52 High-Frequency Ventilation
Over the last 30 years, our understanding of the potential harm of mechanical ventilation has evolved, and it has been clearly demonstrated that lung injury may occur through injurious mechanical forces generated during mechanical ventilation. Potential causes of lung injury include gross air leaks (barotrauma), diffuse alveolar injury due to overdistension (volutrauma), injury due to repeated cycles of recruitment and derecruitment (atelectrauma), and injury due to the release of mediators from the lung (biotrauma).1,2 Importantly, volutrauma and atelectrauma lead to biotrauma, which affects not only the lung but may also contribute to multiple organ dysfunction, the major cause of death in patients with ARDS. Lung-protective mechanical ventilation strategies aim to reduce these injurious forces and subsequent lung damage while providing adequate ventilation and oxygenation. The mechanics of high-frequency ventilation, particularly high-frequency oscillatory ventilation (HFOV), make it particularly well suited to protect the lung, and there is growing clinical experience with the use of high-frequency ventilation as an alternative to conventional mechanical ventilation or as salvage therapy in patients failing conventional ventilation strategies.
 Description and Classification
Description and Classification
High-Frequency Positive-Pressure Ventilation
HFPPV was first described in 1969 as an experimental technique3 and has subsequently found only limited clinical use in specialized upper-airway surgical procedures and bronchoscopy.4 Published clinical experience with HFPPV is largely limited to neonatal populations. One meta-analysis in newborn infants found that synchronized mechanical ventilation delivered as HFPPV was associated with reduced barotrauma and shorter hospital stay compared with conventional mechanical ventilation (CMV),5 but the effect on mortality and chronic oxygen dependency was unclear. In adult patients, HFPPV has been used only in specialized applications in the field of anaesthesia.6–8
High-Frequency Percussive Ventilation
High-frequency percussive ventilation (HFPV) is a hybrid mode that combines the principles of high frequency and CMV using a proprietary mechanical ventilator.9 A conventional ventilation circuit is fitted with a gas-driven piston at the end of the endotracheal tube. The reciprocating piston generates pressure oscillations at 3 to 15 Hz, with short expiratory times that are superimposed on the conventional inspiratory-expiratory pressure waves. The high-frequency beats are delivered in bursts to generate auto-PEEP through breath stacking, and then are interrupted to allow alveolar pressure to return to baseline. It has been hypothesized that the auto-PEEP generated improves alveolar recruitment without exposing the alveoli to the high peak airway pressures that would be generated with comparable CMV. Although the high-frequency pressure oscillations are driven actively in both directions, the bulk of exhalation is passive, from the underlying CMV breaths. The high-frequency percussion also provides some internal mucokinesis, potentially improving pulmonary toilet and reducing endotracheal suctioning requirements.10 Indeed, it may be because of this property that HFPV has been most commonly used in adult patients with inhalational injury, burns, and trauma.
High-Frequency Jet Ventilation
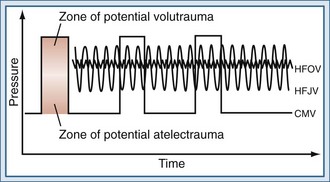
High-frequency jet ventilation (HFJV) employs a small-aperture nozzle inserted into the endotracheal tube in order to direct a high-pressure stream of gas into the lung (Figure 52-1). During inspiration, a high-pressure jet streams into the proximal airways, entraining air from the circuit, and tidal volume is therefore largely dependent on the Venturi and Coanda effects. The parameters controlled by the clinician are frequency, inspiratory time, jet drive pressure, and PEEP applied through the ventilator circuit. Tidal volumes are determined by the jet driving pressure and inspiratory time (i.e., larger tidal volumes can be delivered by increasing jet drive pressure and inspiratory time). Tidal volumes will also be augmented by using a larger jet catheter and a larger endotracheal tube, which increase the amount of jet flow and gas entrainment, respectively. Because expiration is passive, gas trapping may occur at higher frequencies with progressively shorter expiratory times.
