Chapter 25 High-Grade Gliomas
Etiology and Epidemiology
Most malignant brain tumors are high-grade gliomas, and most of these are GBM, a World Health Organization (WHO) grade IV tumor. The remainder are WHO grade III tumors such as anaplastic astrocytoma, anaplastic oligodendroglioma, and anaplastic oligoastrocytoma. Men are more commonly affected than women. The peak incidence occurs in the age range of 65 to 75 years, and the median survival time is inversely proportional to age; these findings have prompted a redoubling of efforts in elderly subpopulations.1
There has been concern for cancer development following exposure to electromagnetic fields, but definitive evidence is lacking. More recently, the use of cellular telephones has been studied extensively in Europe,2 but its importance as a risk factor has not been established. In terms of chemical exposure, nitrosamines have long been regarded as culpable, but causality is far from proven.3 Brain tumors have also been linked with previous exposure to therapeutic ionizing radiation.3 However, the absolute risk is low.
Most gliomas are sporadic, but genetic susceptibility is suspected based on the occurrence of multiple brain tumors in families with germline mutation of the TP53 suppressor gene and patients with neurofibromatosis type I as well as the rare patients who have been diagnosed with Turcot’s syndrome. A heritable syndrome contributes to less than 5% of GBMs.4
Prevention and Early Detection
No viable strategy for screening or early detection of glial tumors has been developed. There is also no convincing evidence demonstrating either improved survival when high-grade gliomas are found early, or a clear rationale for prophylactic strategies to reduce the incidence of these aggressive tumors.3
Biologic Characteristics and Molecular Biology
Investigators around the world are searching for the molecular biologic characteristics of gliomas in an effort to improve therapy. For example, work by the Cancer Genome Atlas Research Network5 and others6 suggests that at least three molecular subclasses of GBM exist with potential therapeutic and prognostic implications. A set of nine genes (derived from a larger set of 38 genes) identifiable from paraffin blocks is also under investigation as a stratification factor in an ongoing phase III trial (Radiation Therapy Oncology Group [RTOG] 0825).7 Mouse modeling has also demonstrated the oncogenic importance of abnormalities in receptor signaling (e.g., epidermal growth factor receptor [EGFR] and platelet-derived growth factor receptor [PDGFR]), signal transduction cascades (e.g., RAS and AKT), and cell cycle regulation.8
In the early 1990s, it was recognized that deletion of the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q) occurs in most oligodendrogliomas.9 Co-deletion of 1p and 19q is prognostic for longer survival,10,11,12 although controversy continues as to whether this finding should alter therapy.13 It is now recognized that an unbalanced chromosomal translocation underlies 1p/19q co-deletion,14 but the specific genes involved and their mechanism of action remain elusive. More recently, mutations in the isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) genes, occurring most often in low-grade gliomas but also in a minority of WHO grade III and IV tumors,15,16 are also prognostic for longer survival.12,15,17,18 Methylation of the MGMT promoter (see the section on Chemotherapy) is emerging as both a predictive factor and a prognostic factor in the treatment of newly diagnosed GBMs.
It is not yet clear how to best incorporate molecular data into the treatment of individual patients. Detailed discussions of the biologic characteristics of glioma and their clinical relevance are beyond the scope of this chapter and can be found elsewhere.19
Pathology and Pathways of Spread
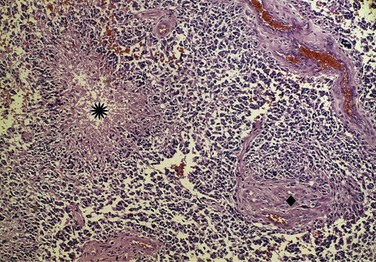
The WHO classification system20 is derived in part from the correlation between pathologic findings and survival rates observed by Bailey and Cushing21 and published in the early 1900s. In current parlance, low grade refers to WHO grade I to II tumors and high grade to WHO grade III to IV tumors (Fig. 25-1). However, the WHO grade I gliomas (e.g., juvenile pilocytic astrocytomas) are biologically different tumors from the others, infrequently occur in adults, and may be amenable to surgical cure. Anaplastic in the context of gliomas refers to WHO grade III tumors such as anaplastic astrocytomas, anaplastic oligodendrogliomas, and anaplastic oligoastrocytomas. GBM is a WHO grade IV astrocytoma, although the most recent WHO classification scheme does account for other rare subtypes, including GBM with oligodendroglial components.
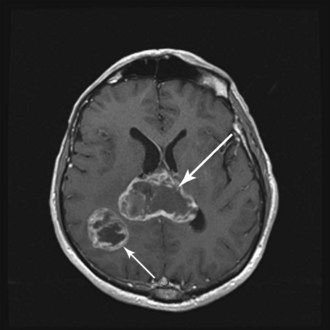
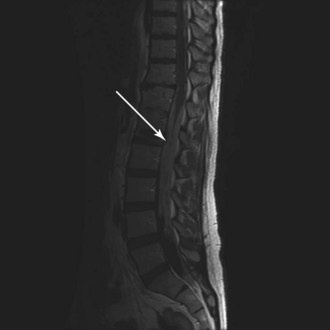
WHO grade II to IV gliomas are characterized by a tendency to directly infiltrate adjacent brain tissue. Lesions with direct access to the corpus callosum may extend across the midline and configure themselves in a classic “butterfly pattern” (Fig. 25-2). MRI underestimates the extent of invasive disease, and the very diffusely infiltrative nature of these tumors makes complete removal of all tumor cells impossible. This “misleading appearance of enucleability” was described over 80 years ago.21 Leptomeningeal spread occurs occasionally (Fig. 25-3). Hematogenous and lymphatic spread are exceedingly uncommon.22
Clinical Manifestations, Patient Evaluation, and Staging
The typical imaging appearance of a GBM is a ring-enhancing or heterogeneously enhancing lesion. The differential diagnosis may include stroke, brain metastasis, primary central nervous system lymphoma, demyelination, and even infectious or other inflammatory diseases. If a brain metastasis is suspected, it is prudent to perform an appropriate extracranial evaluation to identify the primary malignant tumor. If there is a high index of suspicion for primary central nervous system lymphoma, such as in multifocal, periventricular, and/or homogenously enhancing lesions,23 corticosteroids should be withheld preoperatively unless herniation is imminent because their use confounds the histologic diagnosis.
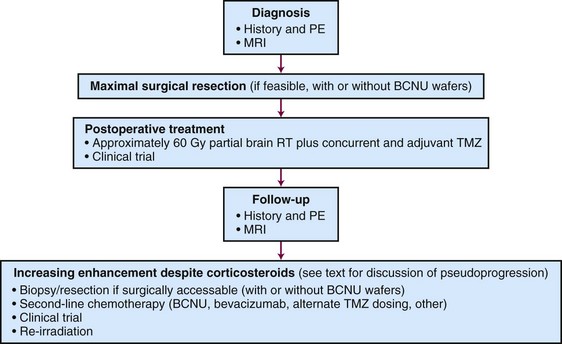
An algorithm for the evaluation and management of patients with GBM is shown in Figure 25-4.
