Chapter 244 Herpes Simplex Virus
Clinical Manifestations
Acute Oropharyngeal Infections
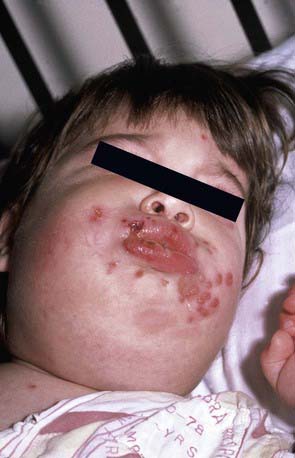
Herpes gingivostomatitis most often affects children 6 mo to 5 yr of age but is seen across the age spectrum. It is an extremely painful condition with sudden onset, pain in the mouth, drooling, refusal to eat or drink, and fever of up to 40.0-40.6°C. The gums become markedly swollen, and vesicles may develop throughout the oral cavity, including the gums, lips, tongue, palate, tonsils, pharynx, and perioral skin (Fig. 244-1). The vesicles may be more extensively distributed than typically seen with enteroviral herpangina. During the initial phase of the illness there may be tonsillar exudates suggestive of bacterial pharyngitis. The vesicles are generally present only a few days before progressing to form shallow indurated ulcers that may be covered with a yellow-gray membrane. Tender submandibular, submaxillary, and cervical lymphadenopathy is common. The breath may be foul as a result of overgrowth of anaerobic oral bacteria. Untreated, the illness resolves in 7-14 days, although the lymphadenopathy may persist for several weeks.
Cutaneous Infections
Herpes whitlow is a term generally applied to HSV infection of fingers or toes, although strictly speaking it refers to HSV infection of the paronychia. Among children, this condition is most commonly seen in infants and toddlers who suck the thumb or fingers and who are experiencing either a symptomatic or a subclinical oral HSV-1 infection (Fig. 244-2). An HSV-2 herpes whitlow occasionally develops in an adolescent as a result of exposure to infectious genital secretions. The onset of the infection is heralded by itching, pain, and erythema 2-7 days after exposure. The cuticle becomes erythematous and tender and may appear to contain pus, although if it is incised, little fluid is present. Incising the lesion is discouraged, as this maneuver typically prolongs recovery and increases the risk for secondary bacterial infection. Lesions and associated pain typically persist for about 10 days, followed by rapid improvement and complete recovery in 18-20 days. Regional lymphadenopathy is common, and lymphangitis and neuralgia may occur. Unlike other recurrent herpes infections, recurrent herpetic whitlows are often as painful as the primary infection but are generally shorter in duration.
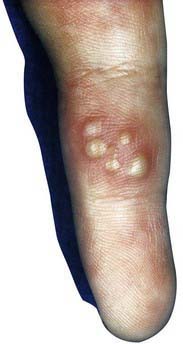
Figure 244-2 Herpes simplex infection of fingertip (whitlow).
(From Schachner LA, Hansen RC, editors: Pediatric dermatology, ed 3, Philadelphia, 1988, Mosby, p 1079.)
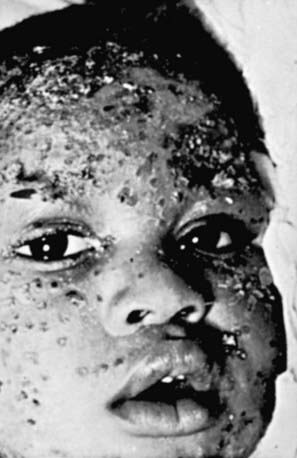
Cutaneous HSV infections can be severe or life threatening in patients with disorders of the skin such as eczema (eczema herpeticum), pemphigus, burns, and Darier disease, and following laser skin resurfacing. The lesions are frequently ulcerative and nonspecific in appearance, although typical vesicles may be seen in adjacent normal skin (Fig. 244-3). If untreated, these lesions can progress to disseminated infection and death. Recurrent infections are common but generally less severe than the initial infection.
Perinatal Infections
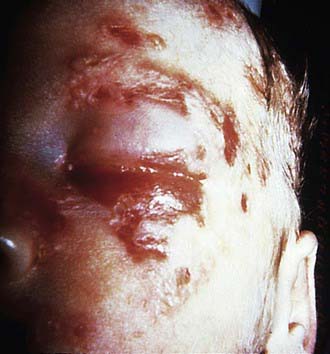
Infants with encephalitis typically present at 8-17 days of life with clinical findings suggestive of bacterial meningitis, including irritability, lethargy, poor feeding, poor tone, and seizures. Fever is relatively uncommon, and skin vesicles occur in only about 60% of cases (Fig. 244-4). If untreated, 50% of infants with HSV encephalitis die and most survivors have severe neurologic sequelae.
Treatment (Chapter 237)
Anderson BJ. Managing herpes gladiatorum outbreaks in competitive wrestling: the 2007 Minnesota experience. Curr Sports Med Rep. 2008;7:323-327.
Caviness AC, Demmler GJ, Almendarez Y, et al. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr. 2008;153:164-169.
Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
Elbers JM, Bitnum A, Richardson SE, et al. A 12-year prospective study of childhood herpes simplex encephalitis: is there a broader spectrum of disease? Pediatrics. 2007;119:e399-e407.
Handsfield HH, Waldo AB, Brown ZA, et al. Neonatal herpes should be a reportable disease. Sex Transm Dis. 2005;32:521-525.
O’Riordan DP, Golden C, Aucott SW. Herpes simplex virus infections in preterm infants. Pediatrics. 2006;118:e1612-e1620.
Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med.. 2009;360:1298-1309.
Xu F, Sternberg MR, Kottiri BJ, et al. Seroprevalence in the United States Trends in herpes simplex virus type 1 and type 2. JAMA. 2006;296:964-973.