Chapter 47 Hepatobiliary Cancer
Hepatobiliary malignant diseases include hepatocellular carcinoma (HCC), gallbladder cancer, extrahepatic cholangiocarcinoma (EHCC), and intrahepatic cholangiocarcinoma (IHCC). In 2010, the expected incidence in the United States was 33,880 cases, with 22,230 deaths.1 HCC, already the fifth most common malignant tumor worldwide,2 is increasing in incidence in the United States as a result of the hepatitis C epidemic.3 A similar increase has been reported in the incidence of IHCC for reasons that are not known.4 The primary therapy for all hepatobiliary tumors is resection, but only a minority of patients present with resectable disease. A number of therapies have been in use for unresectable patients, including systemic and regional chemotherapy (using standard cytotoxic agents and, more recently, sorafenib), chemoembolization, immunotherapy, and various ablative techniques. The role of these modalities has not been defined, and there is currently no accepted standard for the management of unresectable hepatic malignant tumors. Historically, RT has not played a significant role in the treatment of liver malignant diseases because of the low tolerance of the whole liver to radiation.5,6 With the advent of three-dimensional conformal treatment planning and intensity-modulated radiation therapy (IMRT), interest in the use of irradiation for malignant diseases of the liver has increased. Modern-day techniques allow delivery of higher doses to the target lesions than had been previously possible, while minimizing the dose to the noninvolved liver. At the same time, our understanding of the relationships between radiation dose and volume and the risk of radiation-induced liver disease has improved considerably. These developments have led to a body of evidence that now exists in support of the use of RT for unresectable hepatobiliary cancer.
Epidemiology And Etiology
Epidemiology
Cancer of the hepatobiliary system is uncommon in the United States, accounting for only about 2% of new cancer cases.7 Hepatobiliary cancers occur primarily in the elderly; the incidence increases with age. The most common hepatobiliary cancer is HCC, followed by gallbladder cancer, EHCC, and IHCC. Approximately one quarter of the cholangiocarcinomas occur at the bifurcation of the common hepatic duct and are known as hilar tumors or Klatskin tumors. Other cancers of the liver, such as carcinoid tumors, hepatoblastoma, angiosarcoma, and leiomyosarcoma, are extremely rare. HCC and IHCC are three and two times more common in men than women, respectively, whereas gallbladder cancer is about three times more common in women and EHCC occurs with approximately equal frequency.5
There is considerable variation in the incidence of HCC worldwide. In Asia, southeast Asia, and sub-Saharan Africa, HCC is between 15 and 100 times more common than in North America,8 although the incidence is increasing in the United States and Europe.9 Carcinoma of the gallbladder also has shown a substantial geographic variation, and the incidence in some areas, such as northeastern Europe, is more than 20 times higher than in the United States.10 Although EHCC has a fairly uniform incidence, IHCC is also more common in southeast Asia than in other parts of the world, presumably because of the higher incidence of liver fluke infestation in this region.
Considerable variation in the incidence of HCC is also observed in the United States. For example, HCC is five times more common in black men than white women.11 Gallbladder cancer is 15 times more common among Native American women in New Mexico than white women in the same state.10 The other major types of hepatobiliary tumors are of approximately equal distribution or show a slight preponderance among whites.
Etiology
Hepatocellular Carcinoma
In Korea and Taiwan, approximately 90% of patients with HCC have positivity for hepatitis B surface antigen (HBsAg), and prospective studies have found that hepatitis B virus carriers have a 200-fold increase in the relative risk for HCC.8 Indeed, 90% of patients with HCC who are also HBsAg positive have viral DNA integrated into the host genome.12 The greatest evidence of a causal relationship between hepatitis B infection and HCC development, however, is the observation that the incidence of childhood HCC in Taiwan has declined significantly since a nationwide vaccination program was instituted.13
The hepatitis C virus has also been implicated in the pathogenesis of HCC but with a lower incidence of coexistent cirrhosis and without evidence of host genome integration of the viral DNA.8 These findings have suggested that an alternate mechanism may be in play that does not involve either cirrhosis or host DNA alterations.
Cirrhosis without hepatitis infection probably accounts for more cases of HCC in the United States than hepatitis infections.8,14 The development of HCC has been observed in a number of diseases, all of which share the endpoint of cirrhosis.15
Aflatoxin B is a toxic metabolite of fungi that can grow in stored grain and peanuts and has been linked with HCC development in some areas of Africa and Asia.12 A reactive intermediate metabolite of aflatoxin B has been shown to selectively bind to guanine, which is excised and preferentially replaced with thymidine. This guanine-to-thymidine mutation is commonly found in the TP53 tumor suppressor gene in cases of HCC occurring in areas of high aflatoxin ingestion but is not found where aflatoxin levels are low.
Gallbladder Cancer
Epidemiologic studies have found that gallbladder cancer is more common in regions or among populations with a high frequency of cholelithiasis.10,16 Whether the increased risk is related to a direct irritating effect of gallstones or to the presence of carcinogens in bile acids is unknown, however.10,17 Additionally, the incidence rate of cancer in patients with calcified gallbladders has implied that any chronic inflammatory condition could lead to carcinogenesis.18
Cholangiocarcinoma
The development of cholangiocarcinoma has been epidemiologically linked to liver fluke infestation, hepatolithiasis, pyogenic cholangitis, congenital bile duct cysts, past exposure to thorium dioxide, a typhoid carrier state, and ulcerative colitis.8,19 Patients with primary sclerosing cholangitis also have an increased risk, with an incidence of cholangiocarcinoma of about 10%.20 It is possible that EHCCs share a common pathway of carcinogenesis with gallbladder cancer because the risk is lessened 10 years after cholecystectomy.21
Prevention And Early Detection
The identification of patient groups at high risk for HCC and the poor results of therapy have made prevention and early detection strategies attractive.22 The prevention of HCC by vaccination for hepatitis B has been supported by a review of the childhood HCC rates in Taiwan after introduction of a nationwide vaccination program.13 Approximately 85% or more of infants in Taiwan have received hepatitis B vaccination since 1986 compared with none before 1984. On review, the incidence of HCC among 6- to 14-year-olds has significantly declined from 0.7 to 0.36 per 100,000, with corresponding reductions in mortality rates as well. Because the incidence of HCC peaks among people 50 to 60 years of age, it can be anticipated that reductions in HCC incidence will increase as the immunized population ages.
The early detection of HCC in endemic areas of hepatitis B has received considerable attention. One screening tool is serum alpha-fetoprotein (AFP), which has been used in extensive population surveys in China, Taiwan, and South Africa and among native Alaskans.23 Unfortunately, even when screening was limited to people at high risk, the number of HCC cases detected was low. For example, a screening study in South Africa obtained AFP levels in more than 11,000 HBsAg-positive patients but found only 10 with HCC.23 Similarly, in China, of about 2 million people who were screened with AFP, only 300 cases were found. Liver ultrasonography has also been pursued as a screening tool. In a Chinese trial, almost 19,000 people were randomized between screening AFP and ultrasound twice yearly or observation. Mortality rates from HCC were significantly lower in the screened group (83/100,000 vs. 132/100,000).24 Although ultrasonography may be superior to AFP screening,23 it is more expensive22 and its utility is dependent on the expected incidence in the screened population. Still, screening with AFP and ultrasound every 6 to 12 months is recommended by many, including the National Comprehensive Cancer Network (NCCN) in its practice guidelines.25
Given the increased risk of cholangiocarcinoma in patients with primary sclerosing cholangitis and ulcerative colitis, the difficulty in obtaining a diagnosis of malignancy, and the lethality of the cancer when diagnosed, the finding that serum CA19-9 may be useful for screening and early diagnosis is attractive.26 Its sensitivity of 63% and specificity of 50% make it difficult to recommend this test for routine use, however.27
There are no known preventive measures for the biliary tract cancers. Even though cholelithiasis is a risk factor for gallbladder cancer10,16 and may also be related to extrahepatic bile duct cancers, there is no evidence to justify cholecystectomy as a preventive measure.10,21
Biologic Characteristics And Molecular Biology
Molecular techniques have led to significant advances in delineating the process of hepatocarcinogenesis. Frequently, fragments of hepatitis B viral DNA can be found within the genome of the HCC,8,12 leading to the hypothesis that viral integration activates oncogenes or interferes with tumor suppressor genes. In fact, the normal liver of patients with chronic hepatitis B infection will show viral DNA integration early in the course of the disease, suggesting that the HCC originated from clonal expansion of the affected hepatocytes.28 Against these arguments, however, is the finding that the sites of viral DNA integration are not consistent and do not occur near any known oncogenes or tumor suppressor genes. Nor does the viral DNA itself contain any known oncogenes. If, therefore, the integration of viral DNA is necessary for carcinogenesis, the mechanism may be caused by genomic instability from deletions and chromosomal rearrangement rather than specific oncogene activation or disruption of a tumor suppressor gene pathway.28
Hepatocarcinogenesis may not be the result of one particular molecular change but may be related to the ability of the liver to respond to damage by regeneration. The continuous liver damage seen with chronic hepatitis or cirrhosis leads to increased turnover of hepatocytes. During normal hepatocyte regeneration, both proto-oncogene activation and inactivation of suppressor genes occur. Chronic liver damage leads to a nearly continuous cellular reproduction, which may allow carcinogenic molecular changes to accumulate without repair.28 The observation of apparent stepwise progression of small HCCs from regenerating nodules of liver cirrhosis supports this hypothesis.29
Pathology And Pathways Of Spread
Hepatocellular Carcinoma
On gross examination, HCC can appear as expanding or spreading, depending on whether the margin is discrete or poorly defined, or as multifocal, with multiple tumors scattered throughout the liver without an obvious primary-to-secondary relationship.30 The microscopic appearance of HCC resembles that of normal liver tissue, both in its cytologic findings and in its characteristic platelike growth. Nuclear grading is typically judged using a four-grade system, but for prognostic purposes cells are either low grade (grade 1 or 2) or high grade (grade 3 or 4).31 Grade 1 tumors can be difficult to differentiate from adenomas or atypical hyperplastic nodules. Similarly, grade 4 tumors can resemble other anaplastic cancers and require special stains for further characterization.15
HCC may locally invade the portal vein, hepatic vein, or diaphragm. Approximately one-third of patients have regional disease at presentation, with metastases to the portahepatic and celiac axis lymph node chains.11 Intra-abdominal metastasis to the peritoneal lining or liver or extra-abdominal spread to the lungs and bone is found in about one third of patients.11 Metastasis to other sites, such as the brain or muscle, occurs infrequently.