Primary Therapy
Prognostic and Predictive Factors
Historically, all high-grade gliomas were lumped together in most clinical trials, thereby confounding results due to maldistribution of patients with differing prognoses. In 1993, Curran and colleagues24 published a landmark paper describing a prognostic classification scheme based on clinical variables. Data from three RTOG trials that enrolled nearly 1600 high-grade glioma patients from 1974 to 1989 were used. This recursive partitioning analysis (RPA) methodology builds decision trees to model predictors by examining all possible “cut points” for all variables included in the model. Patients were segmented into six distinct groups with different survival outcomes. The key variables included patient age, performance status, histologic tumor type (i.e., anaplastic astrocytoma vs. GBM), mental status, symptom duration antecedent to diagnosis, extent of resection, neurologic function, and radiotherapy dose. Median survival ranged from 4.6 months for class VI patients to almost 5 years for class I patients, underscoring survival heterogeneity. The European Organization for Research and Treatment of Cancer (EORTC) demonstrated the prior RTOG RPA classification remained valid among patients in a more recent phase III trial.25
One of the more controversial factors in the setting of high-grade gliomas has been the extent of surgical resection. Bailey and Cushing observed longer survival following resection in their 1926 publication,21 as did others in the 1960s.26 Numerous series since then also support more complete resection as a prognostic factor.27–31 One of the largest involved over 400 patients at the M.D. Anderson Cancer Center and demonstrated improved median survival (13 months vs. 8.8 months; p < .0001) following at least 98% resection, as defined by postoperative MRI scans.32 One small randomized study of 30 patients older than 65 years demonstrated improved survival rates following resection versus biopsy alone.30
External Beam Irradiation
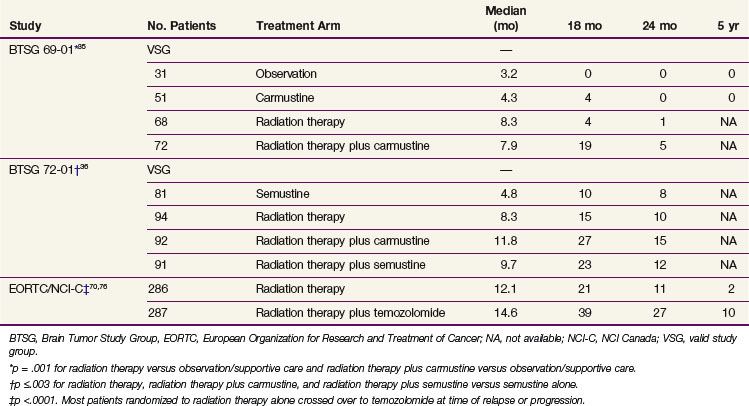
External beam irradiation (EBRT) has historically been the cornerstone of the therapeutic approach to GBM (and anaplastic astrocytoma) for the past half century, and its use in brain tumors was already described by the 1920s.33 In the 1970s and early 1980s, categorical level I data became available from several studies,34 including prospective phase III trials conducted by the Brain Tumor Study Group (BTSG)35,36 (Table 25-1).
TABLE 25-1 Positive Phase III Trials Evaluating the Role of Irradiation, Chemotherapy, or Chemoradiation in the Treatment of Malignant Gliomas
The radiotherapeutic approach to high-grade glioma has evolved. Initially, large opposed lateral fields were employed to cover the entire brain volume. In 1989, Shapiro and colleagues37 published data from Brain Tumor Cooperative Group trial 80-01, in which the randomization was altered during the trial to compare partial brain irradiation with whole-brain radiotherapy. No difference in OS or change in the patterns of failure was seen. Accordingly, whole-brain irradiation is generally not advocated, except perhaps in the scenario of a widespread intracranial process such as gliomatosis cerebri.38
Several lines of evidence have influenced the trend to treat the gross tumor volume along with a margin of approximately 2 cm. In a classic paper published in 1980, Hochberg and Pruitt39 used CT scans to determine that nearly 90% of GBM recurrences occurred within 2 cm of the primary tumor site (although this may be changing with the use of bevacizumab).40 Wallner and associates41 assessed the patterns of recurrence in 32 patients with unifocal malignant gliomas who were treated with primary surgery and postoperative irradiation. Nearly 80% of patients manifested recurrence or progression within 2 cm of the original tumor. Even when 80 Gy of partial brain irradiation was used in a prospective phase I trial,42 90% of patients failed within the high-dose region.
It has been demonstrated on biopsy and autopsy studies that the abnormality detected on T2 or fluid-attenuated inversion-recovery (FLAIR) images harbors microscopic tumor extension. Accordingly, 45 to 50 Gy delivered, in 1.8- to 2-Gy fractions, to the T2/FLAIR abnormality seen on the image, followed by a boost to raise the total dose to 60 Gy based on the T1-enhancing abnormality, is generally prescribed. The MRI abnormalities, however, remain quite nonspecific in terms of histopathologic confirmation, and even when novel strategies such as MRS are used for radiotherapy planning, there can be overestimation or underestimation of the target.43
Rationale for Current Total Irradiation Dose
A pooled analysis of three successive randomized trials conducted by the Brain Tumor Study Group (BTSG 66-01, 69-01, 72-01, respectively) generated data to support doses in excess of 50 Gy.44 A stepwise improvement in survival was observed with doses ranging from less than 45 Gy to 60 Gy, consistent with dose response. A comparison of 70 Gy versus 60 Gy demonstrated no survival or local control advantage for the 70-Gy dose.45,46 These results established 60 Gy as the standard of care.
Dose escalation has remained an important investigational option because there is still a pattern of failure characterized by local progression or recurrence. There are now multiple techniques for dose escalation, including three-dimensional conformal irradiation, radiosurgery, and brachytherapy, but these have not yielded higher rates of disease control or survival. Increasing the radiotherapy dose or reirradiation with radioprotective or antiangiogenic agents may be a useful strategy in the future.47
Altered Fractionation
The RTOG has systematically and rigorously studied hyperfractionation for high-grade gliomas. In trial 83-02, patients were randomized to one of four dose arms (64.8, 72, 76.8, or 81.6 Gy) using twice-daily fractions of 1.2 Gy. Initial results suggested the superiority of 72 Gy,48 but a subsequent phase III trial demonstrated no improvement.49
Prados and colleagues50 used an elegant randomization to assess not only a hyperfractionation schedule of 1.6 Gy twice daily to a total dose of 70.4 Gy, but also to determine the activity of difluoromethylornithine (DFMO), a compound that inhibits sublethal and potentially lethal damage repair. Unfortunately, neither intervention improved survival.
Stereotactic Irradiation
Two provocative small-scale experiences prompted the design of phase III trials to formally evaluate radiosurgery for high-grade gliomas. Loeffler and colleagues51 reported on 37 patients treated with 59.4 Gy fractionated radiotherapy followed by a radiosurgical boost to a median dose of 12 Gy. After a median follow-up period of 19 months, a 76% survival rate was reported. Sarkaria and associates52 described 115 high-grade glioma patients who received conformal radiation therapy and a stereotactic boost. The median survival time was 96 weeks. These results called into question whether they represented a benefit from radiosurgery or simply selection bias.