A complication specific to HFJV is traumatic upper airway injury. The high-velocity inspiratory jet may cause direct trauma to the proximal airways, and necrotizing tracheobronchitis is a recognized complication of HFJV in both infants and adults.11,12 Gas conditioning during HFJV, particularly humidification and warming, is also problematic. Although the gas entrained from the proximal circuit is warmed and humidified, the gas injected from the jet nozzle expands and cools, compromising the overall conditioning of the inspired gas. It has also been hypothesized that high gas flow rates and rapid increases in lung volume could cause lung injury through the generation of shear forces at the interface of adjacent compliant and atelectatic lung units.13
The clinical utility of HFJV is specific to certain clinical settings such as pulmonary air leak syndromes, when the ability to achieve adequate gas exchange with lower peak airway pressures may be advantageous.14 In addition, the decreased reliance on bulk flow with HFJV may improve gas distribution and gas exchange in the presence of large air leaks, although this theoretical advantage has not been borne out in clinical studies.15
The published clinical experience with HFJV in acute respiratory failure remains small, and to date, the greatest clinical experience is in the neonatal and pediatric populations and in anesthesia for airway stability during respiratory tract surgery. There is limited research in its utility in adult respiratory failure, although many intensive care units (ICUs) have sizable anecdotal experience. Comparative clinical trials have shown that high-frequency jet ventilation is safe and offers improved oxygenation and ventilation compared with CMV, while improving respiratory parameters and decreasing required peak pressures.16–18 None of these trials, however, demonstrated a significant survival advantage.
High-Frequency Oscillatory Ventilation
During HFOV, a piston pump oscillates a diaphragm at frequencies between 3 and 15 Hz (180-900 breaths/min) to create pressure waves in the ventilator circuit (see Figure 52-1). Because the diaphragm is actively driven in both directions, the ventilator creates both inspiratory and expiratory pressure waves, meaning that expiration is also active. The use of active expiration distinguishes HFOV from other forms of high-frequency ventilation, in which expiration is passive and dependent on the elastic recoil of the respiratory system. Active expiration may be advantageous in controlling CO2 and preventing hyperinflation. Indeed, HFOV has been shown to be associated with less gas trapping than other forms of high-frequency ventilation.19 The mean airway pressure is maintained by a resistance valve in the circuit, together with the inspiratory bias flow. Changes in alveolar pressure are kept low by small excursions of the piston. Humidification is achieved by passing the bias flow of gas through a humidifier.20
 Mechanisms of Gas Transport with High-Frequency Oscillatory Ventilation
Mechanisms of Gas Transport with High-Frequency Oscillatory Ventilation
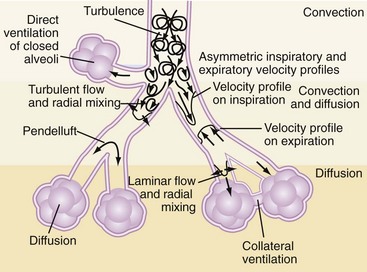
During CMV, gas exchange is largely related to bulk flow of gas to the alveoli. However, since the tidal volumes generated during HFOV may be smaller than the anatomic dead space, ventilation relies on alternative gas exchange mechanisms related largely to enhanced gas mixing within the lung. These gas exchange mechanisms are summarized in Table 52-121 and Figure 52-2. Experimental models suggest that in contrast to CMV, CO2 elimination is a product of the frequency and the square of the tidal volume (VCO2 α = f × VT2)22 such that adequate CO2 elimination may become problematic as tidal volumes decrease, unless accompanied by proportionately larger increases in frequency. Regardless, clinical experience has demonstrated that adequate gas exchange can be achieved in adults with HFOV using frequencies in the 8- to 10-Hz range, delivering tidal volumes that are less than anatomic dead space.23
TABLE 52-1 Mechanisms of Gas Exchange During High-Frequency Oscillatory Ventilation
| Site | Proposed Mechanism |
|---|---|
| Proximal airway | Bulk flow—remains an important mechanism of gas transport in proximal airways |
| Mid-airway | Pendelluft—phenomenon of regional gas movement that occurs as a result of heterogeneity in alveolar filling and emptying rates. Adjacent lung units with different time constants may fill at different rates during inspiration. Following inspiration, there is redistribution of inspired gas from full, fast-filling units to slower-filling units, augmenting gas exchange.9 Taylor dispersion—enhanced diffusion augmented by radial transport mechanisms Asymmetrical velocity profiles—results in a net convective transport of material, especially at airway bifurcations. Fresh gas streams toward alveoli along inner airway walls, while “alveolar” gas streams cranially along outer airway wall. |
| Distal airway | Cardiogenic mixing—rhythmic contraction of heart promotes peripheral gas mixing by generating flow within neighboring parenchymal regions Collateral ventilation—gas exchange between noncommunicating neighboring alveoli via collateral channels |
From Chang HK. Mechanisms of gas transport during ventilation by high-frequency oscillation. J Appl Physiol. 1984;56(3):553-563.
