CHAPTER 5 HIGHER VISUOPERCEPTUAL DISORDERS AND DISORDERS OF SPATIAL COGNITION
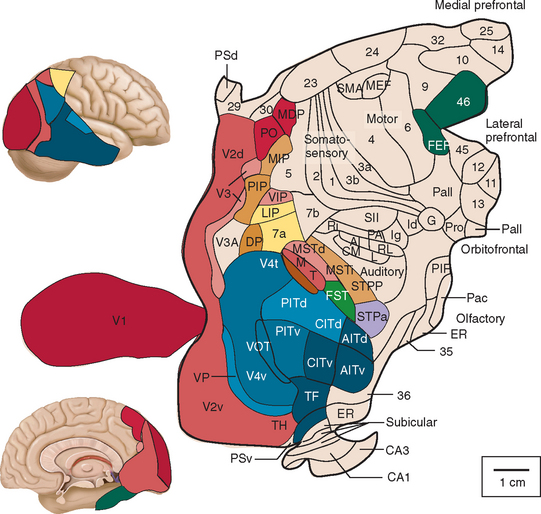
At least one third of the primate brain is devoted to visual perception and visual processing.1 A vast amount has been learned about the visual system in the past 100 years or so, from both laboratory studies and studies of clinical and healthy populations. This research has identified multiple areas in the cerebral cortex (not just the occipital lobe) that receive visual input (Figs. 5-1 through 5-3). There is both parallel and distributed processing of visual stimuli. One of the most famous depictions are the diagrams of Van Essen and colleagues,2 which map the macaque cerebral cortex (Fig. 5-1).
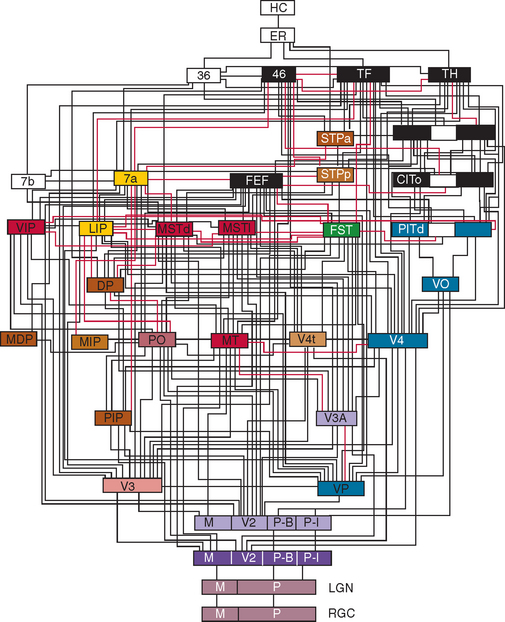
Figure 5-2 Hierarchy of visual areas in the macaque. This diagram shows over 30 visual cortical areas, shaded similarly to Figure 5-1, two subcortical visual stages—the retinal ganglion cell layer (RGC) and the lateral geniculate nucleus (LGN), plus several non-visual areas such as area 7b of the somatosensory cortex, area 36 and the hippocampal complex (HC). These areas are connected by extensive linkages, most of which have been demonstrated to be reciprocal pathways. The patterns of connections help to illustrate two fundamental principles of organization: hierarchical connections and parallel processing.
(From Felleman DJ, Van Essen DC: Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1991; 1:1-47.)
Many disorders of higher visuoperceptual function have been described, some of them in syndromic fashion, and include Balint’s syndrome, cerebral akinetopsia (rare), cerebral dyschromatopsia (somewhat more common), disorders of face recognition (prosopagnosia), environmental disorientation (which overlaps with spatial disorientation: see also Chapter 6), and visual agnosias. Even one of the rarer language disorders may be thought of as a primary visual disturbance: alexia without agraphia (see Chapter 3). Higher-order visual complaints are often one of the first indications of a number of other neurological illnesses such as prion diseases.
