3 Hiccups, sore mouth and bad breath
Case
A 51-year-old post-menopausal woman consults because of a feeling of burning inside her mouth. The pain in her mouth is present every day and persists for most of the day. She also describes dryness of the mouth but no dryness of the eyes. She has otherwise been in excellent health. She denies any history of heartburn or acid regurgitation. Her sense of taste and smell is normal. She has not had any mouth ulcers or trauma. There is no history of arthritis or skin rashes. Her dental history is unremarkable. She has been using mouthwash but only occasionally. She denies symptoms of anxiety or feeling depressed.
Hiccups
Pathophysiology
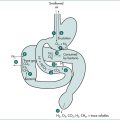
A range of gastrointestinal stimuli can cause reflex excitation of visceral afferent vagal fibres. Vagal afferent receptors in the oesophagus can trigger the responsible medullary centres. This is the proposed mechanism for hiccups occurring during swallowed bolus impaction at the site of a benign stricture or ring, or in response to oesophageal distension by retained food and fluid in oesophageal achalasia, or in the context of pill-induced oesophageal ulceration or stricture. Similar vagal afferent stimuli might originate in cases of hiatus hernia or gastro-oesophageal reflux disease, but the evidence implicating reflux as a cause of hiccups remains inconclusive.
Aetiology of hiccups
The relatively common self-limiting bout of hiccups is frequently induced by gastric distension, emotion, alcohol ingestion or sudden change in temperature. However, most frequently the cause of transient hiccups is unknown. The cause of intractable hiccups can be classified into five groups (Box 3.1):
Approach to the patient with intractable hiccups
A careful history should enquire about neurological symptoms, particularly headache and brainstem symptoms such as diplopia, vertigo, nausea, vomiting, hoarseness, ataxia or clumsiness, and disordered pain sensation. Chest pain, fever and cough are clues to cardiac, respiratory or mediastinal disease. Gastrointestinal causes may be suspected if heartburn, regurgitation, chest pain, dysphagia, vomiting or abdominal pain is reported. A history of metabolic disorders and drug enquiry are also important (Box 3.2).
Box 3.2 Approach to intractable hiccups
History
Physical examination should include examination of cranial nerve, long tract and cerebellar signs. Disordered cognitive function may relate to metabolic derangements or to central causes such as encephalitis. Cardiorespiratory examination should look for signs of pleural or pericardial disease and postural hypotension, which is present in Addison’s disease. The external auditory canals should be examined for foreign bodies. Abdominal examination should specifically look for signs of gastric stasis (e.g. succussion splash), bowel obstruction or tender hepatomegaly, which may indicate hepatic enlargement, or an intrahepatic lesion such as an abscess.
The priorities with respect to investigations will be dictated by the historical and physical findings. Serum electrolytes including sodium, calcium, blood sugar level and liver function tests should be done. A leucocytosis may indicate an underlying infective process. Chest x-ray and electrocardiogram are important to detect pericardial, plural or myocardial disease such as myocardial infarction. A thoracic computed tomography (CT) scan can be performed if mediastinal disease is suspected. Imaging of the abdomen is indicated if a subdiaphragmatic abscess is suspected. Endoscopy is indicated if oesophageal disease or gastric stasis is apparent clinically. The approach to intractable hiccups is outlined in Box 3.2.
Treatment
Primary therapy of any identified underlying cause is appropriate and, in most instances, will lead to rapid resolution of the hiccups. In refractory or idiopathic cases, there are no established guidelines regarding drug therapy for hiccups. A very extensive list of drugs has been described with variable success in hiccups. Notwithstanding, there are numerous reported failures in response to a number of agents and therapy is largely a matter of trial and error. Efficacy studies are largely single case reports and uncontrolled. There is only one randomised, controlled therapeutic trial in hiccups. This study found the GABAB receptor agonist, baclofen, to be effective in four patients. There is little objective evidence suggesting that one pharmacological agent is superior to another. Substantial numbers of favourable case reports support the efficacy of dopaminergic antagonists such as chlorpromazine, haloperidol and metoclopramide in intractable hiccups. Other drugs most frequently reported to be effective include anticonvulsants (phenytoin, sodium valproate and carbamazepine); benzodiazepines (clonazepam); calcium channel blockers (nifedipine and nimodipine), anaesthetic agents (ketamine and lignocaine), and amitriptyline (Box 3.3).
Suggested sequential approach to suppression of hiccups
Sore Mouth
Burning mouth syndrome
Aetiology
Deficiency states cause macroscopic mucosal alterations, but glossodynia can precede these mucosal changes. Glossitis is a characteristic feature of advanced deficiency of vitamins such as folate and B12 (Box 3.4).