 Rationale for High-Frequency Oscillatory Ventilation
Rationale for High-Frequency Oscillatory Ventilation
In the last few decades, there has been an enormous increase in understanding of the effects of mechanical ventilation on the lung and elucidation of VILI and its pathophysiology, namely volutrauma, atelectrauma, and biotrauma. Furthermore, the clinical relevance of VILI was solidified by a landmark study published by the ARDS Network in 2000 that demonstrated a 9% absolute mortality reduction in patients with ARDS ventilated with tidal volumes of 6 mL/kg ideal body weight compared with 12 mL/kg.24
HFOV would appear to be the ideal lung-protective ventilation strategy in patients with ALI/ARDS because of two principal properties: (1) prevention of VILI by delivery of small tidal volumes with limitation of alveolar overdistention and (2) promotion of alveolar recruitment through application of a higher mean airway pressure than can be safely applied with CMV, promoting more alveolar recruitment and avoiding cyclic opening and closing of alveolar units throughout the respiratory cycle. Indeed, there is a wealth of preclinical animal data demonstrating that compared with both injurious and lung-protective conventional ventilation, HFOV is advantageous in terms of gas exchange, markers of inflammation, and lung pathology scores.25
Does High-Frequency Oscillatory Ventilation Truly Deliver Small Tidal Volumes?
Although tidal volumes are not measured directly on the oscillator that is commercially available in the United States, several investigators have measured delivered tidal volumes. In a sheep saline-lavage model of ALI, Sedeek et al. measured delivered tidal volumes with a pneumotachograph26 and found that HFOV applied with a frequency of 4 Hz and pressure amplitude of 60 cm H2O resulted in tidal volumes of 4 mL/kg—not large, but not as small as had been anticipated. More recently, however, Hager and colleagues measured tidal volumes in adults with ARDS receiving HFO using a hot-wire anemometer, which may provide more accurate measurements. These investigators found that usual tidal volumes delivered during adult HFOV were indeed small, in the 1 to 2 mL/kg range, and that frequency27 rather than pressure amplitude was the dominant determinant of tidal volume in adults with ARDS. These authors emphasized that while low tidal volumes can be delivered during HFO, at low frequencies, tidal volumes may be larger than anticipated. This suggests that a strategy that achieves acceptable CO2 clearance while employing the highest tolerated frequency is likely to be most lung protective. To practically achieve these goals, we generally use a relatively high power set to achieve a pressure amplitude (delta P [ΔP]) of 90 cm H2O, and then adjust frequency as high as tolerated to achieve an adequate pH (>7.25), at times using a partial leak around the endotracheal tube cuff to facilitate CO2 clearance and higher frequency tolerance.
Does High-Frequency Oscillatory Ventilation Promote Alveolar Recruitment?
Alveolar recruitment refers to the dynamic process of reopening unstable collapsed alveoli. Easley et al. performed a study in healthy dogs, using computer tomographic imaging to look at the distribution of lung volume.28 They matched the mean airway pressure with conventional CPAP and noted small decreases in total and regional lung volume with HFOV, especially at lower mean airway pressure and accompanying lower frequency. These authors concluded that there was low risk of occult regional overdistention during HFOV in healthy lungs despite the very high respiratory rates.
In a way, HFOV is like any other mode of mechanical ventilation: success relies not only on selecting the mode that makes a difference but on how that mode is employed. A key factor in the use of HFOV that has emerged from both animal and neonatal literature is the need for HFOV to be used as part of a lung recruitment strategy. To achieve this goal, clinicians generally either (1) select a starting mean airway pressure (mPaw) approximately 5 cm H2O above that used on conventional ventilation and titrate mPaw up to achieve adequate oxygenation; or (2) in an approach we favor, begin oscillation with 1 or more sustained inflation recruitment maneuvers followed by downward titration of mPaw from a relatively high mPaw (30-35 cm H2O), using oxygenation as a surrogate for lung recruitment.29
Does High-Frequency Oscillatory Ventilation Improve Outcome in Adult Patients with acute respiratory distress syndrome?
