Chapter 89 High-Risk Pregnancies
High-risk pregnancies are those that increase the likelihood of abortion, fetal death, premature delivery, intrauterine growth restriction, poor cardiopulmonary or metabolic transitioning at birth, fetal or neonatal disease, congenital malformations, mental retardation, or other handicaps (see Table 89-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com ![]() ; Chapter 90). Some factors, such as ingestion of a teratogenic drug in the 1st trimester, are causally related to the risk; others, such as hydramnios, are associations that alert a physician to determine the etiology and avoid the inherent risks associated with excessive amniotic fluid. On the basis of their history, 10-20% of pregnant women can be identified as being at high risk; nearly half of all perinatal mortality and morbidity is associated with these high-risk pregnancies. Although assessing antepartum risk is important in reducing perinatal mortality and morbidity, some pregnancies become high risk only during labor and delivery; therefore, careful monitoring is critical throughout the intrapartum course.
; Chapter 90). Some factors, such as ingestion of a teratogenic drug in the 1st trimester, are causally related to the risk; others, such as hydramnios, are associations that alert a physician to determine the etiology and avoid the inherent risks associated with excessive amniotic fluid. On the basis of their history, 10-20% of pregnant women can be identified as being at high risk; nearly half of all perinatal mortality and morbidity is associated with these high-risk pregnancies. Although assessing antepartum risk is important in reducing perinatal mortality and morbidity, some pregnancies become high risk only during labor and delivery; therefore, careful monitoring is critical throughout the intrapartum course.
Table 89-1 FACTORS ASSOCIATED WITH HIGH-RISK PREGNANCY
ECONOMIC
CULTURAL-BEHAVIORAL
BIOLOGIC-GENETIC
REPRODUCTIVE
MEDICAL
Maternal Factors
The lowest neonatal mortality rate occurs in infants of mothers who receive adequate prenatal care and who are 20-30 yr of age. Pregnancies in both teenagers and women older than 40 yr, particularly primiparous women, are at increased risk for intrauterine growth restriction, fetal distress, and intrauterine death. Advanced maternal age increases the risk of both chromosomal and nonchromosomal fetal malformations (Fig. 89-1).
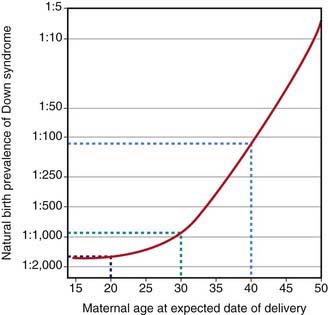
Figure 89-1 Natural birth prevalence of Down syndrome according to maternal age.
(From Wald NJ, Leck I: Antenatal and neonatal screening, ed 2, Oxford, 2000, Oxford University Press.)
Maternal illness (Table 89-2), multiple pregnancies (particularly those involving monochorionic twinning), infections (Table 89-3), and certain drugs (Chapter 90) increase the risk for the fetus. The use of assisted reproductive technology (in vitro fertilization, intracytoplasmic sperm injection) increases the risk of perinatal mortality, infant morbidity, prematurity, low and very low birthweight, and cerebral palsy, largely because of the increase in multiple-fetus pregnancies with such technology; the risks for birth defects are also increased, in part, because of epigenetic effects on gene expression.