Fibrolamellar carcinoma is a unique subtype of HCC that makes up about 5% of the cases in North America.31 It is associated with the longest survival times of any of the HCC subtypes and is typically found in young women. Grossly, fibrolamellar carcinoma is classified as an expanding, sclerosing tumor, characterized by septa of retracted collagenous structures radiating from a central region.30 On cytologic examination, the tumor cells are large and polygonal, with a granular cytoplasm resulting from numerous mitochondria. The abundant fibrous stroma is characteristic and is arranged in parallel lamellae around nests, cords, and sheets of tumor cells.15
Gallbladder Cancer
Most gallbladder cancers are adenocarcinomas.32 The gross appearance can vary from localized nodular growths to involvement of the entire organ.33 Histologically, both grade and vascular invasion are of prognostic value.32 Papillary adenocarcinoma is a variant histologic type that makes up about 6% of the total number of cases and has a much longer median survival time than the more common type. Mucinous carcinoma is another variant of adenocarcinoma, occurring in about 5% of cases and associated with perforation of the gallbladder.33
Direct liver invasion is frequent18 and may be related to the thin wall and single muscular layer of the gallbladder, the presence of Rokitansky-Aschoff sinuses (which themselves can penetrate the muscle layer in cases of chronic cholecystitis), and the fact that the perimuscular connective tissue of the gallbladder is directly contiguous with the interlobular connective tissue of the liver.32 Historically, approximately 40% to 50% of patients will have distant metastases at presentation, usually to the liver or peritoneum.18,32 Despite an increase in the number of cholecystectomies in recent years, driven mostly by the widespread adoption of laparoscopic techniques, the proportion of patients presenting with advanced disease has not declined.34,35 Lymphatic spread to the cystic, pericholedochal, hilar, and celiac lymph nodes is found in 45% of patients,18 but only 5% of patients will have positive regional lymph nodes with tumor confined to the gallbladder.32
Cholangiocarcinoma
Biliary cancer is typically divided according to the site of origin into intrahepatic tumors, extrahepatic tumors, or hilar (Klatskin) tumors. Grossly, biliary cancers are sclerotic, with a diffuse, firm gray-white annular thickening of the duct.36 Nearly all carcinomas of the biliary tree are mucin-producing adenocarcinomas with cell variation from cuboidal to columnar with prominent nucleoli. As with gallbladder cancer, the variant histologic type of papillary adenocarcinoma has a better prognosis.37
Cholangiocarcinoma typically spreads by direct extension along the biliary tree, although separate tumor nodules may represent either multifocal tumor development or metastatic deposits.38 Direct invasion of adjacent organs is more common for intrahepatic and hilar tumors than for extrahepatic tumors.11,37,39 Tumor involvement of the cystic, hilar, or celiac lymph nodes is found in 30% to 50% of patients.11,18,39 The incidence of distant metastases is about 30%, with a lower incidence of peritoneal disease than for gallbladder cancer.39
Clinical Manifestations, Patient Evaluation, And Staging
Symptoms and Signs
The typical presentation of a patient with hepatobiliary cancer depends on the site of origin. Patients with primary liver tumors, whether HCC or IHCC, frequently will complain of right upper abdominal or epigastric pain at presentation.8,39–41 The pain is usually dull but may be sharp and severe with end-stage disease. Rarely, patients with HCC will complain of a sudden, sharp, severe pain related to acute bleeding into the tumor or rupture of the tumor with intra-abdominal bleeding. The patient may also be aware of an abdominal mass or increased girth related to ascites.
Cirrhosis is present in approximately 70% of patients with HCC.14 Occasionally, patients with known cirrhosis will be evaluated because of an unexplained clinical deterioration. Jaundice is unusual with primary liver tumors and may be obstructive because of tumor compression of the biliary system or hepatocellular because of end-stage hepatic replacement with tumor.
In contrast to the primary liver tumors, patients with cholangiocarcinomas of the biliary hilum or distal biliary tree almost invariably present with jaundice.39,40 Abdominal pain and the nonspecific complaints of weight loss, anorexia, fatigue, fever, and night sweats are common in patients with either biliary or hepatic tumors. Patients with gallbladder cancer commonly complain of both abdominal pain and jaundice and may also have weight loss, anorexia, and fatigue but are usually undiagnosed before an attempted cholecystectomy.18,42,43
The most common physical finding in patients with primary hepatic tumors is hepatomegaly,40,41 which is frequently tender. The liver is often smooth but may be nodular as a result of tumor or cirrhosis. Occasionally, patients with HCC may have a hepatic bruit because of the highly vascular nature of the tumor. Ascites is a particularly ominous finding because it represents either hepatic dysfunction resulting from cirrhosis or tumor replacement, Budd-Chiari syndrome from malignant invasion of the hepatic veins, or peritoneal spread of cancer.
Laboratory Studies
The goals of the workup of patients with hepatobiliary malignant tumors are to define the extent of the tumor and to carefully assess liver function. Laboratory testing includes a complete blood count, routine biochemistry panel, and coagulation studies (Table 47-1). In addition, serum CA19-9 may have value as a tumor marker for cholangiocarcinoma.26
TABLE 47-1 Diagnostic Evaluation and Algorithm for Hepatobiliary Cancers
| General |
AFP, alpha-fetoprotein; anti-HCV, antibody to hepatitis C virus; CEA, carcinoembryonic antigen; CT, computed tomography; HBcAb, hepatitis B core antibody; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; MRI, magnetic resonance imaging; PT, prothrombin time; PTT, partial thromboplastin time.
Serum AFP is a widely used tumor marker for HCC; normal values are in the range of 20 µg/L or less, and levels greater than 400 µg/L (4000 µg/L in patients with hepatitis) are considered diagnostic of HCC. There is some evidence that AFP levels are prognostic for patients with tumors 5 cm or smaller as well as those with more than 50% involvement of the liver.44 The major difficulty in using AFP is that elevations to levels between 20 and 400 µg/L may represent either an exacerbation of hepatitis or the presence of a small, curable HCC.22,23 This observation has led to the recommendation that patients with known cirrhosis and/or hepatitis and a rise in AFP levels undergo retesting in 1 to 3 months, after an exacerbation of hepatitis would be expected to have improved. Another difficulty is the false-negative rate, which occurs in 10% to 15% of patients with HCC, presumably because the tumor is either too anaplastic or too well differentiated to produce the protein.22
Patients with HCC also frequently have a history of hepatitis. Although the hepatitis status does not appear to be prognostic,14 response rates to chemotherapy were higher and toxicity was lower in patients who were negative for hepatitis B or C in a trial of hepatic arterial chemotherapy.45 Approximately 5% of patients with HCC have a paraneoplastic syndrome,14 such as hypoglycemia, erythrocytosis, hypercalcemia, or hypercholesterolemia.41
Radiographic Studies
The primary imaging modalities currently being used for characterizing the extent of liver tumors include ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). Patients suspected of hepatic disease, whether IHCC or HCC, are typically first evaluated with ultrasonography. Although findings of focal liver lesions are often nonspecific, recent advances such as the use of Doppler ultrasound, contrast agents, and harmonic imaging have improved the usefulness of this area of examination.46,47 Still, ultrasound may be limited by patient characteristics such as obesity, intestinal distention, or scars, and it is very operator dependent. In evaluation for potential resection, MRI and CT are the dominant imaging modalities because of their ability to display hepatic segmental anatomy and the extent of hepatic disease and evaluate extrahepatic spread. The advent of spiral CT and, more recently, multislice spiral CT has improved hepatic imaging considerably. Now, with faster acquisition times, liver imaging can be accomplished during a single breath hold to improve spatial resolution. With thinner slices, multiplanar reformatting can be used to better assess resectability. State-of-the-art CT scanning involves acquisition of three contrast phases (arterial hepatic, portal venous, and equilibrium) that are very important for assessing lesion vascularity.46 For instance, HCC is hypervascular and typically displays intense enhancement during the arterial-hepatic phase with postcontrast washout, whereas cholangiocarcinoma may demonstrate delayed enhancement.
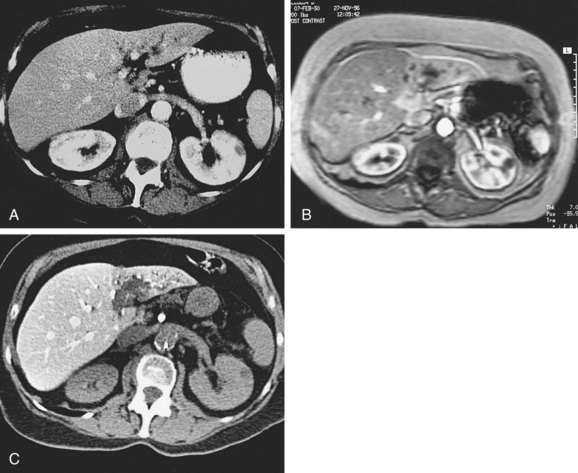
CT scanning can also be performed after administration of contrast via an angiographically placed superior mesenteric artery catheter (CT arterial portography) or a hepatic artery catheter (CT hepatic arteriography). With the former, the bolus of contrast passes through the intestinal vasculature and collects in the portal vein, providing excellent contrast between normal liver tissue and tumor tissue (Fig. 47-1). CT arterial portography and CT hepatic arteriography are considered the most sensitive methods of detecting small liver cancers,48,49 detecting lesions as small as 0.2 cm in diameter.50 However, a high false-positive rate has limited their usefulness.51
MRI plays an important role in detection and characterization of liver tumors. Modern MRI protocols usually include parenchymal imaging with one or more types of contrast agents, MR angiography, and MR cholangiopancreatography (MRCP). The superior contrast resolution and inherent capability of multiplanar evaluation have made MRI the examination of choice for characterization of lesions in cirrhotic patients.46,52
In patients with biliary obstruction, MRCP has limited the role of transhepatic or endoscopic cholangiopancreatography to instances when drainage and decompression of the biliary system are required.47 In addition to being noninvasive, the advantage of MRCP is its ability to define the extent of tumor not only within the intrahepatic and extrahepatic bile ducts but also to provide information on the status of the portal vein and lymph nodes.
The usual appearance of an EHCC is a stricture at the site of involvement with dilatation of the biliary tract proximal to the stricture (Fig. 47-2). The tumor itself may extend for some distance along the biliary tract. One area of particular difficulty is distinguishing bile duct cancer from primary sclerosing cholangitis, with the latter typically showing diffuse rather than focal biliary narrowing.20 Ultrasonography, CT, and MRI of the abdomen frequently show only intrahepatic biliary dilatation (with or without hepatic atrophy) but no identifiable mass.19
Although ultrasonographic examination is frequently obtained in patients suspected of having gallbladder disease, the study is rarely diagnostic of gallbladder cancer.42 Postoperative evaluation using CT scanning can be helpful in defining both residual disease and possible metastases.