RTOG 93-05 compared conformal irradiation plus carmustine with or without a radiosurgical boost for newly diagnosed GBM. No differences were observed in terms of OS (median, approximately 13 months in each arm) or quality of life.53
Radiosurgery may still have a role in the treatment of recurrent disease, particularly if a focal region of recurrence can be defined.47
Brachytherapy
Gutin and associates54 suggested a potential survival benefit in a phase II trial. These findings were not reproduced in subsequent randomized trials.55,56
Interest in this modality was rekindled when the GliaSite radiation therapy system (Proxima Therapeutics, Alpharetta, Georgia) received approval by the Food and Drug Administration (FDA) in 2001. This intracavitary device is implanted at the time of tumor debulking, and a solution of iodine-125 (125I) is injected into an expandable closed-catheter balloon. A retrospective study suggested reasonable safety and promising efficacy.57 A phase I study was conducted.58 However, the implant induces changes in imaging that complicate determination of disease progression.59
Chemotherapy: Concurrent with EBRT, Maintenance Chemotherapy, Other
Early randomized trials of chemotherapy were individually negative, but meta-analysis of these trials showed that 15% to 20% of patients treated with radiation therapy (RT) and nitrosoureas survived at least 18 months versus 5% treated with radiotherapy alone.35,36,60,61 Nitrosoureas, especially carmustine, were the most commonly used drugs, although procarbazine was also used extensively.62 The combination of procarbazine, lomustine (CCNU), and vincristine, also called PCV,63 had no clear benefit (yet much greater toxicity) versus carmustine for anaplastic astrocytoma,64 and this regimen has been largely abandoned.
Intratumoral delivery of chemotherapy for residual postoperative disease is most commonly in the form of carmustine-eluting (Gliadel) wafers. Patients undergoing wafer implantation during surgery for recurrent GBM survived approximately 2 months longer than patients without wafers in one study (p = .02).65 Treatment of newly diagnosed disease also yielded a 2-month prolongation of average survival.66,67 However, this was not statistically significant when the analysis was restricted to patients with GBM histology. Of note, wafer delivery of carmustine versus systemic administration has not been compared for safety or efficacy. Gliadel does, however, carry an FDA label for implantation during resection of recurrent GBM and newly diagnosed malignant glioma. Attempts to treat residual visible or microscopic disease with other local chemotherapies delivered through implanted catheters and using convection-enhanced migration of drug have generally failed.68
Currently, the most widely used chemotherapeutic agent is temozolomide. Whether it is more effective than nitrosoureas has not been investigated, but it is unquestionably better tolerated with significant myelosuppression in less than 20% of patients.69,70 Temozolomide was first approved for recurrent anaplastic astrocytomas following a phase II study.71 A randomized study also demonstrated superior efficacy to procarbazine in recurrent GBM.72
Temozolomide for newly diagnosed GBM has been studied both when given before RT73 and when combined with RT in various dosing schedules.74,75 Its role for newly diagnosed GBM was established by Stupp and associates69,70 on the basis of the EORTC 26981/22981 and NCIC CE.3 trial. In this phase III multicenter study, 573 newly diagnosed GBM patients received RT alone or RT with concurrent temozolomide followed by six adjuvant cycles of temozolomide. The patients who received the combined-modality regimen had significantly longer OS and progression-free survival (PFS) rates without significantly more toxicity (see Table 25-1). The 5-year OS was 10% among those receiving temozolomide versus 2% among those receiving radiotherapy alone (p <.0001).69 Patients in the most favorable RPA class had a 5-year OS rate of 28% following combined therapy.69 In a companion paper, Hegi and colleagues76 reported that methylation of the promoter for the O6-methylguanine DNA methyltransferase (MGMT) gene, which encodes the DNA repair enzyme O6-alkylguanine DNA alkyltransferase (AGT or AGAT, but now commonly also referred to as MGMT), was correlated with prolonged survival.
MGMT repairs DNA damage induced by temozolomide, and methylation of the MGMT promoter silences expression of the protein, thereby accentuating the antineoplastic effects of temozolomide. However, the mechanism by which MGMT promoter methylation leads to an improved outcome is more complex. For example, patients with tumors harboring methylated MGMT survived longer following treatment with RT alone than patients who did not have tumors harboring methylated MGMT treated identically.69 Others reported similar findings in GBM77 and other malignant gliomas.78 Moreover, MGMT protein expression by immunostaining does not predict outcome.79 It remains unclear whether MGMT analysis should alter treatment, although several alternate dosing schedules of temozolomide are under investigation80,81 as a means to overcome this and other potential resistance mechanisms.
Anaplastic Gliomas
Anaplastic astrocytomas, anaplastic oligodendrogliomas, and anaplastic oligoastrocytomas represent the most common WHO grade III tumors.1,20 In anaplastic gliomas, resection appears to improve survival relative to biopsy, as it does for GBM.
It is generally accepted that radiotherapy should be administered postoperatively for astrocytomas. In a German study (NOA-04), survival rates were equivalent whether chemotherapy or radiotherapy was used first among patients with anaplastic astrocytomas, oligodendrogliomas, and mixed tumors.12 However, time to progression following RT was longer than after chemotherapy, and initial radiation therapy achieved more complete and partial responses than initial chemotherapy, suggesting the superiority of radiotherapy.82
Combs and associates83 reviewed the outcome of 191 patients with grade III astrocytic tumors treated at the University of Heidelberg with either RT alone or RT in combination with temozolomide during a 20-year period (from 1988 to 2007). In this retrospective study, no significant advantage in rates of OS or PFS could be attributed to the combination. RTOG trial 9813 randomized patients with anaplastic astrocytomas (or oligoastrocytomas) to radiotherapy with concurrent nitrosourea (carmustine or lomustine) or with temozolomide, and results are pending.
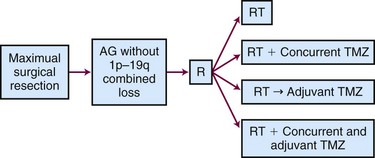
The ongoing CATNON (Concurrent vs. Adjuvant Temozolomide for NON 1p19q co-deleted anaplastic gliomas, also called EORTC 26053-22054) Intergroup trial randomizes patients to receive postoperative RT alone, concurrent temozolomide and RT without adjuvant temozolomide, RT (without concurrent temozolomide) followed by adjuvant temozolomide, or RT with both concurrent and adjuvant temozolomide (Fig. 25-5). Although technically this study allows any WHO grade III glioma without 1p/19q co-deletion, the majority of the tumors will be anaplastic astrocytomas.84
The role of radiotherapy for newly diagnosed anaplastic oligodendroglioma is most controversial because of the tumor’s reported sensitivity to chemotherapy,85 especially tumors with 1p/19q co-deletion.86 In spite of this, two separate randomized studies that compared radiation therapy with radiation therapy plus chemotherapy (RTOG 94-0210 and EORTC 2695111) failed to demonstrate a survival advantage from the addition of chemotherapy. PCV chemotherapy prolonged PFS, and those who failed irradiation were frequently treated with PCV, possibly confounding the results. Both trials provided validation of the prognostic value of the allelic loss of heterozygosity of the 1p and 19q chromosomes.
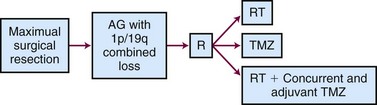
Neither of these trials used chemotherapy alone, which is recommended by 42% of clinicians (typically with temozolomide) for co-deleted tumors.13 The ongoing CODEL (for 1p/19q CO-DELeted tumors) trial randomizes patients to RT alone, RT with concurrent and adjuvant temozolomide, or temozolomide alone to prospectively address this issue (Fig. 25-6).