Gastro-oesophageal reflux is thought in some cases to be associated with glossodynia, but there is little objective evidence to suggest that reflux causes it. Furthermore, the observation that reflux is extremely common and glossodynia relatively uncommon argues against a strong association. Anecdotal experience suggests potent antireflux therapy is ineffective in the treatment of glossodynia.
Diagnosis
The first step is to exclude an underlying treatable cause, although this will only be identified in the minority. History should include an assessment of the patient’s behaviour and life stress factors. A careful inspection of the mouth, tongue and teeth is important. A full physical examination may reveal features of systemic disease such as diabetes mellitus, thyroid disease, haematological or neurological diseases (Box 3.5). An otolaryngological examination is also important. A dental opinion should probably be sought in most cases, unless an underlying, correctable systemic disease is found.
Bad Breath
Pathogenesis
The majority of cases of halitosis arise from oral conditions (see Box 3.6). The odour is a result of sulfur-containing proteins and peptides being hydrolysed by gram-negative bacteria in the alkaline environment of the mouth. The resulting volatile sulfur-containing end-products include hydrogen sulfide and methyl mercaptan. Surprisingly, the usual compounds associated with putrefaction of biological tissues (ammonia, putrescine, indole, skatole and cadaverine) are not contributors to oral malodour. Under normal circumstances the odour of the oral cavity is not static and varies throughout the day and as a function of age, gender, hunger state and, perhaps, menstruation. It is affected by multiple factors, many of which are interdependent, including oral flora, salivary flow, pH, oral musculature and the presence of appropriate substrates. Conditions favouring production of putrid odours include low ambient oxygen concentration, a shift from gram-positive to gram-negative bacterial colonisation, reduced carbohydrates available as bacterial substrates, an alkaline oral pH and reduced salivary flow.
Box 3.6 Causes of halitosis
Oral/dental
Approach to the patient
The approach to the patient is detailed in Box 3.7. The first step is to determine whether the patient does actually have halitosis, by wafting the expired air towards the examiner’s nose with the palm of the hand. It is important to be aware, however, that halitosis can be intermittent or reduced by recent antibiotic use. Therefore, if it is not apparent to the clinician, corroborative evidence should be sought from family members or close contacts. If the problem cannot be substantiated, the patient may have disordered chemoreception or the problem may be psychogenic (halitophobic). Neurosis can lead to compulsive use of mints, mouthwashes and oral deodorants in the absence of objective malodour. In such cases, halitosis is not physically based and counselling or psychiatric help may be needed.
Box 3.7 Approach to halitosis
History
Examination
The next step is to determine whether the odour is perceived predominantly during exhalation via the mouth or via the nose. Ask the patient to breathe out each time through the mouth or the nose and count to 20; place your nose 10 cm away. A stronger odour from nasally expired air indicates a lesion or disease of the nose, nasopharynx, sinuses or respiratory tract. Common causes include local tumours, rhinitis, sinusitis, or nasal foreign body (Box 3.6). Bronchiectasis can cause halitosis. Other respiratory disorders, such as asthma requiring systemic or inhaled corticosteroids, can result in oropharyngeal candidiasis that causes halitosis. Conditions in which oral candidiasis is common include cancer, immune deficiency states, diabetes mellitus and xerostomia. Tongue odour can be tested by using a plastic spoon to scrape the back of the tongue. Check whether the dentures smell foul.
Key Points
Marinella M.A. Diagnosis and management of hiccups in the patient with advanced cancer. J Support Oncol. 2009;7:122-127.
Ramirez F.C., Graham D.Y. Treatment of intractable hiccup with baclofen: results of a double-blind randomized, controlled, cross-over study. Am J Gastroenterol. 1992;87:1789-1791.
Walker P., Watanabe S., Bruera E. Baclofen, a treatment for chronic hiccup. J Pain Symptom Manage. 1998;16:125-132.
Torgerson R.R. Burning mouth syndrome. Dermatol Ther. 2010;23:291-298.
Zakrzewska J.M., Forssell H., Glenny A.M. Interventions for the treatment of burning mouth syndrome. Cochrane Database Syst Rev. 2005;1:CD 002779.
Armstrong B.L., Sensat M.L., Stoltenberg J.L. Halitosis: a review of current literature. J Dent Hyg. 2010;84:65-74.
Porter S.R., Scully C. Oral malodour (halitosis). BMJ. 2006;333:632-635.
Quirynen M., Zhao H., van Steenberghe D. Review of the treatment strategies for oral malodour. Clin Oral Invest. 2002;6:1-10.
Roldan S., Herrera D., Saaz M. Biofilms and the tongue: therapeutical approaches for control of halitiosis. Clin Oral Invest. 2003;7:189-197.
VanderBroek A.M., Feerstra L., deBaat C. A review of the current literature on management of halitosis. Oral Dis. 2008;14:30-39.