Diagnosis
The NCCN guidelines recommend a biopsy for patients suspected of having HCC when the AFP level is 400 µg/L or less (≤4000 µg/L in patients with hepatitis). Fine-needle aspiration can also be used in patients with hepatic tumors to arrive at a diagnosis.15 Cytologic efforts have found an improved ability to confidently diagnose HCC on fine-needle aspiration by examining the nuclear-cytoplasmic ratio, trabecular pattern, and atypical naked hepatocytic nuclei.53
Establishing the diagnosis of cholangiocarcinoma can be difficult without exploration, presumably because of the large amount of sclerosis present. Performing an incisional biopsy or curettage at the time of surgery should be avoided, however, in view of an increased risk of peritoneal failure or wound implant with these procedures.54 When cytologic study only is used, cancer can be confirmed in 50% to 90% of cases.19,55
Staging
The American Joint Committee on Cancer staging manual, seventh edition, has defined separate TNM staging systems for hepatic tumors, IHCCs, gallbladder tumors, and EHCCs, divided into perihilar and distal bile duct lesions56 (Table 47-2). The staging for both gallbladder cancers and EHCC tumors is primarily surgical, reflecting the role of resection.
| Primary Tumor: Liver | |
| T0 | No evidence of primary tumor |
| T1 | Solitary tumor without vascular invasion |
| T2 | Solitary tumor with vascular invasion, or multiple tumors, none more than 5 cm |
| T3a | Multiple tumors more than 5 cm |
| T3b | Single or multiple tumors of any size involving a major branch of the portal or hepatic vein(s) |
| T4 | Tumor(s) with direct invasion of adjacent organs other than the gallbladder or with perforation of the visceral peritoneum |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| Primary Tumor: Intrahepatic Bile Duct | |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ (intraductal tumor) |
| T1 | Solitary tumor without vascular invasion |
| T2a | Solitary tumor with vascular invasion |
| T2b | Multiple tumors, with or without vascular invasion |
| T3 | Tumor perforating the visceral peritoneum or involving the local extrahepatic structures by direct invasion |
| T4 | Tumor with periductal invasion |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis present |
| Primary Tumor: Gallbladder | |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades lamina propria or muscle layer |
| T1a | Tumor invades lamina propria |
| T1b | Tumor invades muscle layer |
| T2 | Tumor invades perimuscular connective tissue; no extension beyond serosa or into liver |
| T3 | Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts |
| T4 | Tumor invades main portal vein or hepatic artery or invades multiple extrahepatic organs or structures |
| N0 | No regional lymph node metastasis |
| N1 | Metastases to nodes along the cystic duct, common bile duct, hepatic artery, and/or portal vein |
| N2 | Metastases to periaortic, pericaval, superior mesentery artery, and/or celiac artery lymph nodes |
| Primary Tumor: Perihilar Bile Ducts/Proximal Extrahepatic Cholangiocarcinoma | |
| Tis | Carcinoma in situ |
| T1 | Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue |
| T2a | Tumor invades beyond the wall of the bile duct to surrounding adipose tissue |
| T2b | Tumor invades adjacent hepatic parenchyma |
| T3 | Tumor invades unilateral branches of the portal vein or hepatic artery |
| T4 | Tumor invades main portal vein or its branches bilaterally, common hepatic artery, or the second-order biliary radicals bilaterally, or unilateral second-order biliary radicals with contralateral portal vein or hepatic artery involvement |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis (including nodes along the cystic duct, common bile duct, hepatic artery, and portal vein) |
| N2 | Metastasis to periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes |
| Primary Tumor: Extrahepatic Cholangiocarcinoma, Distal Bile Duct | |
| Tis | Carcinoma in situ |
| T1 | Tumor confined to the bile duct histologically |
| T2 | Tumor invades beyond the wall of the bile duct |
| T3 | Tumor invades the gallbladder, pancreas, duodenum, or other adjacent organs without involvement of the celiac axis or the superior mesenteric artery |
| T4 | Tumor involves the celiac axis or the superior mesenteric artery |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| Distant Metastasis | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
From Edge SB, Byrd DR, Compton C, et al, editors: AJCC Cancer Staging Manual, ed 7, New York, 2010, Springer.
Primary Therapy
Hepatocellular Carcinoma
Potentially curative treatments include partial hepatectomy, total hepatectomy with a liver transplant, and nonsurgical ablative therapies. Because there are no controlled trials comparing these treatments, recommendations are based on nonrandomized cohort studies rather than on firm evidence. Resection and transplantation appear to result in the best outcomes in well-selected candidates (5-year survival rate of 60% to 70%).* Percutaneous treatments provide good outcomes (5-year survival rate of 40% to 50%),67,68–70 but they are generally inferior to those achieved with surgery. Exceptions to the latter might be patients with carcinoma in situ or single small tumors.
Resection
Hepatic resection is the treatment of choice for HCC in noncirrhotic patients in whom major resections can be accomplished with low rates of complications.71 In contrast, among patients who have cirrhosis, strict selection criteria are required to avoid liver failure. State-of-the-art surgery is associated with operative mortality rates of 1% to 3% and 5-year survival rates of approximately 50%.58 Approximately 70% of patients suffer tumor relapse.71 Positive resection margins, microvascular invasion, poorly differentiated histologic characteristics, and satellite lesions predict for relapse.61,72
Adjuvant therapies have been attempted, but their efficacy has not yet been established. A 2002 review73 of randomized controlled trials identified 13 trials with relapse or survival endpoints reported at 3 years or longer. Three studies involved predominantly systemic adjuvant chemotherapy; four involved predominantly hepatic artery-based chemotherapy or embolization; and six used other therapeutic modalities, including immunotherapy, differentiation agents, and hepatic artery infusion of Lipiodol labeled with iodine-131 (131I). Improved disease-free survival (DFS) or overall survival (OS) rates were noted in six trials, five of which involved modalities other than systemic or hepatic artery chemotherapy or embolization. The authors concluded that systemic and hepatic artery chemotherapy or chemoembolization has not been shown to improve OS or DFS rates after resection of HCC. The other adjuvant modalities appeared more promising. A 2009 Cochrane systematic review of 12 randomized studies concluded that there is limited evidence that adjuvant Lipiodol therapy or immunotherapy may potentially improve DFS rates after resection of HCC.74 A separate meta-analysis of adjuvant interferon found a risk of mortality of 0.65 and of recurrence of 0.86 compared with curative local therapy alone in patients with viral hepatitis.75
Liver Transplantation
The advantage of a total hepatectomy with orthotopic liver transplantation for patients with cirrhosis is that the entire organ is removed, therefore taking all gross and microscopic tumor and premalignant disease. In addition, transplantation simultaneously addresses the underlying cirrhosis. The widespread use of liver transplantation, however, has been limited by the shortage of donor organs, the high cost of the procedure, and the high perioperative mortality rates, initially reported in the range of 10% to 25%.8 The use of stricter selection criteria (one lesion <5 cm in diameter or up to three lesions <3 cm in diameter) has improved outcomes dramatically. Today, 5-year OS of 70% and relapse rates lower than 15% can be expected.57,60,61,62,66 Most centers advocate the use of chemotherapy, chemoembolization, percutaneous ablation, or a combination of these treatments while patients are on the waiting list. The value of these therapies is unknown.
Percutaneous Ablation
Only 30% of patients present with resectable HCC. For those with small unresectable lesions, percutaneous ablation is the most common option and may offer a chance for long-term survival.68–7076 Ablation can be accomplished by the use of chemicals such as alcohol or acetic acid or by techniques using extreme temperatures, such as radiofrequency ablation, microwave, or laser coagulation or cryoablation. Radiofrequency ablation is the most common method used in the United States. A needle electrode is placed in the tumor, and a high-frequency alternating current heats the surrounding tissue to produce necrosis. The best results are produced with tumors less than 4 cm.77 A recent prospective randomized trial demonstrated reduced rates of local progression (2% vs. 11%) and overall recurrence (70% vs. 85%) and improved OS (74% vs. 57%) with radiofrequency ablation as compared with percutaneous ethanol injection.78 In a review of 3670 patients treated by radiofrequency ablation, the mortality rate was 0.5% and the complication rate was 8.9%.79 Subcapsular location and poorly differentiated histologic characteristics have been implicated in needle-track seeding. Cryoablation has fallen out of favor due to a high complication rate and recent data indicating inferior outcomes. Microwave and laser coagulation are still experimental techniques.76
Intrahepatic Cholangiocarcinoma
Complete resection is the most effective treatment and the only potentially curative therapy for IHCC. Tumors are often large at presentation, and even with major hepatic resections (right or left hepatectomy or extended hepatectomy), few achieve negative margins. The median survival time and 5-year survival rate after resection are 12 to 28 months and 22% to 36%, respectively.80–86 Recognized predictors of poor survival are large tumor size, multiple tumors, vascular involvement, and positive resection margins. The negative prognostic value of lymph node involvement and macroscopic subtype (infiltrating periductal cancer) is less well documented, and it is not known if lymph node dissection improves survival rates.81,87,88,89,90
Gallbladder Carcinoma
Carcinoma of the gallbladder is discovered during or after cholecystectomy for benign disease in 1% to 2% of cases. In this setting, information about nodal involvement is usually not available and management depends on the depth of tumor invasion. If the tumor has invaded the muscularis propria (T1b and beyond), a radical cholecystectomy is recommended. Reexploration may reveal residual tumor in 40% to 76% of cases91–93 and regional lymph node metastases in 40% to 80% of cases; 20% have N2-level lymph node metastases.94 Retrospective reports comparing survival after simple cholecystectomy and radical re-resection have suggested major benefit from the latter for tumors invading the muscularis propria or deeper tissues.92,93,95–97
The surgical management of gallbladder carcinoma diagnosed preoperatively depends on the extent of the disease. Patients with organ-confined disease should have a radical cholecystectomy. The management of T3 and T4 disease has been more controversial, but over the past decade, it has become evident that cures are possible with radical surgery.92,93,98–102 The role of more extensive surgery, including pancreaticoduodenectomy, right colectomy, and nephrectomy, has not been established. Para-aortic lymph node metastasis is present in 19% to 25% of patients with locally advanced disease,103 but para-aortic lymphadenectomy is not warranted.104
Adjuvant Therapy
Local failure after cholecystectomy for gallbladder cancer is common. In a review of the literature, 86% of patients dying within 5 years after surgery had a local recurrence.105 Despite the publication of this observation more than 20 years ago, data on the role of adjuvant treatment with RT or chemoradiation are very limited.106,107,108
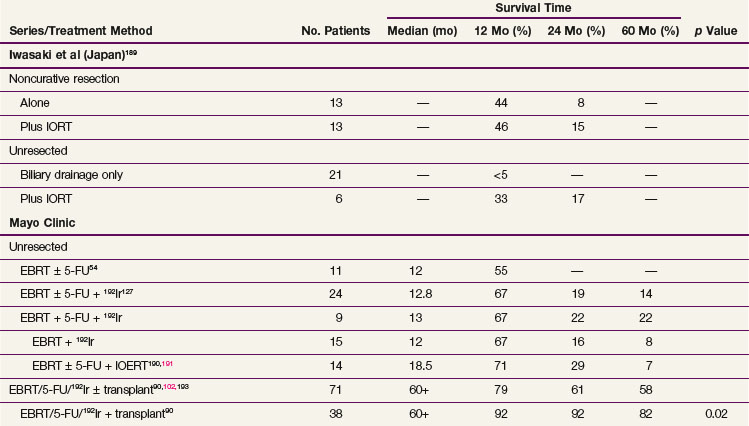
Kresl and colleagues108 reviewed the Mayo Clinic experience with adjuvant EBRT plus concurrent bolus 5-FU. A total of 21 patients (12 with R0 resection, 5 with R1 resection, and 4 with R2 resection) received a median of 54 Gy to the tumor bed and regional lymphatics. For the whole cohort, 5-year OS and local control were 33% and 73%, respectively. These results appeared to be superior to those of historical controls.
