Chapter 90D Hepatic resection for living donor transplantation
Overview
Donor hepatectomy is a major surgical operation performed on a healthy subject only for the benefit of a recipient, who requires liver transplantation (see Chapter 97A). In 1989, Strong (1999) performed donor left hepatectomy and removed segment IV of the liver on the back table prior to implantation of segments II and III into a pediatric recipient. In 1990, Tanaka and coworkers (Yamaoka et al, 1994) used a right liver graft to transplant a pediatric recipient. Living donor left liver transplantation (LDLT) for adults was first performed by Makuuchi in 1993 (Hashikura et al, 1994). The first case of right liver adult LDLT was performed by Fan in 1996 (Lo et al, 1997a). A priori, the right liver graft included the middle hepatic vein (MHV) to address the problems of small-for-size syndrome by providing good venous outflow of the right anterior sector. The first seven recipients who underwent right LDLT all had acute liver failure before the transplantation; one died from candidiasis and the other six survived (Lo et al, 1997b). Subsequently, semiurgent and elective cases were accepted.
Donor Workup
Donor workup is started after indication for LDLT for the potential recipient is ascertained. The workup is to evaluate whether the donor will be psychologically and physically healthy in the long-term after recovery from the organ donation. In a stepwise fashion, expedience is achieved without omission (Chan et al, 2007a). Only healthy individuals who have reached the age of consent are accepted (Abecassis et al, 2000).
Step 1
A detailed medical history is taken to identify any comorbidities. A body mass index of 30 kg/m2 or more (27 kg/m2 for Asians; World Health Organization, 2004) raises the concern for fatty liver– and obesity-related comorbidites. Blood group compatibility is verified. Hepatitis B and C virus and HIV carrier status preclude liver donation, although hepatitis B core antibody positivity by itself does not preclude liver donation, it mandates lifelong prophylaxis with lamivudine in the recipient (Lo et al, 2003).
Step 2b
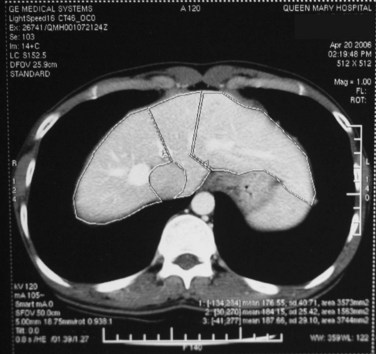
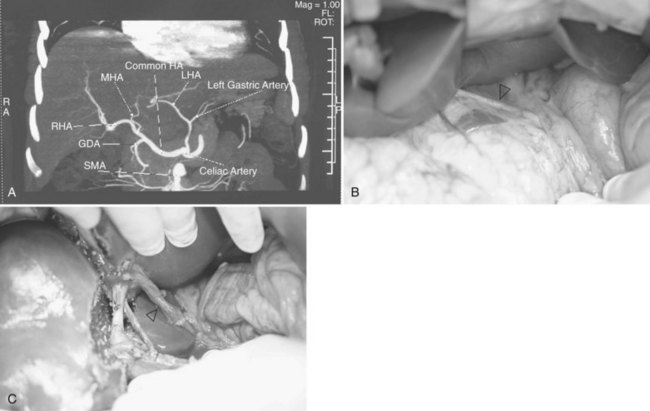
Chest radiographs are taken, and an electrocardiogram is performed. Computed tomography (CT) of the liver under sodium bicarbonate cover (Merten et al, 2004) is also performed, and maximum-intensity projections of the portal veins and hepatic veins are produced. Volumetry of the donor liver by the Heymsfield method (Heymsfield et al, 1979) measures the volumes of the right liver and left liver (for pediatric recipients, segments II and III), using the MHV as a demarcation line on the plane between the right and left livers (Fig. 90D.1). Attenuation of the liver parenchyma in comparison with the spleen on the plain film is appraised for detection of fatty change. Hepatic vein anatomy is determined by the axial cuts in the venous phase and by maximum-intensity projections for easier appreciation. The presence of any inferior hepatic veins allows anticipation at the operation of either preservation or division. Attention to the presence of the segment IVb hepatic vein or segment III hepatic vein draining into the MHV calls for a more caudal division of the MHV to preserve adequate drainage of segment IV of the remnant left liver (Chan et al, 2004a). The right, left, and segment IV hepatic arteries are also illustrated by the three-dimensional (3D) reconstructions for the images obtained during the arterial phase.