EPIDEMIOLOGY
Many of the diseases that underlie these abnormalities are age related—in particular, the dementias and cerebrovascular disease—so these entities are more frequent in older persons and are increasingly commonly encountered as the population ages. Within the neurodegenerative disorders, using Alzheimer’s disease as the prototype, estimates for the prevalence of higher-order visual disturbances are as high as 57%, mainly because of a high prevalence of visual agnosia. The severity of the dementia is strongly correlated with the complexity of the presenting visual disturbance.3
The effects of these higher visuoperceptual disorders are amplified by other disorders of perception that may be present in older people, including visual loss because of anterior visual pathway disease (e.g., cataract, macular degeneration) and impairments of hearing and mobility. Studies have suggested that of people between the ages of 75 and 79, at least 12% have some visual impairment, rising to 23% in the 85- to 89-year-old age range.4 By 90 years of age, at least one in three people will have some visual deficit.4,5
THE TWO-SYSTEMS APPROACH TO HIGHER VISUOSPATIAL FUNCTIONING
Higher visual processing attempts to achieve two primary objectives: the identification of visual stimuli and their localization in space. These two goals of visual analysis are often abbreviated as “what” and “where” (Fig. 5-4). Research has demonstrated that these two goals are achieved relatively independently through two anatomically separated systems known as the ventral (what) and dorsal (where) visual processing pathways.6–8 The fundamental concept is that there is a dorsal stream of information concerned with an object’s location and a ventral stream concerned with its identity. Because this distinction is so widespread in the literature, it must be considered, but even the proponents of this dualistic model freely admit that it is an oversimplification of the true situation, given that strong interactions occur between the two networks.
ANATOMY AND PHYSIOLOGY
In general, higher visuoperceptual and visuospatial disorders reflect damage to one or several multiple brain regions including (but not restricted to) the occipital lobes, temporal lobes, parietal lobes, and underlying white matter. In many of the described syndromes, the right hemisphere appears to be more affected than the left, with many investigators proposing that much of the bilateral activation on higher visual tasks results from the transcallosal influence of the right hemisphere.9,10 The precise nature of the damage for each particular disorder is discussed in the following sections.
Disorders of the Temporal Vision–Related Cortex (Ventral Processing Stream)
Damage to the cortical regions of the ventral processing stream results in a variety of disturbances of visual form and color perception (Table 5-1). These can include dyschromatopsia and alexia without agraphia. Most commonly, however, damage to these regions results in a form of visual agnosia, a clinical syndrome characterized by an inability to recognize a visually presented object despite the presence of adequate cognition, visual acuity, attention, and language skills.11,12
TABLE 5-1 Summary of Clinical Presentation and Location of Lesion in the Temporo-Occipital Vision Disorders
| Condition | Description | Location of Lesion |
|---|---|---|
| Dyschromatopsia | Inability to distinguish colors by hue | Inferior occipital lobe, occipitotemporal lobe |
| Color agnosia (and anomia) | Inability to name colors despite being able to match and sort items by color | |
| Alexia without agraphia or pure alexia | Inability to read despite preserved ability to write | Occipitotemporal lobes |
| Apperceptive (object) | Inability to recognize familiar objects despite intact visual acuity, contrast sensitivity, and often color perception and stereopsis | Bilateral occiptotemporal lobes |
| Associative agnosia | Sensory percept stripped of meaning | Bilateral occiptotemporal lobes |
| Prosopagnosia | Inability to recognize familiar faces | Bilateral (but right > left) occipitotemporal lobes |
| Environmental agnosia | Inability to recognize familiar environments | Right occipitotemporal lobes |
Agnosias are commonly subdivided on the basis of a distinction made by Lissauer,13 who posited in 1890 that the process of recognition has two distinct stages: apperception and association. Apperception is the ability to form a conscious percept of a sensory impression (e.g., an object), which can be thought of as the construction of different visual attributes of the stimulus into a whole percept. Patients who present with these difficulties are said to have the syndrome of apperceptive agnosia, sometimes referred to as apperceptive visual object agnosia.12 Association, on the other hand, refers to the imparting of meaning to the percept, achieved through the use of matching or linking the percept to a previous experience or knowledge.13 Patients with association difficulties, who are said to have the syndrome of associative visual agnosia, often have difficulty accessing the memory of an object’s name or its meaning from the visual stimulus, despite a more or less correctly perceived visual percept, and demonstrably intact knowledge (if accessed via other modalities) of the object’s name and semantic attributes.
