Chapter 143 High Cervical and Occipitocervical Plate, Rod, Wire, and Bone Techniques
Abnormalities at the craniocervical junction were first reported in the early 19th century.1 The craniocervical junction includes the occiput, atlas, and axis vertebrae. Numerous pathologic conditions can lead to instability at the craniocervical junction. Common mechanisms of injury include trauma, rheumatoid arthritis, inflammatory and infectious lesions, tumors, and congenital anomalies.
Stability of the occipitoatlantal joint is provided by the cup-shaped configuration of the occipitoatlantal joint and the thick articular capsule, along with the anterior and posterior atlanto-occipital membranes.2–4 The fibrous capsule of the occipitoatlantal joint is usually thickest laterally and posteriorly and thin if not deficient medially. The tectorial membrane, which is a continuation of the posterior longitudinal ligament, attaches to the ventral foramen magnum and laterally to the medial aspects of the occipitoatlantal joints, playing an important role in the stability of the craniovertebral junction (CVJ).5 The alar ligaments are paired and arise on either side of the dens and have two components: the atlantoalar and the occipitoalar ligaments. These two components connect the dens to the lateral mass of C1 and to the medial aspect of the ipsilateral occipital condyle, respectively.6 These ligaments together with the cruciform and apical ligaments span from the axis vertebra to the occiput, and all provide some degree of stability to the occipitoatlantal joint.7
Causes of Instability in the Upper Cervical Spine
Instability of the upper cervical spine can be caused by congenital, traumatic, inflammatory, or neoplastic involvement.8 Of the several congenital abnormalities that occur in the upper cervical spine, basilar impression (or cranial settling) is the most common. Basilar impression may be progressive and lead to cervicomedullary spinal cord compression. Congenital ligamentous laxity in the upper cervical spine (e.g., Down syndrome, Morquio syndrome) can cause instability and subluxation. Upper cervical spine involvement in patients with rheumatoid arthritis can also lead to atlantoaxial instability and cranial settling, both of which may require surgical decompression and stabilization. Trauma, however, is the most common cause of instability in the upper cervical spine. Most upper cervical spine injuries result from blows to the head (e.g., motor vehicle accidents and falls). The direction of the force vector determines the type of injury (i.e., blows to the head versus deceleration of the torso). Injuries of the occipitoatlantal junction are usually fatal and are only detected postmortem. Atlantoaxial instability, on the other hand, can result from disruption of the bony or ligamentous elements or both. Bony fractures often involve the dens. Fractures of the odontoid process of C2 are classified into three types: I, II, and III.9 Type II odontoid fractures are unstable and notorious for nonunion with conservative management. Published reports of nonunion rates for conservatively managed type II odontoid fractures range from 30% to 60%. Type I fractures are always stable, and type III fractures may be unstable in 10% to 15% of cases.
Craniocervical instability can result from destructive tumors as well as iatrogenically, following resection of such craniocervical tumors. Instability may depend on the extent of condylar destruction or resection in these cases.
Instability at the Craniocervical Junction
There is a paucity of reports in the literature regarding resection of the occipital condyle or the destruction of the condyle with tumors of the craniocervical junction causing instability and the necessity for stabilization in this group of patients. The recommendations to date have been to stabilize the occipitocervical junction when greater than 70% of the occipital condyle has been removed.4,10
Management of Instability in the Upper Cervical Spine
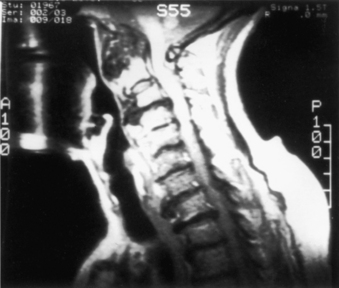
Patients with rheumatoid arthritis should have an MRI to ascertain the presence of inflammatory pannus and/or bony encroachment of the neural elements (Fig. 143-1). Patients who have evidence of neural compromise with occipitocervical instability should be considered for a ventral decompressive procedure before undergoing dorsal stabilization.11
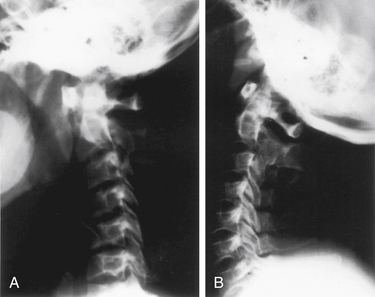
Patients with radiologic evidence of subluxation on neutral radiographs should also be evaluated with dynamic, lateral flexion-extension radiographs to assess the reducibility of the subluxation. Patients with nonreduced subluxations should undergo a trial of cervical traction to reduce the dislocation before a decision is made about the appropriate surgical treatment. Patients with chronic instability should remain in traction for 4 to 5 days with muscle relaxation before being considered for surgical treatment. Patients with a reducible subluxation, reduction achieved with flexion-extension, or with axial traction (Fig. 143-2) and without compromise of the cervicomedullary cord, may be safely stabilized by a dorsal approach.12 In contrast, patients who have a ventral spinal cord compression with a nonreducible subluxation may require a ventral transfacial decompression before undergoing occipitocervical stabilization. Usually, patients with instability related to rheumatoid arthritis or with pannus, but without neurologic deficit, can be stabilized from a dorsal approach without undergoing an initial ventral decompression.13 The pannus typically resolves in 6 to 12 months after abnormal movement has been eliminated.