Side and Size of Graft
The graft to standard liver volume ratio (Urata et al, 1995) is crucial in LDLT. A ratio of more than 35% is required for a predictable recipient success (Fan et al, 2003a). However, a 20% overestimation of the right liver graft size based on CT volumetry is commonly given a conversion factor of 1.19 g/mL (Chan et al, 2006a). Because the left liver is usually one third the size of the total liver, for a donor with a body size no larger than the recipient, the left liver is usually less than 35% of the recipient standard liver volume. For an individual with a larger left liver/right liver ratio, the left liver may be large enough. It is worth noting that in left LDLT, a gram-to-gram equivalence of graft is not applicable, and a left liver graft of the same size as the right is less efficacious (Chan et al, 2007b).
Donor Right Hepatectomy (See Chapter 98B)
Exposure
The donor is placed supine on the operating table with care to avoid pressure sores over the occiput, heels, and sacrum. The position of the donor must be optimal for the surgeon and his first assistant to face the operative field directly, unhindered by the metal bars of the upper hand retractor or those used to set up a fence between the surgeon and anesthetist (Fan, 2007). Access is gained through a right subcostal incision with upper midline extension. The ligamentum teres is ligated and divided, and the falciform ligament is taken down. The two curved blades of the Bookwalter retractor (Codman and Shurtleff, Raynham, MA) pull the rib cage laterally and anteriorly to open up the aperture made by the costal margins. Excising the xiphoid process may improve access to the suprahepatic inferior vena cava (IVC) and the roots of the hepatic veins. Following careful laparotomy, intraoperative ultrasonography (IOUS) is performed to study the junction of the middle and left hepatic veins with the IVC. The relation of the segment IVb hepatic vein to the MHV, already known from CT, is ascertained by IOUS. This also registers the flow characteristics of the hepatic arteries, portal veins, and hepatic veins for reference throughout the operation.
Isolation of Major Vessels and Parenchymal Transection
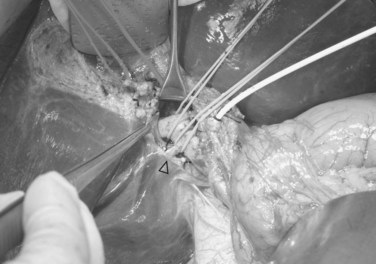
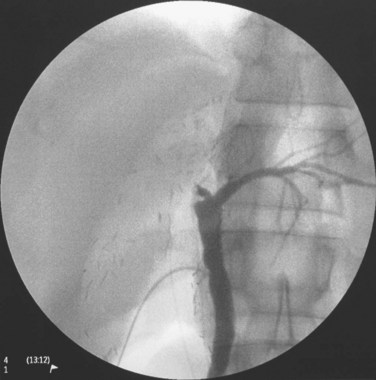
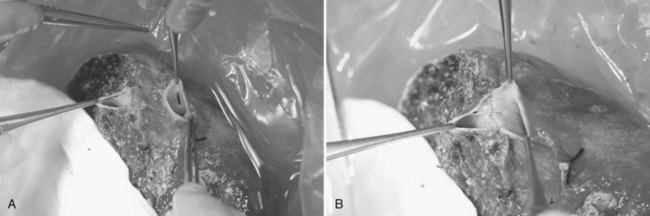
The triangle of Calot is dissected, and the cystic artery is divided between ligatures. The gallbladder is dissected from its fossa, and the cystic duct is cannulated with a 3.5-Fr Argyle catheter (Tyco Healthcare, Mansfield, MA). The cystic duct is then severed at the site of insertion of the catheter for delivery of the gallbladder. Next, the peritoneum overlying the right hepatic duct (RHD) is divided for identification. A large metal LigaClip (Ethicon Endo-Surgery, Cincinnati, OH) is put on the liver capsule at the planned line of division of the RHD, 3 to 4 mm away from the duct confluence. The biliary anatomy is then demonstrated by operative cholangiogram with undiluted contrast under fluoroscopy with a C-arm. The image quality of the cholangiogram can be improved by temporary and gentle occlusion of the distal common bile duct with an atraumatic vascular clamp (Featherlight Bulldog Clamp; Geister, Tuttlingen, Germany) (Fig. 90D.2). To avoid devascularization of the donor distal common bile duct, care must be taken not to dissect more tissue than necessary for application of the clamp; the clamp must be removed once the cholangiogram is finished. The supine donor will have the right posterior sectoral duct demonstrated first, followed by the right anterior sectoral duct and then the left ducts (Fan et al, 2002). The parallax technique executed by rotation of the C-arm to the right clarifies the anteroposterior position of the right anterior and posterior sectoral ducts. This also provides the true anteroposterior view of the biliary system. A marking is made with reference to the LigaClip on the liver capsule with diathermy for the line of division of the RHD.