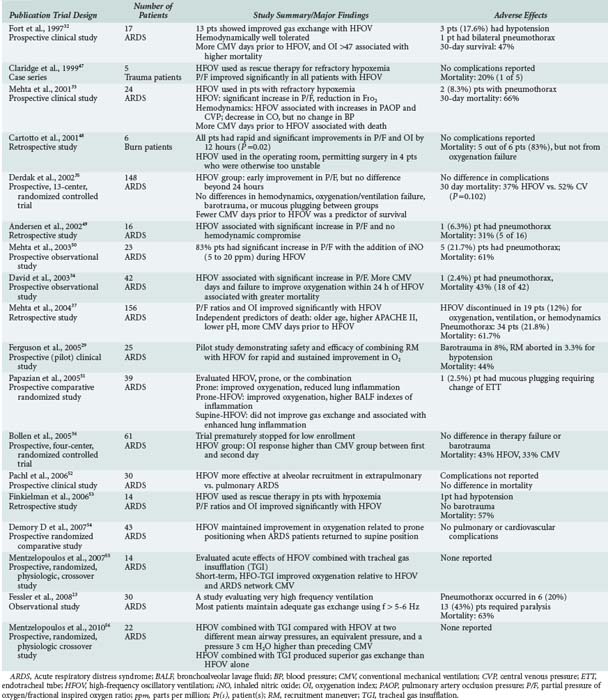
HFOV in principle fulfils the criteria for lung-protective ventilation and can achieve effective ventilation despite very small tidal volumes. However, it is not yet clear whether this ventilatory mode impacts the outcome of adult patients with ARDS. The clinical trials and systematic reviews evaluating HFOV in adults are summarized in Tables 52-2 and 52-3, respectively. Based on the current evidence presented in these two tables, we can conclude the following regarding the use of HFOV in adults with ARDS:
TABLE 52-3 Systematic Reviews Evaluating High-Frequency Oscillatory Ventilation in Adults with Acute Respiratory Distress Syndrome
| Publication | Number of Studies/Patients | Study Summary/Major Findings |
|---|---|---|
| Bollen et al., 200557 Systematic review |
ARDS 2 RCT 7 Observational studies |
Systematic review of determinants of mortality in HFOV in ARDS Prolonged CMV prior to HFOV did not relate to mortality OI associated with mortality independently of other disease markers and could be important for selecting ARDS pt that could benefit from HFOV |
| Wunsch et al., 200458 Cochrane Systematic Review [Abstract] |
ARDS 2 RCTs Children, N=58 Adult, N=148 |
Systematic review comparing HFOV and CMV Inadequate evidence to conclude whether HFOV reduces morbidity or mortality in pts with ALI or ARDS |
| Sud et al., 201044 Systematic review and meta-analysis |
ARDS 8 RCTs N=419 |
HFOV may improve survival; unlikely to cause harm Reduced mortality (RR 0.77, 95% CI 0.61-0.98) and treatment failure (refractory hypoxemia, hypercapnia, hypotension, barotrauma, RR 0.67, 95% CI 0.46-0.99) |
ARDS, Acute respiratory distress syndrome; CI, confidence interval; CMV, conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation; OI, oxygenation index; Pt(s), patient(s); RCT, randomized controlled trial; RR, risk ratio.
 Current High-Frequency Oscillatory Ventilation Practices in Patients with Acute Respiratory Distress Syndrome
Current High-Frequency Oscillatory Ventilation Practices in Patients with Acute Respiratory Distress Syndrome
High-frequency ventilation has been in use for at least 3 decades, and the clinical application has evolved over the years. For a detailed discussion of specific recommendations regarding HFOV settings and monitoring, readers are referred to a recent roundtable report on HFOV use.30
Frequency
In adults, HFOV frequency is most commonly 5 to 7 Hz, while higher frequencies are used in neonates (8-15 Hz).31 If there is a need for greater CO2 clearance, the most common approach is to decrease the frequency to less than 5 Hz after ΔP has been optimized. This approach, however, results in delivery of larger tidal volumes and may not optimize the potential of HFOV to deliver small tidal volumes. A recent study in adults23 demonstrated that frequencies greater than 6 Hz (with 1 patient reaching 15 Hz) can maintain adequate gas exchange. In that study, an endotracheal cuff leak was applied in 30% of patients to aid CO2 clearance. The rationale for exploring higher frequencies during HFOV is the ability to achieve smaller tidal volumes and attenuate potential overdistention injury.