Dyschromatopsia and Color Anomia
Dyschromatopsia is a rare acquired inability to discriminate colors by hue.14 Dyschromatopsia is most often associated with damage to the inferior part of the occipital lobes, in the fusiform gyrus. This corresponds to the area identified in functional experiments as the human color area (V4).15,16 Patients usually have difficulty in tasks such as sorting sets of colored counters. Hemidyschromatopsia may be more common; reflecting the location of damage, there is often an associated superior quadrantanopia on the same side. Color anomia is a disorder in which patients present with an inability to name colors but are still able to sort colored counters and differentiate between colors despite failure to name them.17
Alexia Without Agraphia
Alexia without agraphia (or pure alexia) is an acquired reading disorder in which the patient is unable to read, despite preservation of other aspects of language such as spelling and writing.18 Pure alexia is usually caused by an occlusion of distal (posterior) branches of the left posterior cerebral artery. The resultant damage is believed to interrupt the transfer of neural information from the visual cortex to the language cortex. Stroke is one of the most common causes of pure alexia, but more rarely it may result from closed head injury, tumor, occipital lobectomy, arteriovenous malformations, and Alzheimer’s disease. Further details are given in Chapter 3, Disorders of Language.
Associative Agnosia
Those patients with associative agnosia have apparently preserved sensory capability and may retain the ability to match and copy common objects but still fail to recognize them. Such cases led Teuber19 in 1968 to describe this as “a normal percept that has somehow been stripped of its meaning.” Some patients may have their problem limited to a specific class of objects, of which the best known is prosopagnosia—the failure to discriminate and recognize faces. Other object classes with which specific difficulties have been reported include animals or animal species, plants, foods, clothing, makes of cars, colors, or places. Some examples are discussed in the following paragraphs.
Associative Visual Agnosia for Familiar Faces: Prosopagnosia
Prosopagnosia is characterized by an inability to identify familiar faces. Patients may correctly identify the emotion but still fail to identify the face. Given that differences between faces are subtle, the patient with prosopagnosia has difficulty telling them apart but might be able to do so if there is a highly salient feature such as a birthmark or beard. The patient is often able to recognize familiar people from other attributes, such as gait, clothes, or voice. The particular attributes of faces and the task of their recognition are much debated in the literature, but it seems likely that face recognition requires some abilities that do not overlap with other visual recognition abilities. A number of studies have been made of the ability to recognize faces in the normal, upright orientation in a type of gestalt perception, compared with recognizing faces that are upside-down. The latter is a very much harder task for normal subjects and involves different (feature-by-feature rather than whole-object) visual processing. It is intriguing that some prosopagnosics do not have this face inversion effect and, paradoxically, may perform better with inverted faces, suggesting a specific deficit in whole-object processing.20
Prosopagnosia is usually caused by more anterior lesions in the inferior occipitotemporal cortex (the anterior aspects of the lingual and fusiform gyri).21,22 In most cases, bilateral damage is described, but some cases have occurred with just a right-sided lesion. This damage is most commonly a result of stroke, tumors, demyelination, or degenerative atrophy.