Gold and associates109 updated the Mayo Clinic experience with adjuvant chemoradiation in a review of 73 consecutive patients who underwent curative-intent surgery for gallbladder carcinoma at the Mayo Clinic109; 25 of 73 had adjuvant postoperative chemoradiation (median dose of 50.4 Gy in 28 fractions; 5-FU–based concurrent chemotherapy). The median OS times for patients receiving adjuvant chemoradiation after surgery versus surgery alone did not significantly differ and were 4.8 years and 4.2 years, respectively (logrank p value = .56). Patients receiving adjuvant therapy, however, had disease of a significantly higher stage, with 80% of the patients in the adjuvant group having stage II disease compared with only 21% in the surgery alone group (p <.0001). In the multivariate Cox model, increasing T and N classification as well as histologic type other than adenocarcinoma were significant predictors for worse OS rates. Additionally, adjuvant chemoradiation became statistically significant for improved OS rates after adjusting for these multivariate prognostic factors (hazard ratio [HR] for death, 0.3; 95% CI, 0.13 to 0.69; p = .004).
Extrahepatic Cholangiocarcinoma
Tumors of the hilar area or common bile duct are typically treated with resection and reconstruction using a Roux-en-Y hepaticojejunostomy.39 Tumors of the hilar area are resectable in approximately 14% to 40% of cases109,110,111; reported 5-year OS in resected patients range from 10% to 40%39,112–119 and are substantially better after R0 resection. There is a strong association between performance of an en bloc partial hepatectomy and the rate of negative resection margins.120 Tumors of the distal biliary tract are treated by pancreaticoduodenectomy, which can be performed in up to 90% of patients.39 Survival rates are somewhat higher; 5-year OS ranges from 30% to 40%, and median survival is approximately 22 months.121–124
Adjuvant Therapy
Positive margins after resection are common for tumors of the hilar region, occurring in up to 85% of patients in one report,113 and are a poor prognostic factor, with a 5-year OS of 9% versus up to 40% for those with negative margins.39,122 Positive margins are less common in distal biliary tumors, probably because of the more radical resection possible in this area. Lymph node involvement is found in about 50% of resected patients.39 The rate of local recurrence after complete resection ranges from 25%54 to 40%.125
The poor prognosis associated with pathologic invasion to the perimuscular connective tissue, hepatic invasion, and positive regional lymph nodes has been recognized for some time for both adenocarcinoma of the gallbladder and cholangiocarcinoma.39,42,122 Only recently, however, has attention been directed to potentially important differences in biologic behavior between the two entities.126 In a detailed study of initial disease relapse in 156 patients, Jarnagin and colleagues126 found that cholangiocarcinomas had a much greater tendency to fail locally. The proportion of patients with a locoregional failure as the first site of failure was 59% and 15% for cholangiocarcinoma and gallbladder cancer, respectively. Multivariate analysis confirmed the independent predictive value of positive regional lymph node involvement, moderate to poor tumor differentiation, and a positive resection margin for relapse at any site; a diagnosis of cholangiocarcinoma was the only independent predictor of isolated locoregional recurrence.
The evaluation of adjuvant RT or chemoradiotherapy for cholangiocarcinoma has been very limited.* Adjuvant RT was associated with a statistically significant survival benefit in a retrospective European Organization for Research and Treatment of Cancer (EORTC) report of 55 resected patients in which 52 had positive pathologic margins.55,125 Postoperative RT was given in 38 patients, and 17 were treated with surgery alone. Patients who received RT had a median survival time of 19 months versus 8 months for surgery alone. The survival rate at 2 and 3 years was 42% versus 18% and 31% versus 10%, respectively, favoring irradiated patients (p = .0005). In a prospective, underpowered, nonrandomized trial of adjuvant RT alone after resection at the Johns Hopkins Hospital, 14 patients who elected to receive RT had no difference in survival duration or quality than 17 patients who elected observation.129 The patterns of failure were not noted. A retrospective review of 91 patients with hilar cholangiocarcinoma resected at the University of Amsterdam suggests that postoperative RT may be beneficial. Fourteen percent of patients had R0 resections128 and received no adjuvant therapy. The median survival time was significantly longer in patients who received postoperative RT than in patients who had surgery alone (24 months vs. 8 months; p < .01). Brachytherapy did not contribute to survival rates and was associated with a significantly higher complication rate. It is possible that the benefit suggested in this series is limited to patients who had a noncurative resection, and, indeed, the survival rate of patients who received postoperative RT in this series is comparable to that of patients in other series who had an R0 resection and received no adjuvant therapy.39,82,117–119 Additionally, selection bias is a concern because patients with a poor performance status would not likely receive adjuvant therapy.
In general, approximately one third of resected patients will be left with gross residual disease.39 With the addition of RT, the median survival time in this setting is about 13 months.130
Liver Transplant Alone or Plus Neoadjuvant Chemoradiation or Brachytherapy
Unfortunately, many patients present with disease that cannot be removed with standard resection because of local extension or associated advanced primary sclerosing cholangitis with severe fibrosis and cirrhosis. Patients in the latter category often have multifocal cholangiocarcinoma and are at an exceptionally high risk for developing additional tumors following standard resection. For these reasons, orthotopic liver transplantation has been proposed as an alternative to standard resection. Early experience with liver transplantation alone for cholangiocarcinoma has been disappointing, with 5-year OS of 2% to 23%.88
More recently, Heimbach and colleagues89 and Rea and associates90 reported the Mayo Clinic experience with transplantation for small (<3 cm) perihilar cholangiocarcinomas. The regimen consisted of neoadjuvant EBRT (45 Gy in 1.5-Gy fractions twice daily) with concurrent 5-FU followed by an intraluminal brachytherapy boost (20 to 30 Gy). Protracted venous infusion (PVI) of 5-FU was initiated with brachytherapy and continued until the time of transplantation. PVI 5-FU has largely been replaced by capecitabine. After completion of neoadjuvant treatment, patients underwent exploratory laparotomy with biopsy of perihilar lymph nodes. Only patients with disease confined to the liver and uninvolved lymph nodes remained eligible for the transplant. For all patients intended for transplantation, 3- and 5-year OS were 61% and 58%. For the 38 patients who underwent transplantation, 3- and 5-year OS were 92% and 82%. In the same period, 26 patients underwent standard resection rather than transplantation, with 3- and 5-year OS of 48% and 21% (p = .02 favoring neoadjuvant therapy followed by orthotopic liver transplantation).90 Although promising, these results require further confirmation.
Locally Advanced Disease And Palliation
Hepatocellular Carcinoma
The prognosis for patients with unresectable primary liver tumors is poor. Death usually is due to hepatic dysfunction, gastrointestinal hemorrhage, and spontaneous bacterial peritonitis.131 The high incidence of hepatic dysfunction, whether resulting from the tumor or the cirrhosis, has greatly limited the potential role of palliative liver RT, even though patients usually complain of pain.
A number of prognostic factors have been identified for patients with unresectable HCC, including albumin levels, AFP levels, portal vein obstruction, Child’s class, the presence of serologic markers for hepatitis B or C virus, and the size of the tumor or presence of multiple tumors.45,68,132,133 Given the effects of these features, selection bias inherent in nonrandomized trials makes interpretation of the literature difficult.
Systemic Treatment
Sorafinib, an oral multikinase inhibitor, recently has been shown to improve OS rates in patients with advanced HCC. In the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP trial), 602 patients from the Americas and Australasia were randomized to 400 mg of sorafenib twice daily or placebo.134 Patients randomized to sorafenib had an improved median overall survival time of 10.7 months versus 7.9 months and an improved time to radiologic progression of 5.5 months versus 2.8 months (p <.001 for both). Side effects were mild and included diarrhea, hand-foot skin reaction, and weight loss. Other medical treatments for unresectable HCC have been generally ineffective.135 A recent review of randomized controlled trials found no evidence for benefit from systemic chemotherapy.136 Similarly disappointing were the results of a recent large randomized controlled trial137 showing no benefit of tamoxifen over placebo.
Because hepatic neoplasms derive 80% of their blood supply from the hepatic artery,138 higher concentrations of anticancer drugs can be delivered to the tumor by hepatic artery infusion than by the systemic intravenous route. Indeed, higher response rates and longer median survival rates have been reported with regional chemotherapy in case series.139 However, although the only randomized controlled trial reported to date140 found a higher response rate in the hepatic arterial infusion group, there was no significant difference in survival rates.
Transarterial embolization has been used for large unresectable tumors. Because most of the blood supply to HCCs is derived from the hepatic artery and normal parenchyma from the portal vein, embolization or chemoembolization (TACE) can target disease with some specificity, without causing liver necrosis. Delivery of the embolization material (typically, gelatin sponge, polyvinyl alcohol, or starch microspheres) with or without chemotherapy is accomplished by injection into the right or left hepatic artery or a first-, second-, or third-order branch, depending on the disease extent. Two randomized trials have demonstrated improved survival rates using TACE, compared with symptomatic therapy alone and blind embolization.141,142 TACE is often applied multiple times until the area no longer enhances on imaging after administration of radiologic contrast; unfortunately, no reliable data exist that could suggest the optimal number of treatments needed. Common transient complications from TACE include abdominal pain, nausea, fever, fatigue, and elevation of liver enzyme levels. Rare complications include exacerbation of liver failure, liver abscess, gastrointestinal bleeding, and bile duct injury.
Intrahepatic Cholangiocarcinoma
Few data are available on treatment of unresectable IHCC. Survival rates with or without treatment are poor; the median survival time is approximately 7 months, with no 5-year survivors.39,40 Studies including radiolabeled anti-carcinoembryonic antigen antibodies combined with whole-liver RT and chemotherapy143 or yttrium-90 (90Y) radioembolization144 have been reported, but there is no standard approach to treatment. In an Australian study of 25 patients treated with 90Y radioembolization, median survival was 9.3 months. About 24% of patients had a partial response, 48% had stable disease, and 20% had progressive disease. Side effects were fatigue and transient abdominal pain, with rare grade 3 bilirubin, albumin, or alkaline phosphatase abnormalities.144 In another series of 24 patients from Northwestern University, 27% of patients had a partial response, 68% had stable disease, and 5% had progressive disease. Median survival was 15 months.145
Conformal Irradiation For Intrahepatic Tumors
Developments in three-dimensional RT treatment planning have expanded the role of EBRT for patients with unresectable primary hepatobiliary malignant disease. In a series of prospective studies at the University of Michigan, doses well above the whole-liver tolerance dose have been delivered safely to focal lesions.146–149 This was enabled by the development of a normal tissue complication probability (NTCP) model that quantitatively described the relationship between dose and volumes irradiated and the probability of developing radiation-induced liver disease150 and by the use of conformal techniques. The radiation dose was individualized based on the volume of normal liver that could be spared without exceeding a 10% to 15% risk of radiation-induced liver disease. The prescribed dose ranged from 40 Gy to 90 Gy (median, 60.75 Gy). The total irradiation dose was the most important predictor of survival in a multivariate analysis, and patients who received doses of 75 Gy or more had a significantly higher OS (23.9 months) than those receiving lower doses (14.9 months).