Indications for Instrumentation in Occipitoatlantal Instability
The majority of traumatic occipitoatlantal dislocations are fatal. Some patients who arrive in the emergency department are treated effectively in cervical traction followed by occipitocervical instrumentation and fusion. Chronic instability at the occipitoatlantal junction occurs with rheumatoid arthritis, with tumors, and following tumor resection.14 Chronic instability at the craniocervical junction has been described as “glacial” instability in patients with chronic instability erosion of the occipitoatlantal articulation. This instability may result in cranial settling with associated rotary subluxation. In patients presenting with cranial settling with minimal or no neurologic deficits, reduction with cervical traction may be attempted, and if successful, dorsal occipitocervical instrumentation and fusion can be performed.15,16 In patients with significant neurologic deficits, a ventral decompression may be necessary before dorsal stabilization and fusion. Patients with rheumatoid arthritis, atlantoaxial subluxation, and associated cranial settling are candidates for occipitocervical instrumentation and fusion.
The only scientific evidence for the assessment of instability at the craniocervical junction following tumor surgery comes from the biomechanical study by Shin et al.17 Based on our clinical series, the biomechanical studies available, and new concepts of instability applied to the CVJ,18 we recommend that occipitocervical stabilization and fusion be performed when 50% or more of one condyle is resected or noted to have been destroyed by the tumor. A strong argument supporting this guideline can be made when using the extreme lateral transcondylar approach18 for the resection of these tumors. This approach removes the thickest parts of the capsule of the occipitoatlantal joint, and therefore renders the joint unstable (glacial instability). Thus, patients who have the condyle resected for surgical access and patients with tumor destruction of the condyle equal to or greater than 50% (but <70%) who do not undergo stabilization and fusion may progress to a glacial instability and eventually to overt instability with severe neck pain and torticollis.
Occipitocervical Techniques
In 1959, Perry and Nickel described a simple onlay graft for neck fusion for instability after severe poliomyelitis. In 1969, Newman and Sweetnam19 described the technique for occipitocervical fusion in atlantoaxial instability. Fusion was achieved by decortication and laying down strips of corticocancellous bone obtained from the iliac crest. Patients were kept in cervical traction for 6 weeks and then placed in a high plastic cervical collar until bone fusion was observed.
The combination of internal fixation with onlay bone grafting has reduced the need for postoperative traction and rigid immobilization. Pseudarthrosis rates in a series of 302 occipitocervical fusions and 98 atlantoaxial fusions have been reported to be as low as 1%.7
Occipitocervical Fusion
In occipitocervical junction arthrodesis, a bony bridge is established between the occiput and the upper cervical spine. The techniques may be divided into those using bone alone versus internal fixation and bone grafting. A simple onlay graft alone in occipitocervical fusions was used first by Perry and Nickel and later by Newman and Sweetnam.19 The technique entails decortication and laying down of strips of corticocancellous bone obtained from the iliac crest. The fusion extends from the occipital bone to the atlas and axis. Patients who underwent this procedure were placed postoperatively in a halo vest for 3 to 4 months until bony fusion was noted on radiologic studies. The authors reported good fusion rates; however, the patients in their series were young and healthy.