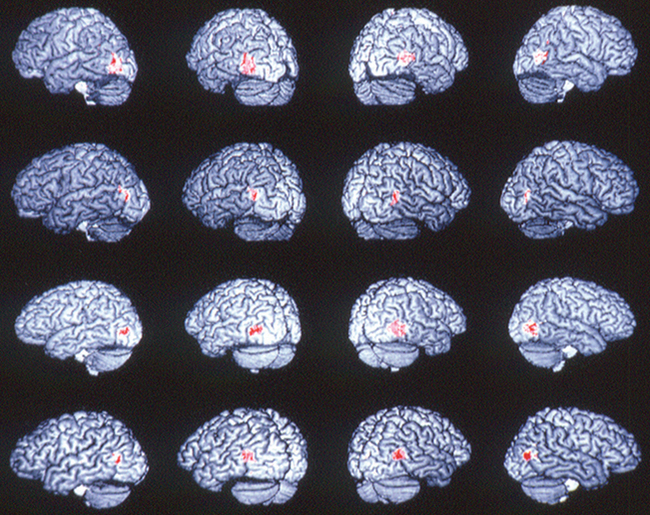
Many face recognition studies on normal subjects have been carried out using functional brain imaging techniques. Haxby and colleagues8 provided some of the key experiments, initially with positron emission topography and then with functional magnetic resonance imaging. Figure 5-5 is an example of the type of stimulus used for these experiments. Figure 5-6 shows the activation along the inferior occipitotemporal cortex in such a face-matching experiment.23
Associative Visual Agnosia: Environmental or Topographical Agnosia
Environmental agnosia, also referred to as topographical disorientation, is an inability to recognize the features or landmarks of a familiar place or region, such as one’s town or even one’s home. Environmental agnosia is associated with lesions in the right medial occipitotemporal region. Unlike the other agnosias, formal testing is not usually required for diagnosis; although disorders such as visual hemineglect may affect navigational tasks, they rarely manifest the profound disorientation observed in this disorder (see Table 5-1).
Disorders of the Parietal Vision–Related Cortex (Dorsal Processing Stream)
Damage to the parietal vision–related cortex commonly results in impairments of the perception, location, and manipulation of items in space (Table 5-2). These can include hemispatial neglect, Balint’s syndrome, impaired spatial relations, and akinetopsia.
TABLE 5-2 Summary of Clinical Presentation and Location of Lesions in Parieto-occipital Vision Disorders
| Condition | Description | Location of Lesion |
|---|---|---|
| Hemispheric neglect | Patients fail to respond to, report, or act on meaningful stimuli that are presented to the side opposite a brain lesion | Unilateral parietal lobe (usually greater on right) |
| Balint’s syndrome | Triad of symptoms of optic ataxia, oculomotor apraxia, and visuospatial inattention (simultanagnosia) | Bilateral damage to the parieto-occipital region |
| Optic ataxia | Inability to guide limbs visually | Bilateral parieto-occipital region |
| Oculomotor apraxia | Inability to execute purposeful eye movements | Bilateral parieto-occipital region |
| Simultanagnosia | Inability to perceive entire picture or integrate separate parts | Bilateral parieto-occipital region |
| Impaired spatial relations | Impaired spatial relations in which patients misestimate size, distance, shape, and orientation. | Bilateral parieto-occipital region |
| Akinetopsia | Inability to detect motion | Damage to the human visual motion area (V5/MT) |
Hemispatial Neglect
Hemispatial neglect is said to be present when patients fail to respond, report, or act on meaningful stimuli that are presented to the side contralateral to a brain lesion. This deficit cannot be attributed to sensory or motor deficits and usually results from unilateral lesions of the posterior parietal region, although other critical regions have also been identified. This disorder is discussed in detail in Chapter 6.