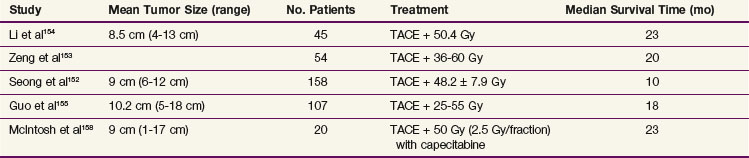
The use of lower doses of radiation with TACE in unresectable HCC has been reported, mostly by Asian investigators151–156; some of the studies are summarized in Table 47-3. A recent systematic review of 17 trials involving almost 1500 patients157 found that patients treated with TACE and RT had improved survival rates compared with patients treated with TACE alone, with an odds ratio of 2.23. Because only five of these trials were randomized, selection bias could have affected these results, but they are promising. Recently, chemotherapy, including capecitabine, has been added to RT in this setting, with encouraging results.158
TABLE 47-3 Results of Radiotherapy with Transcatheter Arterial Chemoembolization (TACE) in Unresectable Hepatocellular Carcinoma
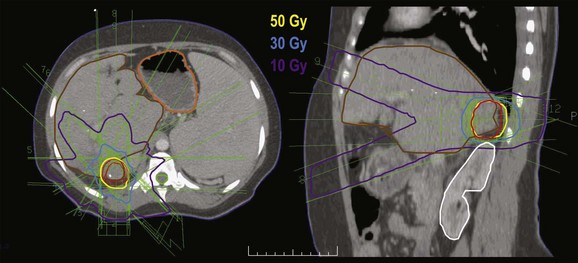
Stereotactic body radiotherapy (SBRT) is also being investigated, both with and without TACE.159–162 The dose per fraction and the total dose range between 6 and 15 Gy and 30 and 50 Gy, respectively (Fig. 47-3). In contrast to patients with metastatic disease to the liver, who typically have normal liver function and tolerate SBRT well, patients with HCC often have liver dysfunction, and radiation tolerance is not established. Considerable variability in patient selection and treatment details, as well as the nonprospective nature of these analyses, makes the interpretation of these results difficult. Nevertheless, similar to the University of Michigan experience, a common finding was that the RT dose was a major determinant of survival rates.
Gallbladder Cancer
Patients with unresectable gallbladder cancer have a median survival of approximately 5 months and a 2-year OS of less than 5%.32,42 A large number of agents, including fluorouracil, mitomycin, methotrexate, etoposide, doxorubicin, and cisplatin, have been tested as single or combination therapies with little effect.163–172 Short-lived partial responses lasting from weeks to months have been observed in approximately 10% to 20% of cases. Although the combinations of cisplatin, epirubicin, and 5-FU (CEF) and gemcitabine and cisplatin are associated with relatively high response rates of 32% to 40%163,173 and 37% to 64%,174,175 respectively, the impact of these regimens, or any chemotherapy, on survival is not known. Similarly, the efficacy of palliative RT for unresectable gallbladder cancer is unknown.
Extrahepatic Cholangiocarcinoma
Unresectable EHCC is typically located in the hilum; distal tumors are usually resectable. Most patients die from hepatic dysfunction resulting from biliary obstruction or cholangitis, or both.111 The median survival time of patients treated palliatively is approximately 6 months, with 3-year survival rates of less than 10%.37,39,109 Palliation can be performed either with a biliary enteric bypass109 or nonsurgically, using transhepatic percutaneous drainage catheters or endoscopically placed indwelling biliary stents.176 Cholangitis is frequent after both of these procedures, occurring in 26% to 47% of patients.176 Complications related to the catheters are also common, and most patients will require at least two exchanges, usually every 3 to 6 months.125 Septic deaths are reported in most series in which indwelling catheters or stents are left in place. Recurrent obstruction develops in 12% to 50% of patients, which can result from progressive tumor growth or biliary sludge.176
Chemotherapy for unresectable cholangiocarcinoma is largely ineffective. An extensive review of 65 clinical trials of nonsurgical therapies failed to document a survival benefit of any such modality in patients with unresectable disease or in the adjuvant setting.177
External Beam Irradiation with or without Chemotherapy or Brachytherapy
The median survival of patients treated with EBRT to a total dose of 45 to 50 Gy, alone or with chemotherapy, has been reported to be 9 to 12 months. Long-term survival has been infrequent.55,125 Both intraoperative irradiation (IORT) with electrons or high-dose-rate brachytherapy, and transcatheter brachytherapy have been used alone or in combination with EBRT to focally irradiate the bile duct to higher total doses of RT. A summary of published reports is provided in Table 47-4. There is a considerable body of published experience with brachytherapy as a component of treatment; median survival rates range from 10 months to 24 months and 3-year OS from 10% to 30%. In all reports that assess control, local failure is still common despite the high total dose of RT possible by including brachytherapy.125 This finding may be related to the difficulty in fully covering unresectable biliary tumors, which are often larger than 2 cm.129 The combination of EBRT, brachytherapy, and 5-FU shows promise. In a Mayo Clinic analysis of 24 patients with proximal extrahepatic bile duct carcinoma treated with EBRT and brachytherapy, 9 patients received concurrent 5-FU. Three patients survived for over 5 years, and two of them had received 5-FU.127 The intriguing Mayo Clinic results with liver transplantation preceded by neoadjuvant chemoradiation/brachytherapy were previously noted.90
Irradiation Techniques
Dose-Limiting Organs
The major dose-limiting organ for any course of irradiation to the hepatobiliary system is the liver. Radiation-induced liver disease typically occurs 4 to 8 weeks after the completion of RT and clinically resembles veno-occlusive disease.178 Patients complain of fatigue and may have vague right upper quadrant discomfort. They may have signs and symptoms of ascites, with rapid weight gain and increased girth. Laboratory studies show a large increase in alkaline phosphatase levels to 3 to 10 times normal and moderate elevations of transaminase levels but little to no increase in bilirubin or lactate dehydrogenase (LDH) levels at first presentation. Evaluation includes an abdominal CT scan and paracentesis of the ascitic fluid to rule out recurrent disease. Of note, differentiation on CT scan of recurrent disease versus radiation change within the liver may be difficult. Although the use of axial beam arrangements shows typical straight-line borders between the liver and areas of radiation change, nonaxial beam arrangements can lead to poorly defined borders and can be confused with infiltrative recurrent disease.179 Radiation-induced liver disease can be treated conservatively using diuretics and steroids, although some have suggested adding anticoagulation.
The risk for radiation-induced liver disease is highly dependent on the volume of liver irradiated.150,179,180 Irradiation of the whole liver to a total dose of 30 Gy in 2-Gy fractions or less has little risk of a complication. The risk rises greatly above a dose of 33 Gy, and it has been estimated that at 42 Gy there is an approximately 50% risk for symptoms.150,180 Partial liver volumes, however, can be irradiated to very high doses without clinically relevant toxicity. For example, trials from the University of Michigan using three-dimensional treatment planning have shown that approximately one-third of the normal liver can tolerate up to 72.6 Gy.150,180 In fact, sufficient data have accumulated in these trials to allow estimation of an individual’s risk for complication based on the distribution of the radiation dose within the liver.150 Although the use of concurrent hepatic arterial fluorodeoxyuridine was not found to increase the risk of radiation-induced liver disease, case reports suggest that previous alkylator therapy increases the chances of such disease.178
The stomach and duodenum are of concern when the left lobe of the liver or the extrahepatic biliary tract is treated to high doses. Of 128 University of Michigan patients with primary hepatobiliary malignant tumors treated in a phase II trial, with 40 to 90 Gy (1.5 Gy per fraction, twice daily), 7% developed upper gastrointestinal bleeding. In that trial, the maximum dose to the stomach or duodenum was not allowed to exceed 68 Gy. Although some authors suggest that a dose of 60 Gy to one third of the stomach carries a 5% risk of ulceration or perforation,181 the Mayo Clinic data for biliary tract cancer suggest that a dose of 55 Gy or less to the stomach or duodenum carries a 5% to 10% risk of severe gastrointestinal complications54; with doses of more than 55 Gy, the risk of severe gastrointestinal complications in the stomach or duodenum increased to 30% to 40%.
Irradiation Technique
Liver
Palliative liver RT may be indicated for both locally advanced hepatic tumors and metastatic disease. The appropriate dose and schedule for palliation were examined in a prospective, nonrandomized trial of more than 100 patients with symptomatic liver metastases.182 Six different dose and fractionation options ranging from 21 Gy in 7 fractions to 25.6 Gy in 16 fractions were tested. Palliation of pain was achieved in 55% of patients, with no difference among the treatment regimens. Although radiation-induced liver disease was not observed, the short median survival time of these patients and the problem of distinguishing this complication from progressive disease make interpretation of this finding difficult. Field arrangement may include opposed anterior-posterior or tangential approaches.
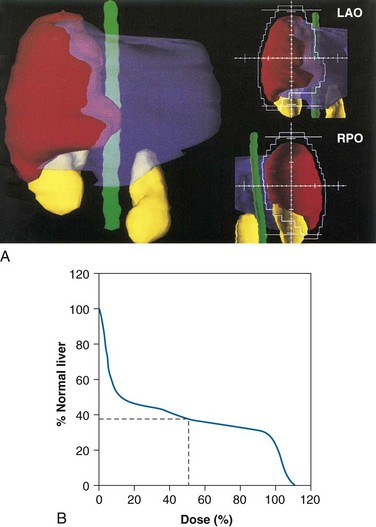
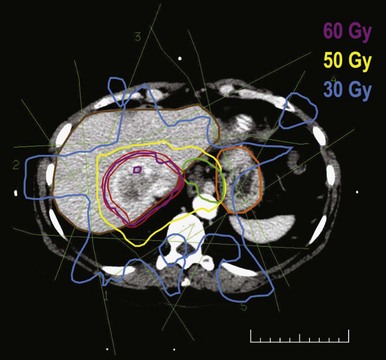
Irradiation of locally unresectable hepatic tumors to doses above the whole-liver tolerance requires three-dimensional treatment planning (Figs. 47-4 and 47-5). The gross tumor volume is typically defined as radiographically abnormal areas seen on CT or MRI. The clinical target volume is defined as the gross tumor volume plus 1 cm based on surgical reports that at least a 1-cm resection margin is necessary for a successful partial hepatectomy.12 The planning target volume includes the clinical target volume plus 0.5 cm for daily patient setup variation and between 0.5 and 2.5 cm (determined under fluoroscopy or four-dimensional CT scanning) in the cranial-caudal dimension to account for liver motion from respiration. The normal liver is defined as the gross tumor volume subtracted from the total liver volume. Using three-dimensional treatment planning, it has been possible to safely irradiate two thirds of the normal liver to 48 to 52.8 Gy and one-third of the liver to 66 to 72.6 Gy (fraction size of 1.5 to 1.65 Gy twice daily with at least 4 hours of separation).148,150,180
Gallbladder and Extrahepatic Cholangiocarcinoma
In the case of gallbladder cancer, the treatment fields should be designed to include the gallbladder fossa and the hilar and celiac lymph node-bearing areas (Fig. 47-6). Accurate definition of the gallbladder fossa can be difficult in the absence of preoperative CT scans or surgical clips placed at the time of resection. Doses in the range of 45 to 50 Gy (1.8- to 2-Gy fractions) for subclinical disease and 60 to 65 Gy for microscopically positive margins are appropriate, although caution must be exercised because irradiation of the gallbladder fossa can include large volumes of liver.