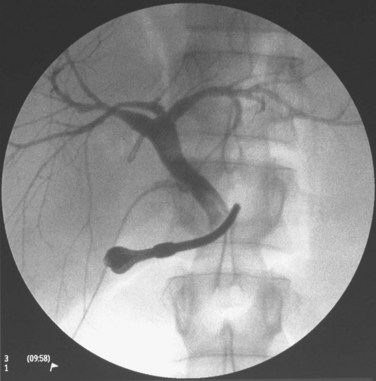
FIGURE 90D.2 Operative cholangiogram with metal clip marking the planned line of division of the right hepatic ducts.
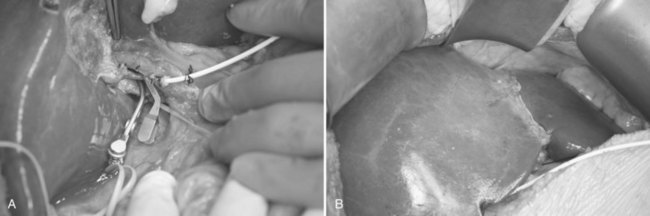
Hilar dissection is continued to isolate the right hepatic artery and right portal vein. The space between the right hepatic artery and right hepatic duct should not be disrupted in order to preserve the blood supply to the latter. To gain an entire length of the right portal vein, branches to the caudate lobe are ligated and then divided. It is important to note that a sizeable branch from the right portal vein may represent vessels supplying segment VI, which should be preserved (Fig. 90D.3). Temporary right liver inflow control is performed (Fig. 90D.4A) to mark the line of transection along Cantlie’s line (Fig. 90D.4B) with electrocautery. The line on the inferior surface is just to the left of the gallbladder fossa, joining the planned line of division of the right hepatic duct marked earlier.
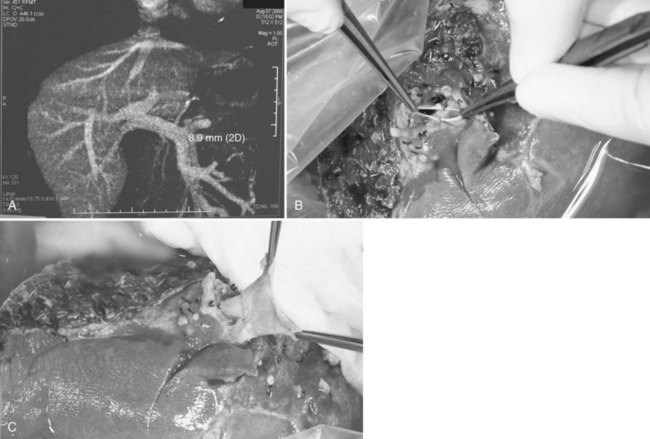
The right triangular ligament is then taken down, leaving the Gerota fascia intact. In a normal donor liver, the right adrenal gland can often be freed from the liver with careful dissection using electrocautery. Minor bleeding from the right adrenal gland is controlled by the argon beam coagulator, and more severe bleeding is addressed by plication with sutures. Short hepatic veins on the right side of the midline of the IVC are divided between ligatures and plicated as required. Inferior right hepatic veins (RHVs) larger than 5 mm are preserved for anastomosis with the IVC in the recipient (Fig. 90D.5).