Timing of High-Frequency Oscillatory Ventilation
In early studies, HFOV was initiated approximately 5 days following the start of CMV.32 However, several observational studies have suggested better outcomes if HFOV is initiated earlier.32,33 David et al.34 observed that mortality was reduced when HFOV was initiated within 3 days following the start of CMV, compared to after 3 days (20% versus 64%, respectively). Thus in recent years, investigators have initiated HFOV earlier: 1.9 days in the trial by Derdak et al.35 and 1.8 days in the trial by Bollen et al.36 Nonetheless, a meta-analysis evaluating determinants of mortality with HFOV in adults with ARDS did not find a relationship between late initiation of HFOV and higher mortality.36
Recruitment Maneuvers
The use of recruitment maneuvers has gathered intense interest in the management of adults with ARDS and has been incorporated by some intensivists with the use of high-frequency ventilation. A pilot study by Ferguson et al. found that the combination of recruitment maneuvers and HFOV can be safely applied and results in rapid and sustained improvement in oxygenation.29
 Complications of High-Frequency Oscillatory Ventilation
Complications of High-Frequency Oscillatory Ventilation
Barotrauma
Given the higher mean airway pressures applied during HFOV, there is a concern regarding the risk of barotrauma. The incidence of pneumothorax with the use of HFOV in observational studies varies from 2.4%34 to 21.8%37; however, HFOV was generally applied as rescue therapy in patients with severe ARDS, who are likely to already be at greater baseline risk of developing barotrauma. In two published randomized controlled trials35,36 comparing HFOV with CMV, there was no difference in the incidence of pneumothorax between CMV and HFOV groups.
Hemodynamic Instability
Another potential effect of a high mean airway pressure, regardless of mode of ventilation, is a reduction in venous return secondary to increased intrathoracic pressure. Thus it is important to consider the patient’s volume status prior to and during the transition from CV to HFOV, and consider judicious volume administration to ensure adequate intravascular volume.20,38
Inadequate Humidification
Inadequate humidification of inspired gas may lead to desiccation of secretions, potentially mucous inspissation and obstruction of the endotracheal tube. This is uncommon during HFOV,32,33 as adequate humidification can usually be achieved by passing the bias flow of gas through a humidifier.39 However, problems with endotracheal tube obstruction may occasionally arise and can be indicated by a sudden rise in generated pressure amplitude for a given power setting.
Sedation and Paralysis
Unlike neonates, the majority of adults require suppression of their respiratory efforts during HFOV so their inspiratory flow rate does not outstrip the provided bias flow. Heavy sedation and occasionally paralysis are required in the majority of patients37; patient selection is therefore important to ensure that the severity of illness is sufficiently high to justify the use of sedation and paralysis, given the adverse effects of these agents.40,41 In contrast to previous literature associating neuromuscular blockade with the development of critical illness polyneuropathy,42 the early short-term use of paralytic agents has recently been reported to improve survival in patients with ARDS.43
 The Future of High-Frequency Oscillatory Ventilation
The Future of High-Frequency Oscillatory Ventilation
One of the most important questions regarding HFOV is whether it truly improves mortality when compared to lung-protective conventional ventilation. To date, several small studies35,36 and a meta-analysis44 suggest that HFOV may be beneficial, but there are issues with many of these studies which used antiquated strategies for both CMV and HFOV. For the definitive answer to this question, we will need to await the results of two large phase 3 studies comparing HFOV with best current conventional ventilation.45,46
Key Points
Derdak S, Mehta S, Stewart TE, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166(6):801-808.
Easley RB, Lancaster CT, Fuld MK, et al. Total and regional lung volume changes during high-frequency oscillatory ventilation of the normal lung. Respir Physiol Neurobiol. 2009;165(1):54-60.
Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2008;36(4):1043-1048.
Hager DN, Fessler HE, Kaczka DW, et al. Tidal volume delivery during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2007;35(6):1522-1529.
Mehta S, Lapinsky SE, Hallett DC, et al. Prospective trial of high-frequency oscillation in adults with acute respiratory distress syndrome. Crit Care Med. 2001;29(7):1360-1369.
Sud S, Sud M, Friedrich JO, et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ. 2010;340:c2327.