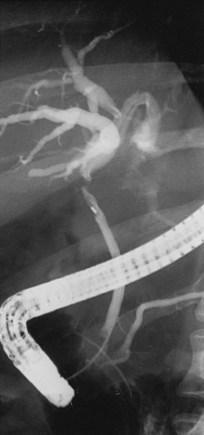
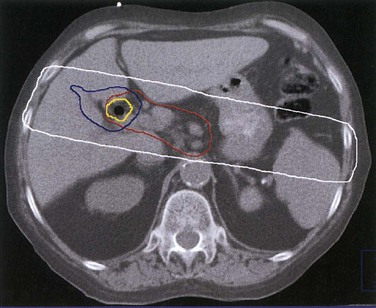
Brachytherapy127,183,125 has frequently been used for EHCC. Usually, brachytherapy is given as a supplement to 45 to 50 Gy (1.8- to 2-Gy fractions) of EBRT. The external biliary drainage catheter is then used to gain access, and fluoroscopy helps to place a guidewire in the treatment area (Fig. 47-7). In general, a boost dose of 15 to 20 Gy is prescribed to a radius of 0.5 to 1 cm from the brachytherapy catheter, although some have suggested an evaluation of boost doses of 30 Gy to a 1-cm radius, depending on the disease location relative to the stomach and duodenum.127
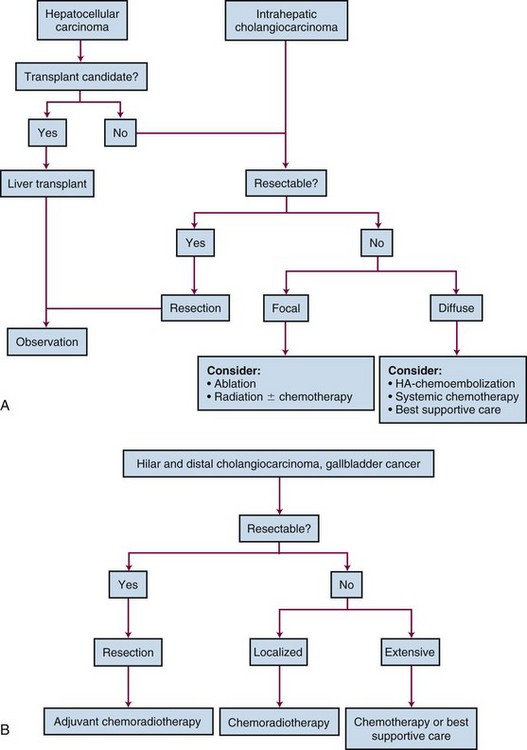
Treatment Algorithm And Future Directions
Figure 47-8 is a treatment algorithm for hepatobiliary cancers. A number of advances suggest avenues for improving RT for patients with intrahepatic cancers. The use of three-dimensional treatment planning has permitted the delivery of much higher doses of radiation for intrahepatic and gallbladder cancers than would have been possible using standard techniques. The addition of brachytherapy to EBRT has done the same for EHCC. Further improvements in treatment may come from even better physical dose delivery and better tumor definition with improved imaging techniques. Because safe RT doses depend on the volume of normal liver irradiated, decreasing the planning target volume could result in higher overall tumor doses and improved local control rates. One approach could include reduction or elimination of the component of the planning target volume allotted for ventilation184 through gating or breath-holding techniques.185,186 The target volume could also be reduced by improving the definition of the target volume with the use of better imaging.
Another method of improving the outcome of treatment for patients with intrahepatic cancer could include the more standard use of radiation sensitizers or radioprotectors. Most studies have used systemic 5-FU or hepatic arterial fluorodeoxyuridine. Other agents could be considered for concurrent systemic or hepatic arterial administration. The latter is particularly attractive for the treatment of intrahepatic tumors given that the dual blood supply of the liver permits the selective perfusion of tumors via the hepatic arterial circulation. Indeed, response rates as high as 50% have been reported with hepatic arterial infusion of fluorodeoxyuridine and mitomycin C187 and with combinations of etoposide and cisplatin with 5-FU (EPF) or doxorubicin (EAP).188 It would also be logical to attempt to combine sorafenib with RT. Conversely, radioprotectors such as amifostine, administered systemically or through the portal vein, could produce selective protection of the normal liver and thereby improve the therapeutic index.
1 Jemal A, Siegal R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
13 Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336(26):1855-1859.
24 Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417-422.
25 NCCN Clinical Practice Guidelines in Oncology 2010. Hepatobiliary Cancers. Available at NCCN.org
54 Buskirk SJ, Gunderson LL, Schild SE, et al. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg. 1992;215(2):125-131.
55 Gonzalez Gonzalez D, Gerard P, Maners AW, et al. Results of radiation therapy in carcinoma of the proximal bile duct (Klatskin tumor). Semin Liver Dis. 1990;10(2):131-141.
56 Edge SBB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual, ed 7. New York: Springer; 2010.
61 Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma. Resection versus transplantation. Hepatology. 1999;30(6):1434-1440.
62 Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
67 Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas. A retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32(6):1224-1229.
74 Samuel M, Chow PK, Chan Shih-Yen E, et al: Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma, Cochrane Database Syst Rev1:CD001199, 2009.
75 Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96(9):975-981.
78 Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122-130.
89 Heimbach JK, Haddock MG, Alberts SR, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10(10 Suppl 2):S65-S68.
90 Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242(3):451-458. discussion 242(3):458-461, 2005
108 Kresl JJ, Schild SE, Henning GT, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(1):167-175.
109 Gold DG, Miller RC, Bhatia S, et al. Adjuvant therapy for gallbladder carcinoma. The Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2009;27:1116-1121.
125 Fritz P, Brambs HJ, Schraube P, et al. Combined external beam radiotherapy and intraluminal high dose rate brachytherapy on bile duct carcinomas. Int J Radiat Oncol Biol Phys. 1994;29(4):855-861.
127 Foo ML, Gunderson LL, Bender CE, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39(4):929-935.
128 Gerhards MF, van Gulik TM, Gonzalez Gonzalez D, et al. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27(2):173-179.
134 Llovet JM, Ricci S, Mazzaferro G, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
140 Tzoracoleftherakis EE, Spiliotis JD, Kyriakopoulou T, Kakkos SK. Intra-arterial versus systemic chemotherapy for non-operable hepatocellular carcinoma. Hepatogastroenterology. 1999;46(26):1122-1125.
141 Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164-1171.
142 Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma. A randomized controlled trial. Lancet. 2002;359(9319):1734-1739.
146 Robertson JM, McGinn CJ, Walker S, et al. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997;39(5):1087-1092.
147 McGinn CJ, Ten Haken RK, Ensminger WD, et al. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16(6):2246-2252.
148 Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18(11):2210-2218.
149 Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23(34):8739-8747.
150 Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810-821.
151 Zhou ZH, Liu LM, Cheng WW, et al. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 2007;80(951):194-201.
152 Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma. A retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55(2):329-336.
153 Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307-316.
154 Li B, Yu J, Wang L, et al. Study of local three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. Am J Clin Oncol. 2003;26(4):e92-e99.
155 Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000;73(874):1091-1097.
156 Cheng JC, Chuang VP, Chang SH, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000;47(2):435-442.
157 Meng MB, Cui YL, Lu Y, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma. A systematic review and meta-analysis. Radiother Oncol. 2009;92(2):184-194.
158 McIntosh A, Hagspiel KD, Al-Osaimi AM, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. 2009;115(21):5117-5125.
159 Choi BO, Choi IB, Jang HS, et al. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer. 2008;8:351.
160 Takeda A, Takahashi M, Kunieda E, et al. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies. Preliminary results for efficacy and toxicity. Hepatol Res. 2008;38(1):60-69.
161 Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657-664.
162 Kopek N, Holt MI, Hansen AT, Hoyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94(1):47-52.
180 Jackson A, Ten Haken RK, Robertson JM, et al. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. Int J Radiat Oncol Biol Phys. 1995;31(4):883-891.
183 Hayes JKJr, Sapozink MD, Miller FJ. Definitive radiation therapy in bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1988;15(3):735-744.
184 Ten Haken RK, Balter JM, Marsh LH, et al. Potential benefits of eliminating planning target volume expansions for patient breathing in the treatment of liver tumors. Int J Radiat Oncol Biol Phys. 1997;38(3):613-617.
185 Balter JM, Ten Haken RK, Lawrence TS, et al. Uncertainties in CT-based radiation therapy treatment planning associated with patient breathing. Int J Radiat Oncol Biol Phys. 1996;36(1):167-174.
189 Iwasaki Y, Todoroki T, Fukao K, et al. The role of intraoperative radiation therapy in the treatment of bile duct cancer. World J Surg. 1988;12(1):91-98.
190 Gunderson LL, Nagorney DN, Garton GR, et al. Pancreas and bile duct cancer results of IORT. In: Abe M, Takahashi M, editors. Intraoperative Radiation Therapy. Tokyo: Pergamon Press; 1991:212-214.
193 Gunderson LL, Haddock MG, Burch P, et al. Future role of radiotherapy as a component of treatment in biliopancreatic cancers. Ann Oncol. 1999;10(Suppl 4):291-295.
194 Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994;28(4):945-951.
195 Flickinger JC, Epstein AH, Iwatsuki S, et al. Radiation therapy for primary carcinoma of the extrahepatic biliary system. An analysis of 63 cases. Cancer. 1991;68(2):289-294.
196 Urego M, Flickinger JC, Carr BJ. Radiotherapy and multimodality management of cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 1999;44(1):121-126.
1 Jemal A, Siegal R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
2 Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan2000. Int J Cancer. 2001;94(2):153-156.
3 El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States. An update. Ann Intern Med. 2003;139(10):817-823.
4 Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353-1357.
5 Wharton JT, Delclos L, Gallager S, Smith JP. Radiation hepatitis induced by abdominal irradiation with the cobalt 60 moving strip technique. Am J Roentgenol Radium Ther Nucl Med. 1973;117(1):73-80.
6 Ingold DK, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol. 1965;93:200-208.
7 American Cancer Society: Cancer Facts and Figures 2004. American Cancer Society, 2004.
8 Nagorney DM, Gigot JF. Primary epithelial hepatic malignancies. Etiology, epidemiology, and outcome after subtotal and total hepatic resection. Surg Oncol Clin North Am. 1996;5(2):283-300.
9 El-Serag HB. Hepatocellular carcinoma. Recent trends in the United States. Gastroenterology. 2004;127(5 Suppl 1):S27-S34.
10 Diehl AK. Epidemiology of gallbladder cancer. A synthesis of recent data. J Natl Cancer Inst. 1980;65(6):1209-1214.
11 Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(1 Suppl):171-190.
12 Khakoo SI, Grellier LF, Soni PN, et al. Etiology, screening, and treatment of hepatocellular carcinoma. Med Clin North Am. 1996;80(5):1121-1145.
13 Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336(26):1855-1859.
14 Stuart KE, Anand AJ, Jenkins RI. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77(11):2217-2222.
15 Anthony P. Tumours and tumour-like lesions of the liver and biliary tract. In: MacSween RN, Anthony PP, Scheur PJ, et al, editors. Pathology of the Liver, 3rd ed. Edinburgh: Churchill Livingstone, 1994.
16 Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer. A case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89(15):1132-1138.
17 Strom BL, Soloway RD, Rios-Dalenz J, et al. Biochemical epidemiology of gallbladder cancer. Hepatology. 1996;23(6):1402-1411.