Occipitocervical Fusion with Internal Fixation
Contoured Rod and Cable Fixation
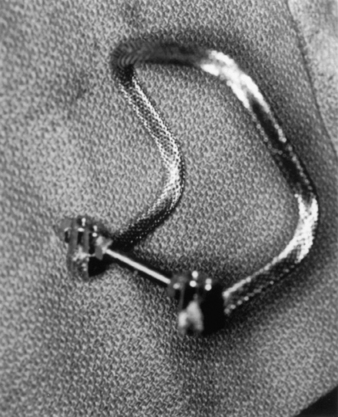
Different smooth and threaded rods have been used for internal fixation in occipitocervical fusions. We use a titanium rod, contoured similar to the “Ransford loop,” with cross-linkage at the caudal end to conform to the craniocervical angle, with the patient’s head in a neutral position (Fig. 143-3).
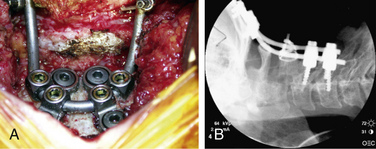
More recently, screw systems have become more popular and provide a more rigid fixation. Cortical screws are placed in the occiput close to the midline where the bone is thickest; this is combined with a rod and or plate system at the occiput and lateral mass and C2 pedicle screws (Figs. 143-4A and B).
After cable placement for cranial fixation, sublaminar placement for spinal fixation is achieved by passing a double cable under the dorsal arch of C1 and the lamina of C2. When separated, a single cable is available on either side of the dorsal arch of C1 and C2. At C3 and C4, a hole may be made at the spinolaminar junction using a right-angled drill. Single cables threaded through Drummond buttons are passed from either side of the spinolaminar junction (Fig. 143-5). Alternatively, sublaminar cables can be passed with due care under the subaxial cervical spine. The contoured rod is placed in the bed, and the cables are torqued sequentially. The distal end of the rod may be cross-fixed, just caudal to the C3 or C4 spinous process, to increase the rigidity of the construct (Fig. 143-6). After decortication, bone graft is obtained through a separate incision from the dorsal iliac crest and is laid as an onlay graft of corticocancellous bone. Care must be taken during head positioning before internal fixation and fusion to obtain the best subluxation reduction with optimal head and neck position, compatible with normal function. Care must be taken to avoid dural perforation during wire passage at the suboccipital bur holes and at C1 or C2. Bevelling the edges of the bur holes and meticulous dissection of the dura mater from the inner table should prevent dural laceration. The leader wire of the single cable should be bent back on itself to present a smooth surface during sublaminar passage. Monitoring the bony surfaces carefully during torquing can help prevent overtightening of the cable. Sequential cable tightening with torque not exceeding 4 to 5 inch-pounds with titanium cables and 8 inch-pounds with stainless steel cables is appropriate in healthy adults. In patients with soft bones and in those with rheumatoid arthritis, the torque is reduced as appropriate (i.e., when cable begins to cut into the bone).
Plate Fixation
Plates and screws have been used for occipitocervical fixation; however, their limiting factor is the variable thickness of the occipital bone. Heywood et al.1 reported that occipital bone thickness measured 9 to 16 mm in the midline and only 3 to 9 mm in the lateral suboccipital bone. No room exists between the dura mater and the cerebellum. Penetration of the inner table could lead to cerebellar injury. Therefore, it is possible that with bicortical purchase during plate fixation, especially laterally in the suboccipital bone, the tip of the screw could perforate the dura mater and the underlying cerebellum.