Table 89-2 MATERNAL CONDITIONS AFFECTING THE FETUS OR NEONATE
| DISORDER | EFFECT(S) | MECHANISM(S) |
|---|---|---|
| Autoantibody against folate receptors | Neural tube defects | Blockage of cellular uptake of folate |
| Cervical neoplasia | Preterm premature rupture of membranes | Associated with loop electrosurgical excision procedure or cone therapy |
| Cholestasis | Preterm delivery, intrauterine fetal demise | Unknown, possibly hepatitis E |
| Cyanotic heart disease | Intrauterine growth restriction | Low fetal oxygen delivery |
| Diabetes mellitus: | ||
| Mild | Large for gestational age, hypoglycemia | Fetal hyperglycemia—produces hyperinsulinemia; insulin promotes growth |
| Severe | Growth restriction | Vascular disease, placental insufficiency |
| Drug addiction | Intrauterine growth restriction, neonatal withdrawal | Direct drug effect plus poor diet |
| Endemic goiter | Hypothyroidism | Iodine deficiency |
| Graves disease | Transient neonatal thyrotoxicosis | Placental immunoglobulin passage of thyroid-stimulating antibody |
| Herpes gestationis (noninfectious) | Bullous rash, intrauterine fetal demise | Unknown |
| Hyperparathyroidism | Neonatal hypocalcemia | Maternal calcium crosses to fetus and suppresses fetal parathyroid gland |
| Hypertension | Intrauterine growth restriction, intrauterine fetal demise | Placental insufficiency, fetal hypoxia |
| Idiopathic thrombocytopenic purpura | Thrombocytopenia | Nonspecific maternal platelet antibodies cross placenta |
| Isoimmune neutropenia or thrombocytopenia | Neutropenia or thrombocytopenia | Specific antifetus neutrophil or platelet antibody crosses placenta after sensitization of mother |
| Malignant melanoma | Placental or fetal tumor | Metastasis |
| Myasthenia gravis | Transient neonatal myasthenia | Immunoglobulin to acetylcholine receptor crosses placenta |
| Myotonic dystrophy | Neonatal myotonic dystrophy, congenital contractures, respiratory insufficiency | Genetic anticipation |
| Obesity | Macrosomia, hypoglycemia | Unknown |
| Phenylketonuria | Microcephaly, retardation | Elevated fetal phenylalanine values |
| Poor nutrition | Intrauterine growth restriction, adult insulin resistance, schizophrenia(?) | Reduced fetal nutrients, nutritional programming |
| Preeclampsia, eclampsia | Intrauterine growth restriction, thrombocytopenia, neutropenia, fetal demise | Uteroplacental insufficiency, fetal hypoxia, vasoconstriction |
| Renal transplantation | Intrauterine growth restriction | Uteroplacental insufficiency |
| Rhesus or other blood group sensitization | Fetal anemia, hypoalbuminemia, hydrops, neonatal jaundice | Antibody crosses placenta and is directed to fetal cells with antigen |
| Sickle cell anemia | Preterm birth, intrauterine growth restriction, stillbirth | Maternal sickling producing fetal hypoxia |
| Systemic lupus erythematosus | Congenital heart block, rash, anemia, thrombocytopenia, neutropenia | Antibody directed to fetal heart, red and white blood cells, and platelets |
Table 89-3 MATERNAL INFECTIONS AFFECTING THE FETUS OR NEWBORN
| INFECTION | MODE(S) OF TRANSMISSION | OUTCOME |
|---|---|---|
| BACTERIA | ||
| Group B streptococcus | Ascending cervical | Sepsis, pneumonia |
| Escherichia coli | Ascending cervical | Sepsis, pneumonia |
| Listeria monocytogenes | Transplacental | Sepsis, pneumonia |
| Ureaplasma urealyticum | Ascending cervical | Pneumonia, meningitis |
| Mycoplasma hominis | Ascending cervical | Pneumonia |
| Chlamydia trachomatis | Vaginal passage | Conjunctivitis, pneumonia |
| Syphilis | Transplacental, vaginal passage | Congenital syphilis |
| Borrelia burgdorferi | Transplacental | Prematurity, fetal demise |
| Neisseria gonorrhoeae | Vaginal passage | Ophthalmia (conjunctivitis), sepsis, meningitis |
| Mycobacterium tuberculosis | Transplacental | Prematurity, fetal demise, congenital tuberculosis |
| Granulocytic ehrlichiosis | Transplacental | Sepsis |
| VIRUS | ||
| Rubella | Transplacental | Congenital rubella |
| Cytomegalovirus | Transplacental, breast milk (rare) | Congenital cytomegalovirus or asymptomatic |
| HIV | Transplacental, vaginal passage, breast milk | Congenital acquired immunodeficiency syndrome |
| Hepatitis B | Vaginal passage, transplacental, breast milk | Neonatal hepatitis, chronic hepatitis B surface antigen carrier state |