The CATNON and CODEL study designs are premised on the belief that the 1p/19q chromosomal status may be more important than the histologic subtype for WHO grade III lesions. However, scientific advances in the interim may outpace the questions posed by these trials as it becomes apparent that MGMT promoter methylation78 and IDH gene mutation12,17 are also powerful markers of outcome in anaplastic gliomas.
Special Topics
Toxicities of Radiation Therapy
Acute and late effects of irradiation are discussed in a subsequent section on Irradiation Techniques and Toxicities.41,83–86,87,88 This includes a discussion of brain necrosis and the risk of normal tissue damage based on the most sensitive 5% of the population.
Elderly Patients with GBM
Over 44% of GBMs occur in patients over 65 years of age,1 but prospective trials often exclude elderly patients.69 Because these patients often have a worse outcome than younger patients, there has been some uncertainty with regard to the aggressiveness of treatment.
A Finnish randomized study of 30 patients over age 65 years with imaging consistent with malignant gliomas demonstrated longer median survival following craniotomy than following biopsy (5.7 months vs. 2.1 months; p = .035).30 The Association of French-Speaking Neuro-Oncologists87 conducted a randomized trial comparing RT alone (50 Gy in conventional fractionation) with supportive care among patients over 70 years of age. The trial was discontinued after 85 patients were registered when it became apparent that radiotherapy was associated with a statistically advantageous outcome (median survival time, 29 weeks vs. 17 weeks; p = .002).
Efforts to abbreviate the duration of RT have been conducted. A randomized study in patients over age 60 years demonstrated that 40 Gy in 15 fractions was not inferior to 60 Gy in 30 fractions.88 A phase II study of 32 patients over age 70 years with newly diagnosed GBM demonstrated a response rate of 31% to temozolomide, with a median PFS time of 5 months and a median survival time of 6.4 months, results that were comparable to those of radiation therapy alone.89,90
The EORTC is currently conducting a trial to assess the benefit of combined temozolomide and RT in elderly patients with GBM. Intravenous temozolomide recently became available as an option for elderly or otherwise impaired patients who cannot swallow capsules or comply with an oral regimen.91
Pseudoprogression
Pseudoprogression confounds interpretation of imaging performed in the first several months following completion of radiotherapy. Descriptions of “pseudoprogression” appeared as early as 1979, when Hoffman and colleagues92 described patients treated with radiotherapy and carmustine. Among patients thought to have experienced disease progression immediately following irradiation, nearly half were shown to have improvement or at least stabilization on subsequent brain imaging.
A report in 2004 described that approximately one third of patients with gliomas stabilized or improved with no change in management.93 Chamberlain and associates94 reported histologically proven treatment injury rather than disease progression in approximately 50% of patients with symptomatic resectable lesions felt to represent worsening disease following concurrent radiotherapy and temozolomide. The incidence of pseudoprogression has been reported to be as high as 75%.81,95,96 Pseudoprogression rather than “true” progression may also correlate with MGMT promoter methylation,95 although this has not yet been prospectively validated.
Multiple imaging techniques have been explored to delineate radiographic pseudoprogression from true progression.95,97–101 However, at this time, histologic analysis is the only validated method of distinguishing the two diagnoses.102
It is presumed that the incidence of pseudoprogression is higher following concurrent radiotherapy and temozolomide than that following radiotherapy alone, although supportive evidence is equivocal.103 One approach to dealing with this issue involves using the MRI study done following radiation therapy as a new baseline rather as a means of designating disease progression, unless there is surgical documentation of recurrent disease or clear worsening outside of the radiation therapy port. This remains an area of active study.104
Recurrent Disease
In brief, efforts to treat recurrent disease with single agents with single molecular targets have generally been unsuccessful, at least in part because of the molecular complexity of the disease.105 Some studies suggest the key may lie in identifying the patients most likely to respond based on molecular profiling of tumor tissue.106
The “BRAIN” trial was a noncomparative phase II study for recurrent GBM that randomized 167 patients to either bevacizumab alone or bevacizumab combined with irinotecan,107 the latter regimen adopted from activity of that combination in colon cancer.108 The 6-month progression-free survival rate was 43% with bevacizumab and 50% with bevacizumab plus irinotecan.107 These results are far superior to those of prior available therapies.
To date, however, there are no data clearly demonstrating a survival advantage for the use of bevacizumab, and major potential toxicities include thromboembolic disease and hypertension. In addition, treatment following progression on bevacizumab may have little to no efficacy,40,109 and the timing of bevacizumab for either first or later recurrence remains controversial. The potential toxicity, as well as the fear of inducing a more invasive tumor phenotype, also tempers enthusiasm for use of this agent in newly diagnosed disease until randomized data become available. RTOG 0825 and the AVAglio trial, as well as antiangiogenic approaches with the anti-integrin cilengitide (CENTRIC) and other agents such as the VEGFR tyrosine kinase inhibitor cedirinib, are currently addressing this issue (Table 25-2).
TABLE 25-2 Major Ongoing Multicenter/International Phase III Trials
| Trial Name | Disease | Notes |
|---|---|---|
| CATNON | Anaplastic gliomas without 1p/19q co-deletion | Efficacy of radiation therapy alone or with concurrent and/or adjuvant temozolomide |
| CODEL | Anaplastic gliomas without 1p/19q co-deletion | Efficacy of radiation therapy with or without concurrent and adjuvant temozolomide, or temozolomide alone |
| RTOG 0525 | GBM | Efficacy of intensive adjuvant temozolomide dosing following radiation therapy |
| RTOG 0825 | GBM | Efficacy of bevacizumab in newly diagnosed disease |
| AVAglio | GBM | Efficacy of bevacizumab in newly diagnosed disease |
| CENTRIC | GBM | Efficacy of cilengitide for newly diagnosed disease |
| REGAL | GBM | Efficacy of cedirinib in recurrent disease |
CATNON, Concurrent vs. Adjuvant Temozolomide for NON 1p/19q co-deleted anaplastic gliomas; CODEL, 1p/19q CO-DELeted tumors; GBM, glioblastoma; RTOG, Radiation Therapy Oncology Group.
Reirradiation
Focal radiotherapy approaches are often employed with limited volume recurrences. In a retrospective analysis of 95 patients with recurrent gliomas treated with the GliaSite device, the median survival time was 36 weeks.110,111 However, whether this is a function of true benefit or patient selection has not been determined.
Fractionated radiotherapy to treat larger-volume recurrent disease has also been employed. Although there has been speculation from animal studies that neural tissue will recover from previous irradiation to a large extent once some time has elapsed (e.g., 1 to 3 years),112 no firm data quantify the degree to which one can assume that a “dose discount” exists. It is most likely that the damage from reirradiation is underestimated because the majority of patients do not live long enough to express such damage. A small study showed good short-term tolerance to intensity-modulated radiation therapy (IMRT) delivered in six daily fractions of 5 Gy.113 A recent single-arm trial from the Memorial Sloan-Kettering Cancer Center demonstrated reasonable safety and efficacy of combined bevacizumab and reirradiation using IMRT to small recurrent malignant gliomas.47 No radionecrosis was observed and survival appeared to be prolonged relative to historic controls, suggesting that bevacizumab may not only treat radionecrosis114 but may protect against it. Another concept was recently reported by investigators from Jefferson Medical College115 who treated 147 patients with recurrent high-grade glioma to 35 Gy in 3.5-Gy fractions with stereotactic radiation therapy. This hypofractionated stereotactic radiation therapy (HSRT) approach was associated with a median survival time in excess of 10 months.