In contrast to hepatectomy for neoplasm (see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90E, Chapter 90F ), continuous inflow control during liver transection is not practiced. Although the Pringle maneuver with intermittent reperfusion of the liver, which is adopted by some centers, has been shown to result in less blood loss in the donors, the difference did not reach statistical significance (Imamura et al, 2002). Possible explanations to this are that bleeding from the hepatic vein tributaries is not controlled by inflow control, and there is bleeding during the 5-minute reperfusion interval. Another potential advantage of the Pringle maneuver is downregulation of the apoptosis pathway by ischemic preconditioning for grafts with a long cold ischemic time (Clavien et al, 2000); however, this has been associated with poorer initial graft function by a prospective trial in deceased donor liver procurement (Azoulay et al, 2005). For adult LDLT, prolonged cold ischemia should not be an issue, because graft delivery matches the explantation of the native liver. In addition, a low central venous pressure (CVP) can be attained through good rapport with the anesthetist, and this lowered pressure has been proven effective in reducing blood loss (Jones et al, 1998). Elevating the head and the trunk by 10 to 15 degrees and complete muscle relaxation of the donor are also helpful.
After mobilization of the right liver from its posterior attachments, a full-thickness suture at the liver edge on both sides of this line helps open up the transection plane by the weight of hemostats. The placement of the laparotomy pad behind the right liver renders the transection plane more vertical, but not excessively so, to compromise retraction of the liver during transection. Liver parenchymal transection is started with electrocautery for the first 1 to 2 cm of parenchyma between segments V and IVb. The rest of the liver transection is done using the Cavitron Ultrasonic Surgical Aspirator (CUSA; Valleylab, Boulder, CO); it exposes the left side of the MHV, which lies two thirds of the depth from the superior surface of the liver (Fan et al, 2000). At a frequency of 23 kHz, the CUSA is set at an amplitude of 60% for the normal donor liver, and irrigation with normal saline continues at around 4 to 6 mL/min. The liver parenchyma is disrupted by cavitation of the aerosol. When the dissector is set at lower amplitudes, there is a tendency for the operator to bring the instrument into direct contact with the liver parenchyma, which will damage small and friable vessels. Suction is set to a moderately high level, just enough to provide a clear operative field. Electrocautery incorporated into the CUSA allows diathermy of vessels less than 1 mm in diameter. Should the segment IVb hepatic vein insert into the MHV, transection is stopped to preserve this vein for adequate drainage (Chan et al, 2004a).
At the liver hilum, the right portal pedicle containing the right hepatic duct is also dissected with minimal use of the CUSA. To denude the right hepatic duct from its blood supply, the dissection should not be overdone. The line of division already marked and verified by operative cholangiogram is followed, and any right hepatic ducts are severed with scissors tangential to the transection plane, which is often quite horizontal. The right hepatic duct stump is repaired by 6-0 polydioxanone continuous suture, and an operative cholangiogram is performed to confirm the patency of the left and main bile ducts (Fig. 90D.6). Liver parenchyma dorsal to the MHV and the caudate lobe are transected, until the IVC is exposed. Lifting up of the caudate lobe with a cotton-tape or a right-angled forceps facilitates the transection, and care must be taken to dissect in a definable plane between the liver capsule and the IVC.