| Hepatitis C | Transplacental | Uncommon, but neonatal hepatitis, chronic carrier state possible |
| Lymphocytic choriomeningitis | Transplacental | Fetal, neonatal death; hydrocephalus, chorioretinitis |
| Herpes simplex type 2 or 1 | Transplacental | Congenital herpes simplex virus |
| Vaginal passage, ascending | Neonatal encephalitis, disseminated viremia | |
| Varicella-zoster | Transplacental: | |
| Early | Congenital anomalies | |
| Late | Neonatal varicella | |
| Parvovirus | Transplacental | Fetal anemia, hydrops |
| Coxsackie B | Fecal-oral | Myocarditis, meningitis, hepatitis |
| Poliomyelitis | Transplacental | Congenital poliomyelitis |
| Epstein-Barr | Transplacental | Anomalies(?) |
| Rubeola | Transplacental | Abortion, fetal measles |
| West Nile | Transplacental | Chorioretinitis, focal cerebral necrosis |
| PARASITES | ||
| Toxoplasmosis | Transplacental | Congenital toxoplasmosis or asymptomatic |
| Malaria | Transplacental | Abortion, prematurity, intrauterine growth restriction |
| Trypanosomiasis | Transplacental | Congenital Chagas disease |
| Hookworm | None | Maternal anemia, low birthweight |
| FUNGI | ||
| Candida | Ascending, cervical | Sepsis, pneumonia, rash |
| PRION | ||
| Creutzfeldt-Jakob disease | Transplacental, colostrum | Hypothetical route, no long-term data |
Preterm birth is common in high-risk pregnancies (Chapter 91). Factors associated with prematurity, noted in Table 89-1, include biologic markers such as cervical shortening, genital infection, fetal fibronectin in cervicovaginal secretions, serum α-fetoprotein, and preterm premature rupture of membranes (PROM). PROM occurs in 1% of pregnancies but is noted in 30-40% of preterm deliveries, and it is a leading identifiable cause of prematurity. Premature delivery is often difficult to predict.
Acute polyhydramnios is rare and is usually associated with premature labor and delivery, before 28 wk of gestation. Chronic polyhydramnios is diagnosed in the 3rd trimester from the discrepancy between uterine size and gestational age; it is occasionally not diagnosed until the patient has dysfunctional labor or an abnormally large amount of amniotic fluid is noted during delivery. Polyhydramnios is associated with premature labor, abruptio placentae, multiple congenital anomalies, and fetal neuromuscular dysfunction or obstruction of the gastrointestinal tract that interferes with reabsorption of the amniotic fluid that is normally swallowed by the fetus (Table 89-4). Increased fetal urination or edema formation is also associated with excessive amniotic fluid volume. Ultrasound demonstrates the increased amniotic fluid surrounding the fetus and detects associated fetal anomalies, hydrops, pleural effusions, and ascites. In 60% of patients, no cause is identified. Symptomatic polyhydramnios may be managed by serial amniocenteses or by short-course maternal indomethacin if the problem is due to excessive fetal urination. Treatment is indicated for acute maternal respiratory distress and threatened preterm labor or to provide time for the administration of corticosteroids to enhance fetal lung maturity.
Table 89-4 CONDITIONS ASSOCIATED WITH DISORDERS OF AMNIOTIC FLUID VOLUME
OLIGOHYDRAMNIOS
POLYHYDRAMNIOS
Oligohydramnios is associated with congenital anomalies; intrauterine growth restriction; severe renal, bladder, or urethral anomalies; and drugs that interfere with fetal urination (see Table 89-4). Oligohydramnios becomes most evident after 20 wk of gestation, when fetal urination is the major source of amniotic fluid. Rupture of the membranes is the most common cause of oligohydramnios and must be ruled out if oligohydramnios is suspected, especially if a normal-sized bladder is seen on fetal ultrasound. Oligohydramnios causes fetal compression abnormalities such as fetal distress, clubfoot, spadelike hands, and a flattened nasal bridge. The most serious complication of chronic oligohydramnios is pulmonary hypoplasia. The risk of umbilical cord compression during labor and delivery is increased in pregnancies complicated by oligohydramnios and may be alleviated by saline amnioinfusion. Prophylactic intrapartum amnioinfusion reduces the need for cesarean section and improves Apgar scores.