18 Abi-Rached B, Neugut AJ. Diagnostic and management issues in gallbladder carcinoma. Oncology (Huntingt). 1995;9(1):19-24. discussion 9(1):24, 27, 30, 1995
19 Van Leeuwen DJ, Huibregtse K, Tytgat GN. Carcinoma of the hepatic confluence 25 years after Klatskin’s description. Diagnosis and endoscopic management. Semin Liver Dis. 1990;10(2):102-113.
20 Rosen CB, Nagorney DM. Cholangiocarcinoma complicating primary sclerosing cholangitis. Semin Liver Dis. 1991;11(1):26-30.
21 Ekbom A, Hsieh CC, Yuen J, et al. Risk of extrahepatic bile duct cancer after cholecystectomy. Lancet. 1993;342(8882):1262-1265.
22 Regan LS. Screening for hepatocellular carcinoma in high-risk individuals. A clinical review. Arch Intern Med. 1989;149(8):1741-1744.
23 Di Bisceglie AM, Rustgi CK, Hoofnagle JH, et al. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988;108(3):390-401.
24 Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417-422.
25 NCCN Clinical Practice Guidelines in Oncology2010. Hepatobiliary Cancers. Available at NCCN.org
26 Nichols JC, Gores GJ, LaRusso NF, et al. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin Proc. 1993;68(9):874-879.
27 Bjornsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver. 1999;19(6):501-508.
28 Okuda K. Hepatocellular carcinoma. Recent progress. Hepatology. 1992;15(5):948-963.
29 Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis. Adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991;22(2):172-178.
30 Okuda K, Peters RL, Simson IW. Gross anatomic features of hepatocellular carcinoma from three disparate geographic areas. Proposal of new classification. Cancer. 1984;54(10):2165-2173.
31 Nzeako UC, Goodman ZD, Ishak KG. Comparison of tumor pathology with duration of survival of North American patients with hepatocellular carcinoma. Cancer. 1995;76(4):579-588.
32 Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70(6):1493-1497.
33 Weedon D. Diseases of the gallbladder. In: MacSween RN, Anthony PP, Scheur PJ, et al, editors. Pathology of the Liver, 3rd ed. Edinburgh: Churchill Livingstone, 1994.
34 Malik IA. Clinicopathological features and management of gallbladder cancer in Pakistan. A prospective study of 233 cases. J Gastroenterol Hepatol. 2003;18(8):950-953.
35 Grobmyer SR, Lieberman MD, Daly JM. Gallbladder cancer in the twentieth century. Single institution’s experience. World J Surg. 2004;28(1):47-49.
36 Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol. 1983;139(2):217-238.
37 Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70(6):1498-1501.
38 Suzuki M, Takahashi T, Ouchi K, Matsuno S. The development and extension of hepatohilar bile duct carcinoma. A three-dimensional tumor mapping in the intrahepatic biliary tree visualized with the aid of a graphics computer system. Cancer. 1989;64(3):658-666.
39 Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463-473. discussion 224(4):473-475, 1996
40 Altaee MY, Johnson PJ, Farrant JM, Williams R. Etiologic and clinical characteristics of peripheral and hilar cholangiocarcinoma. Cancer. 1991;68(9):2051-2055.
41 Kassianides C, Kew MC. The clinical manifestations and natural history of hepatocellular carcinoma. Gastroenterol Clin North Am. 1987;16(4):553-562.
42 Chao TC, Greager JA. Primary carcinoma of the gallbladder. J Surg Oncol. 1991;46(4):215-221.
43 Arnaud JP, Casa C, Georgeac C, et al. Primary carcinoma of the gallbladder—review of 143 cases. Hepatogastroenterology. 1995;42(6):811-815.
44 Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64(8):1700-1707.
45 Patt YZ, Charnsangavej C, Yoffe B, et al. Hepatic arterial infusion of floxuridine, leucovorin, doxorubicin, and cisplatin for hepatocellular carcinoma. Effects of hepatitis B and C viral infection on drug toxicity and patient survival. J Clin Oncol. 1994;12(6):1204-1211.
46 Braga L, Guller U, Semelka RC. Modern hepatic imaging. Surg Clin North Am. 2004;84(2):375-400.
47 Hann LE, Winston CB, Brown KT, Akhurst T. Diagnostic imaging approaches and relationship to hepatobiliary cancer staging and therapy. Semin Surg Oncol. 2000;19(2):94-115.
48 Hori M, Murakami T, Kim T, Takahashi S. Sensitivity of double-phase helical CT during arterial portography for detection of hypervascular hepatocellular carcinoma. J Comput Assist Tomogr. 1998;22(6):861-867.
49 Murakami T, Oi H, Hori M, et al. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169(1):131-135.
50 Li L, Wu PH, Mo YX, et al. CT arterial portography and CT hepatic arteriography in detection of micro liver cancer. World J Gastroenterol. 1999;5(3):225-227.
51 Li L, Liu LZ, Xie ZM, et al. Multi-phasic CT arterial portography and CT hepatic arteriography improving the accuracy of liver cancer detection. World J Gastroenterol. 2004;10(21):3118-3121.
52 Taouli B, Losada M, Holland A, Krinsky G. Magnetic resonance imaging of hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S144-S152.
53 Cohen MB, Haber MM, Holly EA, et al. Cytologic criteria to distinguish hepatocellular carcinoma from nonneoplastic liver. Am J Clin Pathol. 1991;95(2):125-130.
54 Buskirk SJ, Gunderson LL, Schild SE, et al. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg. 1992;215(2):125-131.
55 Gonzalez Gonzalez D, Gerard JP, Maners AW, et al. Results of radiation therapy in carcinoma of the proximal bile duct (Klatskin tumor). Semin Liver Dis. 1990;10(2):131-141.
56 Edge SBB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual, ed 7. New York: Springer; 2010.
57 Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19(3):311-322.
58 Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519-524.
59 Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229(6):790-799. discussion 229(6):799-800, 1999
60 Jonas S, Bechstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33(5):1080-1086.
61 Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma. Resection versus transplantation. Hepatology. 1999;30(6):1434-1440.
62 Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
63 Takayama T, Makuuchi M, Hirohashi S, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998;28(5):1241-1246.
64 Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma. A randomised trial. Lancet. 2000;356(9232):802-807.
65 Wayne JD, Lauwers GY, Ikai I, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235(5):722-730. discussion 235(5):730-731, 2002
66 Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma. Expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-1403.
67 Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas. A retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32(6):1224-1229.
68 Livraghi T, Girogio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients. Long-term results of percutaneous ethanol injection. Radiology. 1995;197(1):101-108.
69 Lencioni R, Pinto F, Armillotta N, et al. Long-term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis. A European experience. Eur Radiol. 1997;7(4):514-519.
70 Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma. Long-term results. Eur Radiol. 2001;11(6):914-921.
71 Bismuth H, Majno PE. Hepatobiliary surgery. J Hepatol. 2000;32(1 Suppl):208-224.
72 Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105(2):488-494.
73 Schwartz JD, Schwartz M, Mandell J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma. Review of the randomised clinical trials. Lancet Oncol. 2002;3(10):593-603.
74 Samuel M, Chow PK, Chan Shih-Yen E, et al: Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma, Cochrane Database Syst Rev 1:CD001199, 2009.
75 Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96(9):975-981.
76 Okada S. Local ablation therapy for hepatocellular carcinoma. Semin Liver Dis. 1999;19(3):323-328.
77 Tanabe KK, Curley SA, Dodd GD, et al. Radiofrequency ablation. The experts weigh in. Cancer. 2004;100(3):641-650.
78 Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122-130.
79 Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89(10):1206-1222.
80 Inoue K, Makuuchi M, Takayama T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127(5):498-505.
81 Weber SM, Jarnagin WR, Klinstra D, et al. Intrahepatic cholangiocarcinoma. Resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384-391.
82 Pichlmayr R, Lamesch P, Weimann A, et al. Surgical treatment of cholangiocellular carcinoma. World J Surg. 1995;19(1):83-88.
83 Jan YY, Jeng LB, Hwang TL, et al. Factors influencing survival after hepatectomy for peripheral cholangiocarcinoma. Hepatogastroenterology. 1996;43(9):614-619.
84 Casavilla FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg. 1997;185(5):429-436.
85 Madariaga JR, Iwatsuki S, Todo S, et al. Liver resection for hilar and peripheral cholangiocarcinomas. A study of 62 cases. Ann Surg. 1998;227(1):70-79.
86 Valverde A, Bonhomme NI, Farges O, et al. Resection of intrahepatic cholangiocarcinoma. A Western experience. J Hepatobiliary Pancreat Surg. 1999;6(2):122-127.
87 Isa T, Kusano T, Shimoji H, et al. Predictive factors for long-term survival in patients with intrahepatic cholangiocarcinoma. Am J Surg. 2001;181(6):507-511.
88 Shimoda M, Farmer DG, Colquhoun SD, et al. Liver transplantation for cholangiocellular carcinoma. Analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7(12):1023-1033.
89 Heimbach JK, Haddock MG, Alberts SR, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10(10 Suppl 2):S65-S68.
90 Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242(3):451-458. discussion 242(3):458-461, 2005
91 de Aretxabala X, Roa I, Burgos I, et al. Gallbladder cancer in Chile. A report on 54 potentially resectable tumors. Cancer. 1992;69(1):60-65.
92 Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992;215(4):326-331.
93 Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224(5):639-646.
94 Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy. Aggressive reresection is beneficial. Cancer. 1998;83(3):423-427.
95 de Aretxabala XA, Roa IS, Burgos LA, et al. Curative resection in potentially resectable tumours of the gallbladder. Eur J Surg. 1997;163(6):419-426.
96 Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer. Comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232(4):557-569.
97 Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg. 1992;163(4):382-386.
98 Nakamura S, Sakaguchi S, Suzuki S, Muro H. Aggressive surgery for carcinoma of the gallbladder. Surgery. 1989;106(3):467-473.
99 Onoyama H, Yamamoto M, Tseng A, et al. Extended cholecystectomy for carcinoma of the gallbladder. World J Surg. 1995;19(5):758-763.
100 Ogura Y, Mizumoto R, Isaji S, et al. Radical operations for carcinoma of the gallbladder. Present status in Japan. World J Surg. 1991;15(3):337-343.
101 Matsumoto Y, Fujii H, Aoyama H, et al. Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens. Am J Surg. 1992;163(2):239-245.
102 Donohue JH, Nagorney DM, Grant CS, et al. Carcinoma of the gallbladder. Does radical resection improve outcome? Arch Surg. 1990;125(2):237-241.
103 Fahim RB, McDonald JR, Richards JC, Ferris DO. Carcinoma of the gallbladder. A study of its modes of spread. Ann Surg. 1962;156:114-124.
104 Kondo S, Nimura Y, Hayakawa N, et al. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87(4):418-422.
105 Kopelson G, Harisiadis L, Tretter P, Chang CH. The role of radiation therapy in cancer of the extra-hepatic biliary system. An analysis of thirteen patients and a review of the literature of the effectiveness of surgery, chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 1977;2(9-10):883-894.
106 Hanna SS, Rider WD. Carcinoma of the gallbladder or extrahepatic bile ducts. The role of radiotherapy. Can Med Assoc J. 1978;118(1):59-61.