Graft Delivery
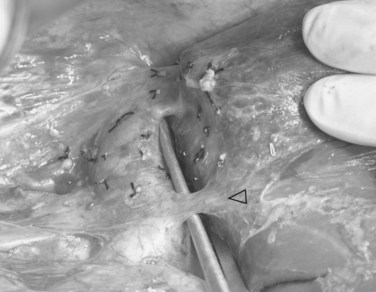
To have short cold ischemic time, the graft is not delivered until the recipient is ready for explanting of the native liver. Graft delivery starts with application of a clamp onto the proximal right hepatic artery, distal to the segment IV hepatic artery. The right hepatic artery is then divided with scissors, and the right portal vein is divided between the vascular clamps, applied at a right angle to the course of the main portal vein. The MHV, RHV, and, if present, the inferior RHV are controlled with the vascular stapler (TA 30; Tyco Healthcare) prior to its division by scissors. To avoid stricture of the portal vein, the right portal vein stump is sutured in a transverse manner with 6-0 Prolene continuous back-and-forth sutures. Biliary leakage and patency of the remnant left hepatic duct are checked with intraoperative cholangiogram and dilute methylene blue instillation; the methylene blue in the bile ducts must be flushed away by normal saline. The cystic duct is ligated with 2-0 Vicryl, and the remnant left liver is maintained in the anatomic position by reconstitution of the falciform ligament with nonabsorbable sutures (Fig. 90D.7). Patency of the vessels is verified with IOUS. The hepatic flexure of the colon and the corresponding portion of the greater omentum are allowed to ascend into the right subphrenic space for prevention of adherence of the small bowel to the transection surface of the remnant left liver, and the abdomen is closed without drainage (Liu et al, 2005).
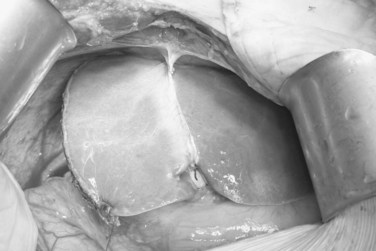
FIGURE 90D.7 Remnant left liver is prevented from dropping into the right subphrenic space by suturing the falciform ligament.
The outcomes of donor right hepatectomy including the MHV have improved with accumulation of experience. In our consecutive series of 200 donor right hepatectomies, all including the MHV, the operation time was shortened, and blood loss decreased with maturity of these techniques (Chan et al, 2007c).
Including the Middle Hepatic Vein
An area of ongoing controversy in right LDLT is whether or not to include the MHV. Deleterious effects of no drainage to segments V and VIII include severe venous congestion and necrosis of these segments (Lee et al, 2001). The decision to exclude the MHV requires demonstration of collaterals between segment V and VIII tributaries and the RHV (Cescon et al, 2002).
Researchers at Kyoto University devised an algorithm that includes the MHV when the graft is MHV dominant, or the graft to recipient weight is less than 1%, and in all cases, the remnant left liver is larger than 35% (Tanaka & Yamada, 2005). Chang Gung Memorial Hospital includes the MHV when the graft to estimated standard liver volume is 50% or less, or when the segment V and VIII hepatic veins are large, and the RHV is small (de Villa et al, 2003). Researchers at Tokyo University observed congestion of segments V and VIII of the graft after temporary clamping of the right hepatic artery before determining venous interpositional grafting (Sano et al, 2002).
We include the MHV in all right liver grafts for the simplicity and familiarity of the technique (Fan et al, 2003b). Irrespective of the venous drainage pattern of segment IV of the remnant left liver, the segment IVb hepatic vein is preserved. Utmost care is needed for its preservation, when it drains into the MHV (Chan et al, 2004a). On the back table, the outflow capacity is guaranteed by venoplasty of the MHV and RHV into a single cuff (Liu et al, 2003). The venoplasty is further marked by a more expedient hepatic vein to the IVC anastomosis and higher outflow capacity of the right liver graft (Liu et al, 2003).