Balint’s Syndrome
Patients with Balint’s syndrome usually present with a triad of features—optic ataxia, oculomotor gaze apraxia, and simultanagnosia/visual inattention—as a result of bilateral damage to the parieto-occipital regions. Causes include strokes, tumor, trauma, Creutzfeldt-Jakob disease, diffuse cerebral hypotension/hypoxia, Alzheimer’s disease (especially its posterior cortical atrophy variant),24–26 and human immunodeficiency virus–associated encephalitis. Optic ataxia refers to the inaccurate reaching for a stationary object in extrapersonal space, and although it usually manifests concurrently with oculomotor gaze apraxia and simultanagnosia, it may exist independently. The differential diagnosis includes cerebellar ataxia and proprioceptive deficits. The distinction can usually be made by comparing the reaching inaccuracy to an object in the patient’s own space (e.g., his or her nose) with that to an item that is specifically extrapersonal (e.g., the examiner’s finger held at arm’s length). The second component of Balint’s syndrome is oculomotor gaze apraxia, also called psychic paresis of gaze, which is a defect of volitional pursuit movements and saccadic movements to visual targets, not attributable to visual inattention, in the presence of preserved spontaneous and reflexive saccades. Completing the triad is visual inattention (or simultanagnosia), which is a reduced ability to detect more than one visual object at the same time, regardless of size. The most common complaints that might flag such a diagnosis included persistent complaints of bumping into things, “tunnel vision,” and reading difficulties. Impaired spatial relations are also found in this syndrome, including difficulty in estimating the size, distance, orientation, and shape of objects.
Akinetopsia
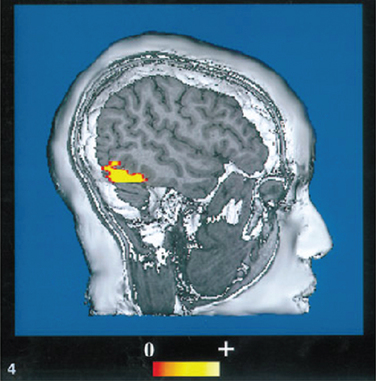
Akinetopsia is an inability to detect motion, so that a moving object such as a car will appear to “jump” from one stationary position to another. Compared with the other higher visuospatial disorders, cases of cerebral akinetopsia are very rare. The human visual motion area (V5/MT) has been identified to lie in the lateral cortex at the junction of the occipital, parietal, and temporal lobes (Fig. 5-7).27 The only two cases of akinetopsia from bilateral lesions have been well described in the literature.28,29
DIAGNOSIS
As with much of clinical medicine, and in particular clinical neurology, a detailed history is of paramount importance in achieving an accurate diagnosis of higher visuoperceptual and visuospatial spatial disorders. Indeed, despite the descriptions of the classical, “pure” syndromes in the previous section, such isolated deficits are distinctly uncommon, and patients more usually present with elements of two or more of these syndromes. Many patients may be dismissed as having psychiatric conditions. A number of patients in our experience have been seen by optometrists and ophthalmologists and have been told that there was nothing wrong with their eyes, without the possibility of an organic process affecting the brain being considered.
Cerebral imaging may be useful, including computed tomography and particularly magnetic resonance imaging. This may show generalized or focal atrophy, evidence of nonfocal and/or discrete cerebral ischemic changes, space occupying lesions such as tumors or abscesses, or even the characteristic appearances of Creutzfeldt-Jakob disease (especially on diffusion-weighted images). Other forms of cerebral imaging that may help include single-photon emission computed tomography and positron emission tomography scans, although the latter are often not as accessible or affordable. Patients with such disorders may from time to time also undergo electroencephalograms and visual evoked responses, but in our experience these are not often helpful.
A Screening Battery for Detecting and Diagnosing Disorders of Higher Visual Function
Neuropsychologists have a wide range of specialized tools for assessing particular aspects of higher visual disorders, with a variety of normed tests. These can be classified according to whether they focus on visuoperceptional, visuospatial, or visuoconstructional impairments (Tables 5-3 and 5-4).