Irradiation Techniques and Toxicities
Whole-brain radiation therapy has been replaced with partial brain techniques by consensus. Although the dose computation component of treatment planning is still based on CT imaging, effective image registration with MRI has made this the modality of choice for contouring. The notion of a dedicated MRI simulator has also been proposed as a valuable adjunct in the radiotherapeutic management of high-grade gliomas.116 However, treatment plans based only on MRI are not able to take into account tissue density variations, an inability that may lead to slightly inaccurate dose calculations.
For high-grade gliomas, especially GBM, the T1 contrast-enhanced sequences are used to define the gross tumor volume (GTV) and the T2 or FLAIR sequences plus a margin define the microscopic disease extent, or clinical target volume (CTV), which reflects the bulk of microscopic infiltration. To arrive at a planning target volume (PTV), both organ motion and setup error must be taken into account. Organ motion in the brain is quite minimal during therapy (e.g., <1 mm). The PTV may be further modified to exclude normal tissue in areas where gliomas are unlikely to infiltrate. Bokstein and colleagues117 recently demonstrated that when anatomic barriers such as the temporal bone can serve as a border to impede tumor spread, failure is likely to be seen in less than 5% of cases even when the customary 2-cm margins are not added to the abnormalities seen on MRI (Fig. 25-7).
In general, there are two major schools of thought (with numerous institutional variations based on these) that provide guidance for the prescription of the radiation regimen. The RTOG approach is a biphasic technique that includes an initial PTV (PTV 1) followed by a second PTV (PTV 2) that represents the cone down. In the lexicon of the RTOG, the PTV 1 includes the T2 or FLAIR CTV with a margin and is treated to 46 Gy in 2-Gy fractions. The PTV 2 includes the T1-enhancing GTV with a margin and is treated to an additional 14 Gy. The EORTC recommends a single-phase technique using one treatment volume throughout the course of therapy. Table 25-3 shows the partial brain volumes advocated by several cooperative groups for the successive phases of partial brain irradiation.
TABLE 25-3 Radiotherapy Volume Used in Current Clinical Trials
| RT Dose | Block Edge Dosimetry Margin* | Source for RT Dose |
|---|---|---|
| 46 Gy | T2 + 2 cm | RTOG |
| 14 Gy | T1 + 2.5 cm | |
| 60 Gy† | T1 + 2-3 cm | EORTC |
| 50 Gy | T2 + 2 cm | NCCTG |
| 10 Gy | T1 + 2 cm |
EORTC, European Organization for Research and Treatment of Cancer; Gy, Gray; NCCTG, North Central Cancer Treatment Group; RT, irradiation dose; RTOG, Radiation Therapy Oncology Group; 3D, three-dimensional.
* Both RTOG and EORTC prescribe dose to block edge (geometric) margin, whereas NCCTG prescribes dose to dosimetry (3D) margin.
† CENTRIC studies: either RTOG or EORTC volumes and RT doses are used, at individual physician discretion.
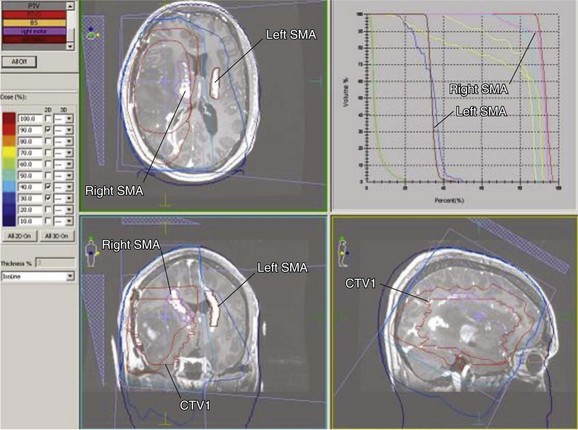
With the advent of functional imaging tools (e.g., functional MRI [fMRI]) it may be possible to specifically modify irradiation doses to functional brain areas. Figure 25-8 displays a treatment plan wherein the region governing motor control (e.g., finger tapping) is delineated to enable an accounting for dose deposition. In this case, this region in the right hemisphere (i.e., governing tapping by the left upper extremity) is included in the high-dose region but the contralateral side is well spared.
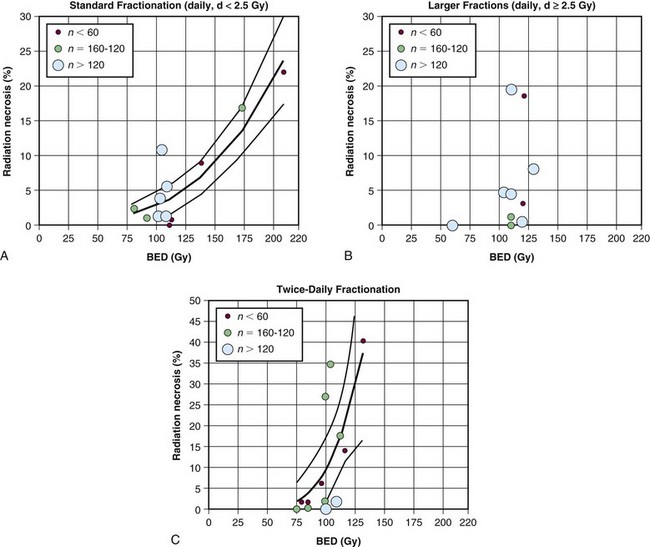
Table 25-4 summarizes the tolerance of various organs at risk according to the new QUANTEC (Quantitative Analysis of Normal Tissue Effects in the Clinic) guidelines. Lawrence and colleagues118 postulated that the original estimates of Emami and colleagues,119 suggesting a 5% risk of chronic brain damage at 5 years when one-third of the brain is irradiated to 60 Gy, were overly conservative. Instead, they hypothesized (Fig. 25-9) that the dose correlated with a 5% risk of damage at 5 years following conventionally fractionated irradiation to the partial brain is 72 Gy.
TABLE 25-4 Selected Organs at Risk for Treatment Planning of Malignant Glioma
| Organ | Dose Limit | Comments |
|---|---|---|
| Brain parenchyma118 | 72 Gy | Brain appears to be more sensitive to fraction size >2 Gy and to twice-daily radiation therapy |
| Optic apparatus132 | 60 Gy | 12 Gy for single-fraction radiosurgery |
| Brainstem133 | 54 Gy | 59 Gy if small volumes (i.e., 1-10 mL) |
| Parotid gland134 | 20 Gy | Severe xerostomia vis-à-vis sparing of one parotid gland |
| 25 Gy | Severe xerostomia vis-à-vis sparing of both parotid glands |
There may sometimes be a tendency to overlook structures that, if damaged, would have led to noncatastrophic sequelae. For instance, although it is true that radiation-induced cataracts are easily repairable,120 avoidance of entrance and exit dose to the eye may be a relatively simple means of preventing not only cataracts but also conjunctivitis and a dry eye. Similarly, when one contours the ear canals, there is now a greater awareness of the risks of developing otitis externa as well as otitis media.
