil Steril. 1995;64:511-517.
151 Cusan L, et al. Comparison of flutamide and spironolactone in the treatment of hirsutism: A randomized controlled trial. Fertil Steril. 1994;61:281-287.
152 Wysowski DK, et al. Fatal and nonfatal hepatotoxicity associated with flutamide. Ann Intern Med. 1993;118:860-864.
153 Rittmaster RS. Hirsutism. Lancet. 1997;349:191-195.
154 Venturoli S, et al. A prospective randomized trial comparing low dose flutamide, finasteride, ketoconazole, and cyproterone acetate–estrogen regimens in the treatment of hirsutism. J Clin Endocrinol Metab. 1999;84:1304-1310.
155 Rittmaster RS. Finasteride. NEJM. 1994;330:120-125.
156 Faloia E, et al. Effect of finasteride in idiopathic hirsutism. J Endocrinol Invest. 1998;21:694-698.
157 Wong IL, et al. A prospective randomized trial comparing finasteride to spironolactone in the treatment of hirsute women. J Clin Endocrinol Metab. 1995;80:233-238.
158 Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078-3082.
159 Asuncion M, Calco RM, San Millan JL, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434-2438.
160 Zargar AH, Wani AI, Masoodi SR, et al. Epidemiologic and etiologic aspects of hirsutism in Kashmiri women in the Indian subcontinent. Fertil Steril. 2002;77:674-678.