TABLE 5-3 Neuropsychological Tests Used to Assess Visuoperceptual, Visuospatial, and Visuoconstructional Abilities
| Visuoperceptual | Visuospatial | Visuoconstructional |
|---|---|---|
| Benton Visual Form Discrimination Test | Line Bisection Test | Clock drawing test (Addenbrooke’s Cognitive Examination) |
| Hooper Visual Organization Test | Mesulam’s Cancellation Test | Rey-Osterrieth Complex Figure Test |
| Embedded Figures Test | Benton Judgment of Line Orientation | Block Design Test (Wechsler Adult Intellegence Scale) |
| Visual Object and Space Perception Battery | Visual Object and Space Perception Battery | |
| Benton Facial Recognition Test |
TABLE 5-4 Screening Battery for Higher Visual Disorders for the Neurologist
| Disorder | Practical Screening Batteries for the Differential Diagnosis |
|---|---|
| Dyschromatopsia | Ask the patient to name colors presented to them. Those with dyschromatopsia will fail this task. |
| Ask the patient to read Ishihara color plates. Patients with the more severe cases of dyschromatopsia will have difficulty on this task. | |
| Ask the patient to match colored counters (Farnsworth tests). | |
| Patients will generally have difficulty with this task if they have dyschromatopsia. | |
| Color anomia/color agnosia | Ask the patient to name colors presented to them. They should be unable to do this. |
| Color agnosia | Ask the patient to match colored counters. Unlike patients with dyschromatopsia, they will be able to complete the task. |
| Ask the patient “What color is the sky?” and similar questions. The patient will be able to give the correct answer. | |
| Ask the patient to color in a picture giving them colored pens. If the patient colors with appropriate colors (e.g., a tree trunk as brown) he is more likely suffering from color anomia. If unable to do so, the differential diagnosis includes color agnosia. | |
| Alexia without agraphia | Ask the patient to read a paragraph from a magazine or newspaper, progressing down to single words and letters; if impaired, proceed to Step 2. |
| Ask the patient to trace the letters and try to identify each of them. | |
| Someone with alexia without agraphia should be able to identify them by tracing. | |
| Visual agnosia | Hold up an object such as glasses or a stopwatch and ask patients to name the object. Those with visual agnosia will be unable to do so. |
| Ask the patient to mime an object’s use (e.g., a toothbrush or hammer). He will generally be unable to mime the object’s use. | |
| Give the object to the patient to hold, and ask the patient to name it. Those with visual agnosia will be able to name the object with the help of perceptual cues. | |
| Formal neuropsychological testing is usually required to determine the specific type of visual agnosia. | |
| Prosopagnosia | Ask the patient to identify pictures of famous people from a magazine or other pictures. A patient with prosopagnosia will have difficulty naming famous people. |
| If the patient is able to name family members, ask a spouse or family member to wear an unusual item such as large glasses and a hat and return to the room without speaking. Failure to identify the family member is strongly suggestive of prosopagnosia. | |
| Environmental agnosia | Family members report disorientation and confusion in familiar places or areas. |
| Exclude hemispatial neglect. | |
| Hemispatial neglect | Ask the patient to copy a simple drawing of a house or tree. A patient with true hemispatial neglect will generally fail to draw a particular side of the picture or will only draw one part of the object. |
| Ask the patient to search for a target or targets in an array of shapes or letters. Patients with hemispatial neglect will miss targets in the affected part of their visual field. | |
| Balint’s syndrome | Simultagnosia: Ask the patient to describe what is going on in a complex picture or copy a complex drawing (such as the Rey figure). A person with simultagnosia will tend to focus on one small area and is generally unable to integrate the parts of the picture into the whole. |