Donor Left Hepatectomy
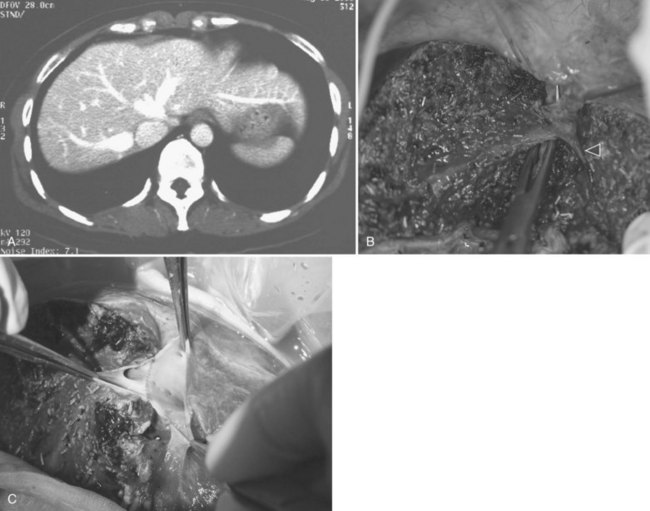
For a donor with a low body mass index, an upper midline incision can provide adequate access for the procedure. The left coronary ligament is taken down, and adequate mobilization of the left liver requires division of the ligamentum venosum or Arantius ligament between ligatures. The left hepatic duct, which may have a short extrahepatic course, is marked by a large metal clip, and the planned line of division is verified by cholangiogram under fluoroscopic guidance. Special attention is given to identify an insertion of the right posterior sector duct into the left hepatic duct (Fig. 90D.8). In this situation, the left hepatic duct can only be divided to the left of the right posterior sectoral duct. The middle hepatic artery should be preserved, when it arises from the left hepatic artery. A middle hepatic artery that is not large and arises from the right hepatic artery can usually be sacrificed, and segment IV will be supplied adequately by collaterals from the left hepatic artery. A replaced left hepatic artery arising from the left gastric artery allows a good length of isolated artery (Fig. 90D.9). Temporary inflow control of the left hepatic artery and portal vein by the vascular clamp reveals the Cantlie line. Liver transection is to the right of the MHV by CUSA. A low insertion of the segment VIII hepatic vein into the MHV calls for caution in the final part of the liver transection; this segment VIII vein should be preserved for venous outflow of the right anterior sector of the remnant right liver, and inadvertent damage of the vein results in torrential bleeding. When compared with donor right hepatectomy also including the MHV, blood loss is more significant, and the operation time is longer; these are likely the results of a larger liver transection surface (Chan et al, 2007b).
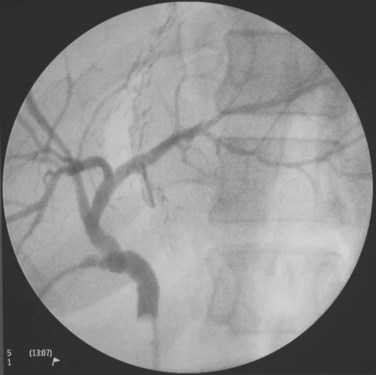
FIGURE 90D.8 Operative cholangiogram with a large metal clip marking the planned line of division of the left hepatic duct.
Anatomic Anomalies of the Donor (See Chapter 1B)
Besides inadequacy of size and proportion of the donor liver, occasional anatomic anomalies of the donor liver may preclude liver donation. When a major segmental branch of the right portal vein arises from the left portal vein intrahepatically, both right and left hepatectomy become too hazardous for the donor, so such a finding contraindicates donation. On the other hand, in the case of trifurcation of portal vein branches, if these are extrahepatic, donor operation is still feasible (Fig. 90D.10A); however, the right anterior and posterior branches of the portal veins require venoplasty to conform into a single cuff prior to graft implantation (Fig. 90D.10B). If the two branches are too short to merge into one, the portion of the recipient’s portal vein at the bifurcation can be used as an interpositional graft, using the recipient portal vein (Fig. 90D.10C). These are done on the back table. The segment III or IVb hepatic vein could at times drain into the MHV (Fig. 90D.11A and B); however, MHV to RHV venoplasty is still feasible (Fig. 90D.11C).
Biliary tract anomalies occasionally render a liver graft unsuitable, as in the case of multiple small branches in the graft (Liu et al, 2004).