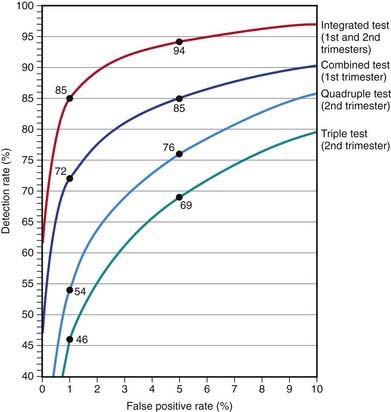
Several effective screening strategies can be used to detect Down syndrome (Fig. 89-2), including a combination of maternal age, nuchal translucency on ultrasound, and a number of serum markers: α-fetoprotein, unconjugated estriol, total human chorionic gonadotropin (HCG), the free β subunit of HCG, inhibin A, and pregnancy-associated plasma protein A. The most effective strategy, the integrated test, combines 1st- and 2nd-trimester screening and can identify 94% of affected pregnancies with a 5% false-positive rate or 85% with a 1% false-positive rate (Chapter 76.1). Absence of the fetal nasal bone is also noted in trisomy 21. Chromosomal analysis of cells obtained by amniocentesis or chorionic villus sampling makes the diagnosis.
ACOG Practice Bulletin. Assessment of risk factors for preterm birth. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2001;98:709-716.
Benn PA, Chapman AR. Practical and ethical considerations of noninvasive prenatal diagnosis. JAMA. 2009;301:2154-2156.
Chitty LS, Kagan KO, Molina FS, et al. Fetal nuchal translucency scan and early prenatal diagnosis of chromosomal abnormalities by rapid aneuploidy screening: observational study. BMJ. 2006;332:452-454.
Chiu RWK, Akolekar R, Zheng YWL, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
Cunniff C. Prenatal screening and diagnosis for pediatricians. Pediatrics. 2004;114:889-894.
Driscoll DA, Gross S. Prenatal screening for aneuploidy. N Engl J Med. 2009;360:2556-2562.
Ecker JL, Frigoletto FDJr. Cesarean delivery and the risk-benefit calculus. N Engl J Med. 2007;356:885-888.
Ekelund CK, Jørgensen FS, Petersen OB, et al. Impact of a new national screening policy for Down’s syndrome in Denmark: population based cohort study. BMJ. 2009;338:449-452.
Goldenberg RL, Iams JD, Mercer BM, et al. What we have learned about the predictors of preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Semin Perinatol. 2003;27:185-193.
Guise JM, McDonagh MS, Osterweil P, et al. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. Br Med J. 2004;329:19-22.
Hansen M, Kurinczuk JJ, Bower C, et al. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725-730.
Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Eng J Med. 2004;351:2581-2589.
Klemetti R, Gissler M, Sevón T, et al. Children born after assisted fertilization have an increased rate of major congenital anomalies. Fertil Steril. 2005;84:1300-1307.
Klemetti R, Sevón T, Gissler M, et al. Health of children born as a result of in vitro fertilization. Pediatrics. 2006;118:1819-1827.
Malone FD, Canick JA, Ball RH, et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001-2011.
Pitt C, Sanchez-Ramos L, Kauntiz AM, et al. Prophylactic amnioinfusion for intrapartum oligohydramnios: a meta-analysis of randomized controlled trials. Obstet Gynecol. 2000;96:861-866.
Steer PJ, Modi N. Elective caesarean sections—risks to the infant. Lancet. 2009;374:675-676.