There are reports of cytopenias arising from cranial irradiation even among patients who have not received chemotherapy.121 The hypothesized mechanism of this consequence is either irradiation of circulating blood (e.g., within the cavernous sinus), injury to marrow in the cranium, or the use of vertex beams that exit the spinal axis. The latter mechanism has also been invoked to explain radiation-induced fatigue by some investigators.122
Toxicities of Radiation Therapy
Acute radiation morbidity includes fatigue, erythema, alopecia, headache, and, rarely, nausea with or without vomiting; these are generally not severe and are usually self-limiting.41 Some have cautioned that the combination of cranial radiotherapy and phenytoin as well as other anticonvulsants could give rise to the Stevens-Johnson syndrome,123,124 but this dermatologic emergency is an exceedingly rare event.
Late effects of radiation (e.g., somnolence and, especially, cognitive impairments) are more worrisome and may become manifest many years later.125 The impact of partial brain irradiation on neurocognitive decline continues to be a hotly debated topic. The confounding factor is always the extent to which there is baseline cognitive impairment or decreased mentation secondary to uncontrolled tumor.
Brain necrosis is a serious and uncommon late toxicity, and recently bevacizumab has been explored as a treatment.114 In a small trial, all patients showed improvement of MRI abnormalities as well as a reduction in corticosteroid requirements.126
We currently estimate the risk of normal tissue damage based on the most sensitive 5% of the population. Accordingly, we bias our recommendations in a manner that is not germane to most individuals, and preliminary efforts at predicting the likelihood of toxicities based on risk are ongoing.127,128
Treatment Algorithm, Controversies, Challenges, and Future Possibilities
Treatment Algorithm (Fig. 25-4)
For adults up to age 70 years with newly diagnosed GBM, maximal surgical resection (with or without Gliadel wafer implantation) followed by concurrent EBRT (approximately 60 Gy in 30 fractions) with temozolomide and followed by at least 6 months of adjuvant temozolomide is the standard of care. For suspected progression that occurs within 3 months of completing radiotherapy, the possibility of pseudoprogression should be strongly considered.129 Relapsed disease may be treated with re-resection, second-line chemotherapy, or experimental therapy in a clinical trial. Reirradiation is also a possibility but is used infrequently. Bevacizumab is approved for relapsed disease.107
For newly diagnosed anaplastic gliomas, radiotherapy is typically administered as part of the initial therapy. Some advocate chemotherapy first, especially in oligoendrogliomas.12,13
Controversies
Treatment for elderly patients (variably defined as older than 60, 65, or 70 years of age, depending on the study) remains controversial. Patients with a good performance status and few comorbidities are often treated according to the same algorithm used for younger patients. However, abbreviated radiotherapy courses appear to be non-inferior88 and are often employed. Whether chemoradiation using a rapid course of radiotherapy is superior to radiotherapy alone is under investigation.
Challenges
Local control of high-grade gliomas remains a vexing problem for radiation oncologists. In addition, the increased recognition of pseudoprogression as an entity and the possibility of pseudoresponse to antiangiogenic therapy such as bevacizumab130 complicate matters further. A revised set of consensus criteria were recently developed in part to address some of these issues.104,129 Certainly, correlative clinical follow-up to provide the proper perspective on the health status of the patient will never be abandoned, because imaging will always represent an imperfect surrogate for survival. Although new developments in diagnostic imaging continue to hold out promise for resolution of the diagnostic dilemmas faced by the neuro-oncology team, to date even the most sophisticated imaging studies (e.g., FDG PET, MRS) have not provided a consistently reliable solution to these and other vexing problems.
Future Possibilities
For most tumors that have demonstrated radiosensitivity but defy control, the reflex recommendation of the radiation oncologist is usually dose escalation. Ostensibly, dose escalation as a solution to the problem of local failure of high-grade gliomas has been put to rest by virtue of trials discussed earlier. However, it is conceivable that the notion of dose escalation now needs to be revisited in the backdrop of potential radioprotection offered by bevacizumab,47 both for newly diagnosed and recurrent disease.
Many new strategies to improve outcome in high-grade gliomas have been proposed. The RTOG awaits analysis of trial 0525, which randomized newly diagnosed GBM patients between the conventional Stupp regimen and the same regimen with dose-intensive temozolomide. The brisk accrual of large numbers of glioma patients to recent studies such as RTOG 0525 and 0825 is a testimony to the renewed interest in studying high-grade gliomas. The success of antiangiogenic therapy has also reinvigorated study of this disease.107
The 5-year survival rate from the EORTC-NCIC study of patients with GBM treated with radiotherapy and temozolomide was approximately 10%, and for patients with favorable prognostic factors it approached 30%.69 Moreover, a patient who lived more than 20 years following the diagnosis of GBM was described, perhaps the longest documented survivor.131 He had been treated with surgery and partial brain irradiation (59 Gy of 6-MV photons in conventional fractionation delivered via the shrinking-field technique). The authors speculated that the outcome may have stemmed from the fact that he had a favorable molecular profile (e.g., methylated MGMT promoter, PTEN positive, and TP53 positive, which the authors termed “triple positive,” similar to the nomenclature of breast cancer). Whether this explained the long survival time is unclear. More importantly, these observations prove that one may strive to create and sustain hope for patients diagnosed with high-grade glioma.
6 Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157-173.
7 Colman H, Zhang L, Sulman EP, et al. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 2010;12:49-57.
9 Reifenberger J, Reifenberger G, Liu L, et al. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994;145:1175-1190.
10 Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma. Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-2714.
11 van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas. A randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715-2722.
14 Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852-9861.
15 Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765-773.
24 Curran WJJr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704-710.
34 Shapiro WR, Young DF. Treatment of malignant glioma. A controlled study of chemotherapy and irradiation. Arch Neurol. 33, 1976. 494–450
35 Walker MD, Alexander EJr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333-343.
36 Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323-1329.
38 DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
39 Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907-911.
44 Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731.
47 Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156-163.
49 Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients. A report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40:51-55.
60 Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585-2597.
61 Stewart LA. Chemotherapy in adult high-grade glioma. A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011-1018.
62 Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121-132.
69 Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study. 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
70 Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
76 Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
82 DeAngelis LM. Anaplastic glioma. How to prognosticate outcome and choose a treatment strategy (corrected). J Clin Oncol. 2009;27:5861-5862.
87 Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527-1535.
88 Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme. A prospective randomized clinical trial. J Clin Oncol. 2004;22:1583-1588.
93 de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535-537.
107 Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740.
112 Ang KK, Jiang GL, Feng Y, et al. Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys. 2001;50:1013-1020.
113 Voynov G, Kaufman S, Hong T, et al. Treatment of recurrent malignant gliomas with stereotactic intensity modulated radiation therapy. Am J Clin Oncol. 2002;25:606-611.
116 Kristensen BH, Laursen FJ, Logager V, et al. Dosimetric and geometric evaluation of an open low-field magnetic resonance simulator for radiotherapy treatment planning of brain tumours. Radiother Oncol. 2008;87:100-109.
117 Bokstein F, Kovner F, Blumenthal DT, et al. A common sense approach to radiotherapy planning of glioblastoma multiforme situated in the temporal lobe. Int J Radiat Oncol Biol Phys. 2008;72:900-904.
118 Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20-S27.
119 Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122.
127 Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy. Towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134-142.
129 Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas. Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972.
1 CBTRUS. Central Brain Tumor Registry of the United States Statistical Report. Primary brain and central nervous system tumors in the United States. www.cbtrus.org.