1 Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104-2112.
2 Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721-1725.
3 Oberg PA, Sjostrand U. Studies of blood-pressure regulation. II. On-line simulation as a method of studying the regulatory properties of the carotid-sinus reflex. Acta Physiol Scand. 1969;75:287-300.
4 Borg U, Eriksson I, Sjostrand U. High-frequency positive-pressure ventilation (HFPPV): a review based upon its use during bronchoscopy and for laryngoscopy and microlaryngeal surgery under general anesthesia. Anesth Analg. 1980;59:594-603.
5 Greenough A, Milner AD, Dimitriou G. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst Rev 2000:CD000456.
6 Heifetz M, De-Myttenaere S, Copel J. [Clinical evaluation of high-frequency positive pressure ventilation (HFPPV) in morbid obesity]. Anaesthesist. 1982;31:670-673.
7 Larsson S, Nordberg G. Emergency one-lung high-frequency positive-pressure ventilation (HFPPV). Anesth Analg. 1987;66:471-474.
8 Tsui SL, Chan CS, Chan AS, et al. A comparison of two-lung high frequency positive pressure ventilation and one-lung ventilation plus 5 cm H2O non-ventilated lung CPAP, in patients undergoing anaesthesia for oesophagectomy. Anaesth Intensive Care. 1991;19:205-212.
9 Davis KJ, Hurst J, Branson R. High frequency percussive ventilation. Probl Respir Care. 1989;2:39-47.
10 Freitag L, Long WM, Kim CS, et al. Removal of excessive bronchial secretions by asymmetric high-frequency oscillations. J Appl Physiol. 1989;67:614-619.
11 Boros SJ, Mammel MC, Lewallen PK, et al. Necrotizing tracheobronchitis: a complication of high-frequency ventilation. J Pediatr. 1986;109:95-100.
12 Circeo LE, Heard SO, Griffiths E, et al. Overwhelming necrotizing tracheobronchitis due to inadequate humidification during high-frequency jet ventilation. Chest. 1991;100:268-269.
13 Ihra G, Gockner G, Kashanipour A, et al. HFJV in European and North American Institution: development and clinical practice. Eur J Anaesth. 2000;17:418-430.
14 Carlon GC, Kahn RC, Howland WS, et al. Clinical experience with high frequency jet ventilation. Crit Care Med. 1981;9:1-6.
15 Bishop MJ, Benson MS, Sato P, et al. Comparison of high-frequency jet ventilation with conventional mechanical ventilation for bronchopleural fistula. Anesth Analg. 1987;66:833-838.
16 Carlon GC, Howland WS, Ray C, et al. High-frequency jet ventilation. A prospective randomized evaluation. Chest. 1983;84:551-559.
17 Gluck E, Heard S, Patel C, et al. Use of ultrahigh frequency ventilation in patients with ARDS. A preliminary report. Chest. 1993;103:1413-1420.
18 MacIntyre NR, Follett JV, Deitz JL, et al. Jet ventilation at 100 breaths per minute in adult respiratory failure. Am Rev Respir Dis. 1986;134:897-901.
19 Leipala JA, Sharma A, Lee S, et al. An in vitro assessment of gas trapping during high frequency oscillation. Physiol Meas. 2005;26:329-336.
20 Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest. 2007;131:1907-1916.
21 Chang HK. Mechanisms of gas transport during ventilation by high-frequency oscillation. J Appl Physiol. 1984;56:553-563.
22 Boynton BR, Hammond MD, Fredberg JJ, et al. Gas exchange in healthy rabbits during high-frequency oscillatory ventilation. J Appl Physiol. 1989;66:1343-1351.
23 Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2008;36:1043-1048.
24 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
25 Ferguson ND, Slutsky AS. Point: High-frequency ventilation is the optimal physiological approach to ventilate ARDS patients. J Appl Physiol. 2008;104:1230-1231.
26 Sedeek KA, Takeuchi M, Suchodolski K, et al. Determinants of tidal volume during high-frequency oscillation. Crit Care Med. 2003;31:227-231.
27 Hager DN, Fessler HE, Kaczka DW, et al. Tidal volume delivery during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2007;35:1522-1529.
28 Easley RB, Lancaster CT, Fuld MK, et al. Total and regional lung volume changes during high-frequency oscillatory ventilation (HFOV) of the normal lung. Respir Physiol Neurobiol. 2009;165:54-60.