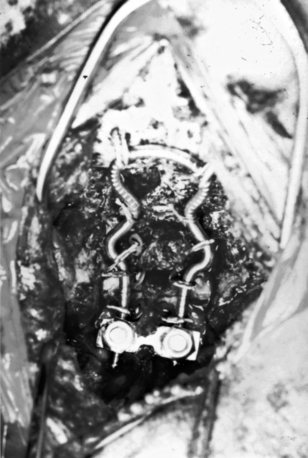
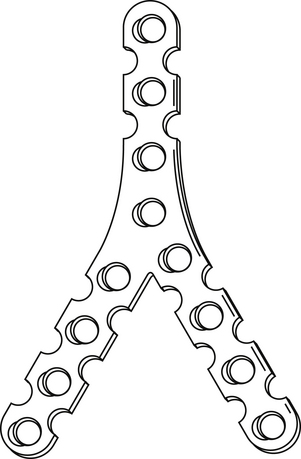
With the Y-plate system of plate and screws for occipitocervical fusion, the stem of the Y is laid over the midline of the occiput (Fig. 143-7). The occipital screws are 2.7 mm in diameter and 8 to 10 mm long. If subaxial fusion is necessary, transfacet screws are placed in the C1-2 facet joint (Magerl technique) and in the lateral masses of the subaxial spine.
Because of the risk of screws perforating the dura matter and the cerebellum, the use of the Y-plate and the lateral occipitocervical plate (see Fig. 143-4) is limited due to the need to place screws in the lateral suboccipital bone. Occipital plates with screws placed in the midline and connected to rod systems that connect to the screws placed in the C2 pedicle and lateral masses in the lower cervical spine are more rigid biomechanically than the wiring techniques.
Atlantoaxial Fusion
In 1910, Mixter and Osgood described the first atlantoaxial stabilization procedure using internal fixation. Gallie popularized wiring and bone grafting techniques for dorsal atlantoaxial arthrodesis.20 A loop of wire is passed beneath the dorsal arch of the atlas from caudal to rostral. The loop of wire is then pulled caudally over the spinous process of C2. The free ends of the wire are run downward and around the spinous process of C2 and are twisted together. The dorsal surfaces of C1 and C2 are decorticated, and onlay bone graft from the iliac crest is placed to achieve bony union. This construct provides minimal rotational stability. In 1978, Brooks and Jenkins modified this technique.10 They described passing two wires on either side, underneath the dorsal arch of C1 and the lamina of C2. Two unicortical wedges of iliac crest bone are placed between C1 and C2. This improves rotational stability. A modification of the Gallie technique described by Papadopoulos et al.21 uses a bicortical H-graft placed between the dorsal arch of C1 and the spinous process and lamina of C2.
Preoperative evaluation and management for this technique is identical to that described with occipitocervical fusion. In the modified Gallie technique, a loop of cable is passed under the dorsal arch of C1 from caudal to rostral. The caudal edge of the dorsal arch of C1 and the rostral edge of the C2 lamina and spinous process are decorticated. A bicortical graft, notched on its caudal surface to hug the contour of the spinous process and lamina of C2, is placed between C1 and C2. The cancellous surfaces of the graft abut the decorticated areas of C1 and C2. The cable passes as with the Gallie technique. The loop is dorsal to the graft, and the free ends run ventral to the graft. This ensures that the graft does not migrate dorsally or ventrally while providing the necessary rotational stability. Further decortication and onlay bone grafting of the dorsal surfaces of C1 and C2 is carried out. Overtightening of the cable around the graft can lead to graft erosion and loosening. In the presence of dorsal subluxation of the dens, there is a risk of pulling the dens dorsally into the spinal cord during wire tightening. This risk is minimized by using a larger wedge of bone between the dorsal elements of C1 and C2. The tip of the leader wire should be doubled back to present a smooth surface during sublaminar wire passage to prevent dural laceration.
Summary
Guidelines for occipitocervical stabilization following resection of tumors at the CVJ have been based on anecdotal evidence with very little reported in the literature. Based on our clinical series21 and the biomechanical studies available, we recommend that occipitocervical stabilization and fusion be performed where 50% or more of one condyle is resected or noted to have been destroyed by the tumor.
Bohlman H.H. Acute fractures and dislocations of the cervical spine: an analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg [Am]. 1979;61:1119-1142.
Menezes A.H. Surgical approaches to the craniocervical junction. In: Frymoyer J., editor. The adult spine. New York: Lippincott-Raven; 2003:523-551.
Menezes A.H., VanGilder J.C. Transoral-transpharyngeal approach to the anterior craniocervical junction: 10-year experience with 72 patients. J Neurosurg. 1988;69:895-903.