2 Myung SK, Ju W, McDonnell DD, et al. Mobile phone use and risk of tumors. A meta-analysis. J Clin Oncol. 2009;27:5565-5572.
3 Fisher JL, Schwartzbaum JA, Wrensch M, et al. Epidemiology of brain tumors. Neurol Clin. 2007;25:867-890.
4 Farrell CJ, Plotkin SR. Genetic causes of brain tumors. Neurofibromatosis, tuberous sclerosis, von Hippel-Lindau, and other syndromes. Neurol Clin. 2007;25:925-946. viii
5 Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061-1068.
6 Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157-173.
7 Colman H, Zhang L, Sulman EP, et al. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 2010;12:49-57.
8 Lassman AB, Holland EC. Molecular biology and genetic models of glioma and medulloblastoma. In: McLendon RE, Rosenblum MK, Bigner DD, et al, editors. Russell and Rubinstein’s pathology of tumors of the nervous system. ed 7. London: Hodder Arnold Health Sciences; 2006:1039-1091.
9 Reifenberger J, Reifenberger G, Liu L, et al. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994;145:1175-1190.
10 Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma. Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-2714.
11 van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas. A randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715-2722.
12 Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874-5880.
13 Abrey LE, Louis DN, Paleologos N, et al. Survey of treatment recommendations for anaplastic oligodendroglioma. Neuro Oncol. 2007;9:314-318.
14 Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852-9861.
15 Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765-773.
16 Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age. A study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469-474.
17 van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors. A report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16:1597-1604.
18 Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150-4154.
19 Hofer S, Lassman AB. Molecular markers in gliomas. Impact for the clinician. Target Oncol. 2010;5:201-210.
20 Louis DN, Ohgaki H, Wiestler OD, et al. WHO classification of tumours of the central nervous system, ed 4. Lyon, France: International Agency for Research on Cancer; 2007.
21 Bailey P, Cushing H. A classification of the tumors of the glioma group on a histo-genetic basis with a correlated study of prognosis. Philadelphia, J.B.: Lippincott; 1926.
22 Rajagopalan V, El Kamar FG, Thayaparan R, et al. Bone marrow metastases from glioblastoma multiforme. A case report and review of the literature. J Neurooncol. 2005;72:157-161.
23 Buhring U, Herrlinger U, Krings T, et al. MRI features of primary central nervous system lymphomas at presentation. Neurology. 2001;57:393-396.
24 Curran WJJr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704-710.
25 Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma. Recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24:2563-2569.
26 Jelsma RK, Bucy PC. The treatment of glioblastoma multiforme. Trans Am Neurol Assoc. 1967;92:90-93.
27 Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma. A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392-401.
28 Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma. Data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467-473.
29 Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371-379.
30 Vuorinen V, Hinkka S, Farkkila M, et al. Debulking or biopsy of malignant glioma in elderly people. A randomised study. Acta Neurochir (Wien). 2003;145:5-10.
31 Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993;78:767-775.
32 Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme. Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-198.
33 Bailey P. The results of roentgen therapy on brain tumors. Am J Roentgenol Radium Ther. 1925;13:48-53.
34 Shapiro WR, Young DF. Treatment of malignant glioma. A controlled study of chemotherapy and irradiation. Arch Neurol. 33, 1976. 494–450
35 Walker MD, Alexander EJr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333-343.
36 Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323-1329.
37 Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71:1-9.
38 DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
39 Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907-911.
40 Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200-1206.
41 Wallner KE, Galicich JH, Krol G, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405-1409.
42 Lee SW, Fraass BA, Marsh LH, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas. A quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43:79-88.
43 Pirzkall A, McKnight TR, Graves EE, et al. MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2001;50:915-928.
44 Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731.
45 Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52:997-1007.
46 Nelson DF, Diener-West M, Horton J, et al. Combined modality approach to treatment of malignant gliomas. Re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up. A joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988;6:279-284.
47 Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156-163.
48 Nelson DF, Curran WJJr, Scott C, et al. Hyperfractionated radiation therapy and bis-chlorethyl nitrosourea in the treatment of malignant glioma. Possible advantage observed at 72.0 Gy in 1.2 Gy B.I.D. fractions. Report of the Radiation Therapy Oncology Group Protocol 8302. Int J Radiat Oncol Biol Phys. 1993;25:193-207.
49 Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients. A report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40:51-55.
50 Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluoromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;49:71-77.
51 Loeffler JS, Alexander E3rd, Shea WM, et al. Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol. 1992;10:1379-1385.
52 Sarkaria JN, Mehta MP, Loeffler JS, et al. Radiosurgery in the initial management of malignant gliomas. Survival comparison with the RTOG recursive partitioning analysis. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1995;32:931-941.
53 Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme. Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853-860.
54 Gutin PH, Prados MD, Phillips TL, et al. External irradiation followed by an interstitial high activity iodine-125 implant “boost” in the initial treatment of malignant gliomas. NCOG study 6G-82-2. Int J Radiat Oncol Biol Phys. 1991;21:601-606.
55 Florell RC, Macdonald DR, Irish WD, et al. Selection bias, survival, and brachytherapy for glioma. J Neurosurg. 1992;76:179-183.
56 Selker RG, Shapiro WR, Burger P, et al. The Brain Tumor Cooperative Group NIH Trial 87-01. A randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51:343-355. discussion Neurosurgery 51:355-357, 2002
57 Welsh J, Sanan A, Gabayan AJ, et al. GliaSite brachytherapy boost as part of initial treatment of glioblastoma multiforme. A retrospective multi-institutional pilot study. Int J Radiat Oncol Biol Phys. 2007;68:159-165.
58 Stieber VW, Tatter S, Mikkelsen T, et al. NABTT 2105. A Phase I dose-escalation trial of GliaSite brachytherapy with conventional radiation therapy for newly diagnosed glioblastoma multiforme [abstract]. J Clin Oncol. 23, 2005. Abstract #1570
59 Kleinberg L, Yoon G, Weingart JD, et al. Imaging after GliaSite brachytherapy. Prognostic MRI indicators of disease control and recurrence. Int J Radiat Oncol Biol Phys. 2009;75:1385-1391.
60 Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585-2597.
61 Stewart LA. Chemotherapy in adult high-grade glioma. A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011-1018.
62 Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121-132.
63 Levin VA, Edwards MS, Wright DC, et al. Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat Rep. 1980;64:237-244.
64 Prados MD, Scott C, Curran WJJr, et al. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma. A retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17:3389-3395.
65 Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008-1012.
66 Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79-88.
67 Westphal M, Ram Z, Riddle V, et al. Gliadel wafer in initial surgery for malignant glioma. Long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148:269-275. discussion Acta Neurochir (Wien) 148:275, 2006
68 Kunwar S, Westphal M, Medhorn M, et al. Results from PRECISE. A randomized phase 3 study in patients with first recurrent glioblastoma multiforme (GBM) comparing cinttredekin besudotox (CB) administered via convection-enhanced delivery (CED) with Gliadel wafers (GW) [abstract MA-61]. Neuro Oncol. 2007;9:531.
69 Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study. 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
70 Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
71 Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group [Erratum in J Clin Oncol 17(11):3693, 1999]]. J Clin Oncol. 1999;17:2762-2771.
72 Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588-593.