| Optic ataxia: Ask the patient to reach out or point to a target object in space. A patient with optic ataxia will be unable to do this accurately, a form of visual disorientation. | |
| Oculomotor apraxia: Observe the patient’s gaze while the patient is asked to make voluntary eye movements in different directions. | |
| Impaired spatial relations | Ask the patient to draw a clock face with the hands at a particular time. A patient with impaired spatial relations will have difficulty arranging the hands, circle, and numbers. One must always remember impaired semantic function (such as in Alzheimer disease or focal lobar atrophy of the temporal lobes) can affect such tasks as drawing a clock in a similar way. |
| Ask the patient to complete a simple maze task. Patients with impaired spatial relations will have trouble staying within the lines and completing the task. | |
| Akinetopsia | Patient reports seeing the world in “snapshots” or as if strobe lighting is present. Ask the patient about difficulties in crossing the road, anticipating speeds of cars, and so on. |
Assessment Strategies for the Non–neuropsychologist
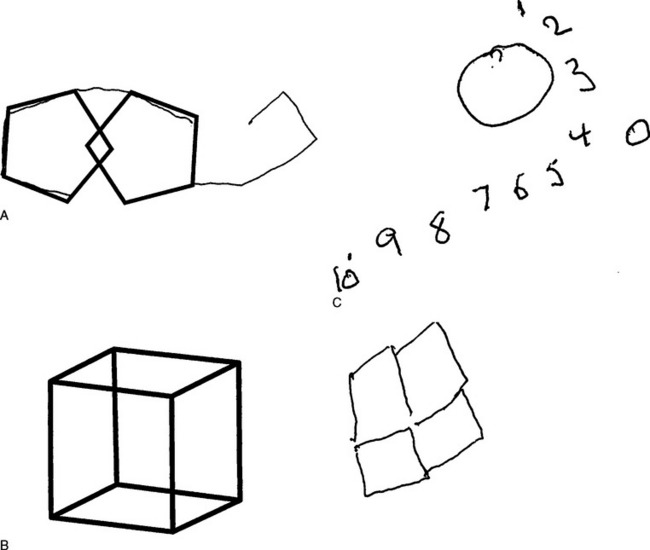
While a neurologist frequently requests a neuropsychological assessment to assess visual impairments formally, in the context usually of a dementia, there are simple ways to screen for suspected disorders within the course of a neurological examination.30 The commonly used screening instrument for dementia, the Folstein Mini-Mental State Examination (MMSE), is inadequate for an assessment of this type. The only test items in this or in similar screening examinations that are especially relevant to higher visuoperceptual and visuospatial disorders are the copying of the interlocking pentagons and cube, the drawing of a clock face, the naming of objects such as the watch and pencil, the reading and subsequent carrying out of a simple command, and the writing of a sentence (although the last is more relevant to language testing). One can also examine for neglect phenomena (see Chapter 6) by asking the patient to copy diagrams, bisect lines, draw and copy a clock, and search for a target or targets in an array of shapes or letters (Fig. 5-8).
The more complex copying of the Rey-Osterrieth figure is extremely sensitive to higher visuoperceptual and visuospatial disorders (Figs. 5-9 and 5-10). It has the advantage of a scoring system, with norms being widely available. Object recognition can be tested by having people name and describe visually presented objects, match objects out of an array, and copy and spontaneously draw objects. The patient’s verbal knowledge of named objects and ability to name objects by touch or other sensory inputs rather than sight should also be checked, to ensure that difficulties with visual recognition are not merely manifestations of a broader dissolution of semantic knowledge about the objects, rather than a visual agnosia. These functions can be more formally assessed by a number of neuropsychological tests.
TREATMENT AND ASSISTANCE
The first important task of the clinician in this situation is to make a diagnosis or institute steps that will lead to a diagnosis; the condition must be recognized for what it is, delineated, labeled appropriately, and explained to the patient, family members, and others. If investigations demonstrate a treatable cause (e.g., a tumor), then correct treatment should be implemented.
CONCLUSIONS
Farah MJ. Visual Agnosia, 2nd ed. Cambridge: MIT Press/Bradford Books, 2004.
Heilman KM, Valenstein E, editors. Clinical Neuropsychology, 4th ed., New York: Oxford University Press, 2003.
Barton JJS, Rizzo M, editors. Vision and the Brain, Parts 1 and 2. Neurologic Clinics, Vol. 21, parts 2 and 3. Philadelphia: WB Saunders, 2003.
1 Sereno MI, Dale AM, Reppas JB, et al. Borders of multiple visual areas in human revealed by functional magnetic resonance imaging. Science. 1995;268:889-893.