107 Houry S, Schlienger M, Huguier M, et al. Gallbladder carcinoma. Role of radiation therapy. Br J Surg. 1989;76(5):448-450.
108 Kresl JJ, Schild SE, Henning GT, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(1):167-175.
109 Gold DG, Miller RC, Bhatia S, et al. Adjuvant therapy for gallbladder carcinoma. The Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2009;27:1116-1121.
110 Klempnauer J, Ridder GJ, Werner M, et al. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79(1):26-34.
111 Chao TC, Greager JA. Carcinoma of the extrahepatic bile ducts. J Surg Oncol. 1991;46(3):145-150.
112 Klempnauer J, Ridder GJ, von Wasielewski R, et al. Resectional surgery of hilar cholangiocarcinoma. A multivariate analysis of prognostic factors. J Clin Oncol. 1997;15(3):947-954.
113 Cameron JL, Pitt HA, Zinner MJ, et al. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159(1):91-97. discussion 159(1):97-98, 1990
114 Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223(4):384-394.
115 Hadjis NS, Blankham JI, Alexander N, et al. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107(6):597-604.
116 Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507-517. discussion 234(4):517-519, 2001
117 Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230(6):808-818. discussion 230(6):819, 1999
118 Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230(5):663-671.
119 Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14(4):535-543. discussion 14(4):544, 1990
120 Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):189-199.
121 Wade TP, Prasad CN, Virgo KS, Johnson FE. Experience with distal bile duct cancers in U.S. Veterans Affairs hospitals. 1987-1991. J Surg Oncol. 1997;64(3):242-245.
122 Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128(8):871-877. discussion 128(8):877-879, 1993
123 Fong Y, Blumgart LH, Lin E, et al. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83(12):1712-1715.
124 Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s. Pathology, complications, and outcomes. Ann Surg. 1997;226(3):248-257. discussion 226(3):257-260, 1997
125 Fritz P, Brambs HJ, Schraube P, et al. Combined external beam radiotherapy and intraluminal high dose rate brachytherapy on bile duct carcinomas. Int J Radiat Oncol Biol Phys. 1994;29(4):855-861.
126 Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma. Implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689-1700.
127 Foo ML, Gunderson LL, Bender CE, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39(4):929-935.
128 Gerhards MF, van Gulik TM, Gonzalez Gonzalez D, et al. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27(2):173-179.
129 Pitt HA, Nakeeb A, Abrams RA, et al. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221(6):788-797. discussion 221(6):797-798, 1995
130 Mahe M, Romestaing P, Talon B, et al. Radiation therapy in extrahepatic bile duct carcinoma. Radiother Oncol. 1991;21(2):121-127.
131 A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma, Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. N Engl J Med. 1995;332(19):1256-1261.
132 Yamashita Y, Takahashi M, Koga Y, et al. Prognostic factors in the treatment of hepatocellular carcinoma with transcatheter arterial embolization and arterial infusion. Cancer. 1991;67(2):385-391.
133 Stillwagon GB, Order SE, Guse C, et al. Prognostic factors in unresectable hepatocellular cancer. Radiation Therapy Oncology Group Study 83-01. Int J Radiat Oncol Biol Phys. 1991;20(1):65-71.
134 Llovet JM, Ricci S, Mazzaferro G, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
135 Mathurin P, Rixe O, Carbonell N, et al. Review article. Overview of medical treatments in unresectable hepatocellular carcinoma—an impossible meta-analysis? Aliment Pharmacol Ther. 1998;12(2):111-126.
136 Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma. A systematic review of randomized controlled trials. Ann Oncol. 1997;8(2):117-136.
137 Chow PK, Tai BC, Tan CK, et al. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma. A multicenter randomized controlled trial. Hepatology. 2002;36(5):1221-1226.
138 Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30(5):969-977.
139 Yamashita T. Chemotherapy for advanced hepatocellular carcinoma. Systemic chemotherapy or hepatic arterial infusion chemotherapy? J Gastroenterol. 2004;39(4):404-406.
140 Tzoracoleftherakis EE, Spiliotis JD, Kyriakopoulou T, Kakkos SK. Intra-arterial versus systemic chemotherapy for non-operable hepatocellular carcinoma. Hepatogastroenterology. 1999;46(26):1122-1125.
141 Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164-1171.
142 Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma. A randomized controlled trial. Lancet. 2002;359(9319):1734-1739.
143 Stillwagon GB, Order SE, Haulk T, et al. Variable low dose rate irradiation (131I-anti-CEA) and integrated low dose chemotherapy in the treatment of nonresectable primary intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 1991;21(6):1601-1605.
144 Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma. A preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17(2):484-491.
145 Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres. Results from a pilot study. Cancer. 2008;113(8):2119-2128.
146 Robertson JM, McGinn CJ, Walker S, et al. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997;39(5):1087-1092.
147 McGinn CJ, Ten Haken RK, Ensminger WD, et al. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16(6):2246-2252.
148 Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18(11):2210-2218.
149 Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23(34):8739-8747.
150 Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810-821.
151 Zhou ZH, Liu LM, Cheng WW, et al. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 2007;80(951):194-201.
152 Seong J, Park HC, Han KH, Chen CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma. A retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55(2):329-336.
153 Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307-316.
154 Li B, Yu J, Wang L, et al. Study of local three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. Am J Clin Oncol. 2003;26(4):e92-e99.
155 Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000;73(874):1091-1097.
156 Cheng JC, Chuang VP, Chang SH, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000;47(2):435-442.
157 Meng MB, Cui YL, Lu Y, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma. A systematic review and meta-analysis. Radiother Oncol. 2009;92(2):184-194.
158 McIntosh A, Hagspiel KD, Al-Osaimi AM, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. 2009;115(21):5117-5125.
159 Choi BO, Choi IB, Jang HS, et al. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma. Preliminary analysis. BMC Cancer. 2008;8:351.
160 Takeda A, Takahashi M, Kunieda E, et al. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies. Preliminary results for efficacy and toxicity. Hepatol Res. 2008;38(1):60-69.
161 Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657-664.
162 Kopek N, Holt MI, Hansen AT, Hoyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94(1):47-52.
163 Ishii H, Furuse J, Yonemoto N, et al. Chemotherapy in the treatment of advanced gallbladder cancer. Oncology. 2004;66(2):138-142.
164 Harvey JH, Smith FP, Schein PS. 5-Fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the biliary tract. J Clin Oncol. 1984;2(11):1245-1248.
165 Taal BG, Audisio RA, Bleiberg H, et al. Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma. An EORTC Gastrointestinal Tract Cancer Cooperative Group Study. Ann Oncol. 1993;4(7):607-609.
166 Okada S, Ishii H, Nose H, et al. A phase II study of cisplatin in patients with biliary tract carcinoma. Oncology. 1994;51(6):515-517.
167 Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of cisplatin, interferon alpha-2b, doxorubicin, and 5-fluorouracil for biliary tract cancer. Clin Cancer Res. 2001;7(11):3375-3380.
168 Patt YZ, Jones DVJr, Hoke A, et al. Phase II trial of intravenous fluorouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14(8):2311-2315.
169 Jones DVJr, Lozano R, Hoque A, et al. Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol. 1996;14(8):2306-2310.
170 Sanz-Altamira PM, Ferrante K, Jenkins RL, et al. A phase II trial of 5-fluorouracil, leucovorin, and carboplatin in patients with unresectable biliary tree carcinoma. Cancer. 1998;82(12):2321-2325.
171 Pazdur R, Royce ME, Rodriguez GI, et al. Phase II trial of docetaxel for cholangiocarcinoma. Am J Clin Oncol. 1999;22(1):78-81.
172 Penz M, Komek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12(2):183-186.
173 Ellis PA, Norman A, Hill A, et al. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer. 1995;31A(10):1594-1598.
174 Malik IA, Aziz Z, Saidi SH, et al. Gemcitabine and cisplatin is a highly effective combination chemotherapy in patients with advanced cancer of the gallbladder. Am J Clin Oncol. 2003;26(2):174-177.
175 Doval DC, Sekhon JS, Gupta SK, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90(8):1516-1520.
176 Lokich JJ, Kane RA, Harrison DA, McDermott WV. Biliary tract obstruction secondary to cancer. Management guidelines and selected literature review. J Clin Oncol. 1987;5(6):969-981.
177 Hejna M, Pruckmayer M, Raderer M. The role of chemotherapy and radiation in the management of biliary cancer. A review of the literature. Eur J Cancer. 1998;34(7):977-986.
178 Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31(5):1237-1248.
179 Yamasaki SA, Marn CS, Francis IR, et al. High-dose localized radiation therapy for treatment of hepatic malignant tumors. CT findings and their relation to radiation hepatitis. AJR Am J Roentgenol. 1995;165(1):79-84.
180 Jackson A, Ten Haken RK, Robertson JM, et al. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. Int J Radiat Oncol Biol Phys. 1995;31(4):883-891.
181 Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109-122.
182 Borgelt BB, Gelber R, Brady LW, et al. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7(5):587-591.
183 Hayes JKJr, Sapozink MD, Miller FJ. Definitive radiation therapy in bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1988;15(3):735-744.
184 Ten Haken RK, Balter JM, Marsh LH, et al. Potential benefits of eliminating planning target volume expansions for patient breathing in the treatment of liver tumors. Int J Radiat Oncol Biol Phys. 1997;38(3):613-617.
185 Balter JM, Ten Haken RK, Lawrence TS, et al. Uncertainties in CT-based radiation therapy treatment planning associated with patient breathing. Int J Radiat Oncol Biol Phys. 1996;36(1):167-174.
186 Balter JM, Brock KK, Litzenberg DW, et al. Daily targeting of intrahepatic tumors for radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(1):266-271.
187 Atiq OT, Kemeny N, Niedzwiecki D, Botet J. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992;69(4):920-924.
188 Yodono H, Sasaki T, Tarusawa K, et al. Arterial infusion chemotherapy for advanced hepatocellular carcinoma using EPF and EAP therapies. Cancer Chemother Pharmacol. 1992;31(Suppl):S89-S92.
189 Iwasaki Y, Todoroki T, Fukao K, et al. The role of intraoperative radiation therapy in the treatment of bile duct cancer. World J Surg. 1988;12(1):91-98.
190 Gunderson LL, Nagorney DN, Garton GR, et al. Pancreas and bile duct cancer results of IORT. In: Abe M, Takahashi M, editors. Intraoperative Radiation Therapy. Tokyo: Pergamon Press; 1991:212-214.
191 Monson JR, Donohue JH, Gunderson LL, et al. Intraoperative radiotherapy for unresectable cholangiocarcinoma—the Mayo Clinic experience. Surg Oncol. 1992;1(4):283-290.
192 De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoradiation for cholangiocarcinoma. Liver Transpl. 2000;6(3):309-316.
193 Gunderson LL, Haddock MG, Burch P, et al. Future role of radiotherapy as a component of treatment in biliopancreatic cancers. Ann Oncol. 1999;10(Suppl 4):291-295.
194 Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994;28(4):945-951.
195 Flickinger JC, Epstein AH, Iwatsuki S, et al. Radiation therapy for primary carcinoma of the extrahepatic biliary system. An analysis of 63 cases. Cancer. 1991;68(2):289-294.
196 Urego M, Flickinger JC, Carr BI. Radiotherapy and multimodality management of cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 1999;44(1):121-126.