73 Gilbert MR, Friedman HS, Kuttesch JF, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol. 2002;4:261-267.
74 Combs SE, Gutwein S, Schulz-Ertner D, et al. Temozolomide combined with irradiation as postoperative treatment of primary glioblastoma multiforme. Phase I/II study. Strahlenther Onkol. 2005;181:372-377.
75 Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375-1382.
76 Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
77 Pellosk CE, Rivera AL, De La Cruz Guerrero C, et al. MGMT promoter methylation is an independent prognostic factor in the absence of alkylating chemotherapy in glioblastoma [abstract]. Int J Radiat Oncol Biol Phys. 2008;72:S9.
78 van den Bent MJ, Dubbink HJ, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors. A report from EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2009;27:5881-5886.
79 Preusser M, Charles Janzer R, Felsberg J, et al. Anti-O6-methylguanine-methyltransferase (MGMT) immunohistochemistry in glioblastoma multiforme. Observer variability and lack of association with patient survival impede its use as clinical biomarker. Brain Pathol. 2008;18:520-532.
80 Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11:69-79.
81 Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861-3867.
82 DeAngelis LM. Anaplastic glioma. How to prognosticate outcome and choose a treatment strategy [corrected]. J Clin Oncol. 2009;27:5861-5862.
83 Combs SE, Nagy M, Edler L, et al. Comparative evaluation of radiochemotherapy with temozolomide versus standard-of-care postoperative radiation alone in patients with WHO grade III astrocytic tumors. Radiother Oncol. 2008;88:177-182.
84 Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma. A report on underlying mechanisms. Clin Cancer Res. 2006;12:4738-4746.
85 Cairncross G, Macdonald D, Ludwin S, et al. Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12:2013-2021.
86 Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma. Implications for patient management at diagnosis. Clin Cancer Res. 2001;7:839-845.
87 Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527-1535.
88 Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme. A prospective randomized clinical trial. J Clin Oncol. 2004;22:1583-1588.
89 Chinot OL, Barrie M, Frauger E, et al. Phase II study of temozolomide without radiotherapy in newly diagnosed glioblastoma multiforme in an elderly population. Cancer. 2004;100:2208-2214.
90 Glantz M, Chamberlain M, Liu Q, et al. Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer. 2003;97:2262-2266.
91 Diez BD, Statkevich P, Zhu Y, et al. Evaluation of the exposure equivalence of oral versus intravenous temozolomide. Cancer Chemother Pharmacol. 2010;65:727-734.
92 Hoffman WF, Levin VA, Wilson CB. Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg. 1979;50:624-628.
93 de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535-537.
94 Chamberlain MC, Glantz MJ, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81-83.
95 Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192-2197.
96 Taal W, Brandsma D, de Bruin HG, et al. The incidence of pseudo-progression in a cohort of malignant glioma patients treated with chemo-radiation with temozolomide [abstract 2009]. J Clin Oncol. 2007;25:2009.
97 Schlemmer HP, Bachert P, Henze M, et al. Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology. 2002;44:216-222.
98 Schlemmer HP, Bachert P, Herfarth KK, et al. Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. AJNR Am J Neuroradiol. 2001;22:1316-1324.
99 Clarke JL, Abrey LE, Karimi S, et al. Pseudoprogression (PsPr) after concurrent radiotherapy (RT) and temozolomide (TMZ) for newly diagnosed glioblastoma multiforme (GBM). J Clin Oncol. 26, 2008. Abstract 2025
100 Catalaa I, Henry R, Dillon WP, et al. Perfusion, diffusion and spectroscopy values in newly diagnosed cerebral gliomas. NMR Biomed. 2006;19:463-475.
101 Neuwelt E, Raslan A, Gahramanov S, et al. DSC-MRI using ferumoxytol may help differentiate pseudoprogression from true progression in patients with glioblastoma. A preliminary report. Neuro Oncol. 2008;10:894. Abstract RO-844
102 Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment. Pitfalls in neurooncology. Neuro Oncol. 2008;10:361-367.
103 Gerstner ER, McNamara MB, Norden AD, et al. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol. 2009;94:97-101.
104 van den Bent MJ, Vogelbaum MA, Wen PY, et al. End point assessment in gliomas. Novel treatments limit usefulness of classical Macdonald’s criteria. J Clin Oncol. 2009;27:2905-2908.
105 Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492-507.
106 Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012-2024.
107 Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740.
108 Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma [abstract 342]. Neuro Oncol. 2005;7:369.
109 Quant E, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on a bevacizumab-containing regimen [abstract]. J Clin Oncol. 26, 2008. Abstract 2008
110 Gabayan AJ, Green SB, Sanan A, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas. A retrospective multi-institutional analysis. Neurosurgery. 2006;58:701-709.
111 Chan TA, Weingart JD, Parisi M, et al. Treatment of recurrent glioblastoma multiforme with GliaSite brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62:1133-1139.
112 Ang KK, Jiang GL, Feng Y, et al. Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys. 2001;50:1013-1020.
113 Voynov G, Kaufman S, Hong T, et al. Treatment of recurrent malignant gliomas with stereotactic intensity modulated radiation therapy. Am J Clin Oncol. 2002;25:606-611.
114 Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323-326.
115 Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy. An effective therapy for recurrent high grade gliomas. J Clin Oncol. May 17, 2010. [Epub ahead of print]
116 Kristensen BH, Laursen FJ, Logager V, et al. Dosimetric and geometric evaluation of an open low-field magnetic resonance simulator for radiotherapy treatment planning of brain tumours. Radiother Oncol. 2008;87:100-109.
117 Bokstein F, Kovner F, Blumenthal DT, et al. A common sense approach to radiotherapy planning of glioblastoma multiforme situated in the temporal lobe. Int J Radiat Oncol Biol Phys. 2008;72:900-904.
118 Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20-S27.
119 Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122.
120 Payne JF, Hutchinson AK, Hubbard GB3rd, et al. Outcomes of cataract surgery following radiation treatment for retinoblastoma. J AAPOS. 2009;13:454-458.e3.
121 Hughes MA, Parisi M, Grossman S, et al. Primary brain tumors treated with steroids and radiotherapy. Low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423-1426.
122 Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma. Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691-3697.
123 Khafaga YM, Jamshed A, Allam AA, et al. Stevens-Johnson syndrome in patients on phenytoin and cranial radiotherapy. Acta Oncol. 1999;38:111-116.
124 Micali G, Linthicum K, Han N, et al. Increased risk of erythema multiforme major with combination anticonvulsant and radiation therapies. Pharmacotherapy. 1999;19:223-227.
125 Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma. Long-term follow-up. Lancet Neurol. 2009;8:810-818.
126 Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487-1495.
127 Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134-142.
128 Anscher MS, Marks LB, Shafman TD, et al. Risk of long-term complications after TFG-beta1-guided very-high-dose thoracic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:988-995.
129 Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas. Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972.
130 Robins HI, Lassman AB, Khuntia D. Therapeutic advances in malignant glioma. Current status and future prospects. Neuroimaging Clin North Am. 2009;19:647-656.
131 Sperduto CM, Chakravarti A, Aldape K, et al. Twenty-year survival in glioblastoma. A case report and molecular profile. Int J Radiat Oncol Biol Phys. 2009;75:1162-1165.
132 Mayo C, Martel MK, Marks LB, et al. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76:S28-S35.
133 Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76:S36-S41.
134 Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58-S63.