2 Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1-47.
3 Mendez MF, Mendez MA, Martin R, et al. Complex visual disturbances in Alzheimer’s disease. Neurology. 1990;40:439-443.
4 Evans R, Fletcher AE, Wormald RPL, et al. Prevalence of visual impairment in people aged 75 years and older in Britain: results from the MRC trial of assessment and management of older people in the community. Br J Ophthalmol. 2002;86:795-800.
5 Taylor HR, Keeffe JE, Vu HT, et al. Vision loss in Australia. Med J Aust. 2005;182:565-568.
6 Ungerleider LG, Haxby JV. “What” and “where” in the human brain. Curr Opin Neurobiol. 1994;4:157-165.
7 Haxby JV, Grady CL, Horwitz B, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci U S A. 1991;88:1621-1625.
8 Haxby JV, Horwitz B, Ungerleider LG, et al. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336-6353.
9 Horwitz B, Soncrant TT, Haxby JV. Covariance analysis of functional interactions in the brain using metabolic and blood flow data. In: Gonzalez-Lima F, Finkenstaedt T, Scheich H, editors. Advances in Metabolic Mapping Techniques for Brain Imaging of Behavioral and Learning Functions. Dordrecht: Kluwer; 1992:189-217.
10 McIntosh AR, Grady CL, Ungerleider LG, et al. Network analysis of cortical visual pathways mapped with PET. J Neurosci. 1994;14:655-666.
11 Bauer RM, Demery JA. Agnosia. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 4th ed. New York: Oxford University Press; 2003:236-295.
12 Farah MJ. Visual Agnosia: Disorders of Object Recognition and What They Tell Us About Normal Vision. Cambridge: MIT Press, 1990.
13 Lissauer H. Ein fall von seelenblindheit nebst einem Beitrage zur Theori derselben [A case of visual agnosia with a contribution to theory]. Arch Psychiatr Nervenkrankheiten. 1890;21:222-270.
14 Heilman KM, Valenstein E. Clinical Neuropsychology, 4th ed. New York: Oxford University Press, 2003.
15 Lueck CJ, Zeki S, Friston KJ, et al. The colour centre in the cerebral cortex of man. Nature. 1989;340:386-389.
16 Zeki S, Watson JDG, Lueck CJ, et al. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11:641-649.
17 Denes G, Semenza C, Stoppa E, et al. Unilateral spatial neglect and recovery from hemiplegia: a follow-up study. Brain. 1982;105:543-552.
18 Warrington EK, Shallice T. Word-form dyslexia. Brain. 1980;103:99-112.
19 Teuber HL. Alteration of perception and memory in man. In: Weiskrantz L, editor. Analysis of Behavioral Change. New York: Harper and Row, 1968.
20 Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? J Exp Psychol Hum Percept Perform. 1995;21:628-634.
21 Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology. 1982;32:331-341.
22 Farah M, Levinson KL, Klein KL. Face perception and within-category discrimination in prosopagnosia. Neuropsychologia. 1995;33:661-674.
23 Clark VP, Keil K, Maisog JM, et al. Functional magnetic resonance imaging (fMRI) of human visual cortex during face matching: a comparison with positron emission tomography (PET). Neuroimage. 1996;4:1-15.
24 Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dementia Geriatr Cogn Disord. 2002;14:33-40.
25 Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789-793.
26 Caine D. Posterior cortical atrophy: a review of the literature. Neurocase. 2004;10:382-385.
27 Watson JDG, Myers R, Frackowiak RSJ, et al. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79-94.
28 Zihl J, von Cramon D, Mai N. Selective disturbance of movement vision after bilateral brain damage. Brain. 1983;106:313-340.
29 Vaina LM. Functional segregation of color and motion processing in the human visual cortex: clinical evidence. Cereb Cortex. 1994;45:555-572.
30 Hodges JR. Cognitive Assessment for Clinicians. Oxford: Oxford University Press, 1994.