29 Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med. 2005;33:479-486.
30 Fessler HE, Derdak S, Ferguson ND, et al. A protocol for high-frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med. 2007;35:1649-1654.
31 Greenough A, Donn SM. Matching ventilatory support strategies to respiratory pathophysiology. Clin Perinatol. 2007;34:35-53. v-vi
32 Fort P, Farmer C, Westerman J, et al. High-frequency oscillatory ventilation for adult respiratory distress syndrome–a pilot study. Crit Care Med. 1997;25:937-947.
33 Mehta S, Lapinsky SE, Hallett DC, et al. Prospective trial of high-frequency oscillation in adults with acute respiratory distress syndrome. Crit Care Med. 2001;29:1360-1369.
34 David M, Weiler N, Heinrichs W, et al. High-frequency oscillatory ventilation in adult acute respiratory distress syndrome. Intensive Care Med. 2003;29:1656-1665.
35 Derdak S, Mehta S, Stewart TE, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801-808.
36 Bollen CW, van Well GT, Sherry T, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care. 2005;9:R430-R439.
37 Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest. 2004;126:518-527.
38 David M, von Bardeleben RS, Weiler N, et al. Cardiac function and haemodynamics during transition to high-frequency oscillatory ventilation. Eur J Anaesthesiol. 2004;21:944-952.
39 Downar J, Mehta S. Bench-to-bedside review: high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care. 2006;10:240.
40 Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609-2615.
41 Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
42 De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859-2867.
43 Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113-119.
44 Sud S, Sud M, Friedrich JO, et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ. 2010;340:c2327.
45 Young JD. Conventional positive pressure ventilation or High Frequency Oscillatory Ventilation (HFOV) for adults with acute respiratory distress syndrome: OSCAR: High Frequency Oscillation in ARDS. ISRCTN10416500.
46 Ferguson N. High frequency oscillation versus best current conventional ventilation to reduce acute respiratory distress syndrome (ARDS) mortality: a multicentre randomised controlled trial: OSCILLATE. ISRCTN87124254.
47 Claridge JA, Hostetter RG, Lowson SM, et al. High-frequency oscillatory ventilation can be effective as rescue therapy for refractory acute lung dysfunction. Am Surg. 1999;65:1092-1096.
48 Cartotto R, Cooper AB, Esmond JR, et al. Early clinical experience with high-frequency oscillatory ventilation for ARDS in adult burn patients. J Burn Care Rehabil. 2001;22:325-333.
49 Andersen FA, Guttormsen AB, Flaatten HK. High frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome–a retrospective study. Acta Anaesthesiol Scand. 2002;46:1082-1088.
50 Mehta S, MacDonald R, Hallett DC, et al. Acute oxygenation response to inhaled nitric oxide when combined with high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2003;31:383-389.
51 Papazian L, Gainnier M, Marin V, et al. Comparison of prone positioning and high-frequency oscillatory ventilation in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:2162-2171.
52 Pachl J, Roubik K, Waldauf P, et al. Normocapnic high-frequency oscillatory ventilation affects differently extrapulmonary and pulmonary forms of acute respiratory distress syndrome in adults. Physiol Res. 2006;55:15-24.
53 Finkielman JD, Gajic O, Farmer JC, et al. The initial Mayo Clinic experience using high-frequency oscillatory ventilation for adult patients: a retrospective study. BMC Emerg Med. 2006;6:2.
54 Demory D, Michelet P, Arnal JM, et al. High-frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenation. Crit Care Med. 2007;35:106-111.
55 Mentzelopoulos SD, Roussos C, Koutsoukou A, et al. Acute effects of combined high-frequency oscillation and tracheal gas insufflation in severe acute respiratory distress syndrome. Crit Care Med. 2007;35:1500-1508.
56 Mentzelopoulos SD, Malachias S, Kokkoris S, et al. Comparison of high-frequency oscillation and tracheal gas insufflation versus standard high-frequency oscillation at two levels of tracheal pressure. Intensive Care Med. 2010;36:810-816.
57 Bollen CW, Uiterwaal CS, van Vught AJ. Systematic review of determinants of mortality in high frequency oscillatory ventilation in acute respiratory distress syndrome. Crit Care. 2006;10:R34.
58 Wunsch H, Mapstone J. High-frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004:CD004085.