Menezes A.H., VanGilder J.C. Abnormalities of the craniovertebral junction. In: Youmans J., editor. Neurological surgery. ed 3. Philadelphia: WB Saunders; 1990:1359-1420.
Menezes A.H., VanGilder J.C., Graf C.J., McDonnell D.E. Craniocervical abnormalities: a comprehensive surgical approach. J Neurosurg. 1980;53:444-455.
Shin H., Barrenechea I.J., Lesser J., et al. Occipitocervical infusion after resection of craniovertebral junction tumors. J Neurosurg Spine. 2006;4:137-144.
1. Heywood A.W., Learmonth I.D., Thomas M. Internal fixation for occipito-cervical fusion. J Bone Joint Surg [Br]. 1988;70:708-711.
2. Beighton P., Craig J. Atlanto-axial subluxation in the Morquio syndrome. J Bone Joint Surg [Am]. 1979;61:478-481.
3. Dawson E.G., Smith I. Atlanto-axial subluxation in children due to vertebral anomolies. J Bone Joint Surg [Am]. 1978;61:582-587.
4. Fielding J.W., Hawkins R.J., Ratzan S.A. Spine fusion for atlanto-axial instability. J Bone Joint Surg [Am]. 1976;58:400-407.
5. Grob D., Dvorak J., Panjabi M., et al. Posterior occipitocervical fusion. A preliminary report of a new technique. Spine (Phila Pa 1976). 1991;16(Suppl 3):S17-S24.
6. Graham A., Hockley A.D., McMillan J.J., Thompson A.G. A new method of occipitocervical fusion, using internal fixation. Neurosurgery. 1987;21:947-950.
7. Menezes A.H. Surgical approaches to the craniocervical junction. In: Frymoyer J., editor. The adult spine. New York: Lippincott-Raven; 2003:523-551.
8. Bohlman H.H. Acute fractures and dislocations of the cervical spine: an analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg [Am]. 1979;61:1119-1142.
9. Anderson L.D., D’Alonzo R.T. Fracture of the odontoid process of the axis. J Bone Joint Surg [Am]. 1974;56:1663-1674.
10. Brooks A.L., Jenkins E.B. Atlanto-axial arthrodesis by wedge compression method. J Bone Joint Surg [Am]. 1978;60:279-284.
11. Menezes A.H., VanGilder J.C. Abnormalities of the craniovertebral junction. In: Youmans J., editor. Neurological surgery. ed 3. Philadelphia: WB Saunders; 1990:1359-1420.
12. Menezes A.H., VanGilder J.C. Transoral-transpharyngeal approach to the anterior craniocervical junction: 10–year experience with 72 patients. J Neurosurg. 1988;69:895-903.
13. Menezes A.H., VanGilder J.C., Graf C.J., McDonnell D.E. Craniocervical abnormalities: a comprehensive surgical approach. J Neurosurg. 1980;53:444-455.
14. Pierce D.S., Barr J.S. Fractures and dislocations at the base of the skull and upper cervical spine. In: Cervical Spine Research Society, editor. The cervical spine. Philadelphia: Lippincott-Raven; 1983:196-205.
15. Hadley M.N., Dickman C.A., Browner C.M., Sonntag V.K. Acute axis fractures: a review of 229 cases. J Neurosurg. 1989;71:642-647.
16. Rana N.A., Hancock D.O., Taylor A.R., Hill A.G. Atlanto-axial subluxation in rheumatoid arthritis. J Bone Joint Surg [Br]. 1973;55:458-470.
17. Shin H., Barrenechea I.J., Lesser J., et al. Occipitocervical infusion after resection of craniovertebral junction tumors. J Neurosurg Spine. 2006;4:137-144.
18. Cregan J.C. Internal fixation of the unstable rheumatoid cervical spine. Ann Rheum Dis. 1966;25:242-252.
19. Newman P., Sweetnam R. Occipitocervical fusion: an operative technique and its indications. J Bone Joint Surg [Br]. 1969;51:423-431.
20. Gallie W.E. Fractures and dislocations of the cervical spine. Am J Surg. 1939;46:495.
21. Papadopoulos S.M., Dickman C.A., Sonntag V.K. Atlantoaxial stabilization in rheumatoid arthritis. J Neurosurg. 1991;74:1-7.