Liver Graft Back-Table Procedure: Perfusion and Trimming
To shorten the cold ischemic time, the right liver graft is not delivered until the recipient is almost ready for graft implantation. Once delivered, the graft is flushed with three times the graft volume of a cold solution of histidine-tryptophan-ketoglutarate (HTK; Dr. Franz Köhler, Chemie GmbH, Alsbach-Hähnlein, Germany), while being immersed in an ice-sludge basin on the back table. The right portal vein is cannulated and adapted to the vein wall with fingers and not ligature (Fig. 90D.12A; Chan et al, 2004b). The right liver graft with separate right anterior and posterior portal veins requires simultaneous flushing with two cannulae (Fig. 90D.12B). The right hepatic artery is flushed with 100 drops of HTK solution through a 21-Fr angiocatheter by gravity (Fig. 90D.12C). Inadvertent damage to the artery intima can result in unrecoverable graft loss. The right anterior and posterior sectoral ducts are also flushed with cold HTK solution to minimize duct damage during preservation. The graft is weighed and transferred to another basin with cold HTK solution. Venoplasty of the MHV with the RHV of the graft is then performed (Liu et al, 2003). Even though the MHV and the RHV are often at a distance of up to 2 cm (Fig. 90D.13A), they can be drawn together for fashioning of a single venous cuff (Fig. 90D.13B; Chan et al, 2005). Often misled by planar schematic drawings, some have a false perception of the inadequate length of the MHV wall portion of the venoplasty for anastomosis to the IVC. In practice, the IVC—which is tubular, with a venotomy made over the RHV stump—faces the venoplasty of the MHV and RHV. The portion of the recipient IVC, in between the RHV and MHV, also makes up for the deficit in length on the MHV not included in the graft to preserve the segment IVb hepatic vein for venous drainage of the remnant left liver of the donor.
Donor Postoperative Care
Donor hepatectomy, both right and left, is a major surgical procedure undertaken on a healthy volunteer. Meticulous postoperative care is the least that can be offered by the health care profession. Postoperatively, the donor is transferred to the intensive care unit for close monitoring of hemodynamics and respiratory function. Early mobilization and institution of chest physiotherapy lower the risks of deep vein thrombosis and chest infection. The donor will also receive a 6-week course of proton pump inhibitor for peptic ulcer prophylaxis (Chan et al, 2007c). Females on hormone replacement therapy and those taking oral contraceptives require aggressive prophylaxis against deep vein thrombosis.
Donor Mortality and Morbidity
Donor safety is fundamental to LDLT (see Chapter 98B). As the application of LDLT has been extended from the child to the adult, and from using the left liver to using the right, the dilemma between recipient success and donor risk has come to the spotlight. The reported overall complication rate of donors is around 20% but has been reported as high as 67% in one series (Beavers et al, 2002). A unified system of complication reporting (Dindo et al, 2004) may narrow this range. Although even one donor mortality is too many, there are at least 19 known donor deaths (Trotter et al, 2006). The estimated mortality for donor right and left hepatectomy is 0.1% and 0.5% respectively (Barr et al, 2006), although mortality is lower in a multicenter series in Japan (Hashikura et al, 2009). In achieving a 5-year recipient survival rate of 80%, it takes one donor life to save 160 recipients. Less tangible are the quality of life changes of the donor in comparison to the predonation state (Chan et al, 2006b). The long-term biologic consequences of donor hepatectomy are not fully known. Nevertheless, decreases in white cell count, platelet counts, and elevation of liver transaminases are demonstrable even 2 years after right liver donation (Chan et al, 2006c).
Conclusion
Donor mortality after LDLT remains a reality; however, by lowering the donor mortality and morbidity and improving recipient survival, the justification of LDLT becomes stronger. Donor hepatectomy is a major surgical operation on a healthy person who does not require operation, and it is thus an ethical challenge, and this procedure was once viewed by the medical community and society with caution and skepticism (Cronin et al, 2001; Strong, 1999; Surman, 2002). Donor procedures can only be partially justified by the benefit to a recipient who has no other treatment options. Our common ground is the commitment to provide care of the highest standard to the living liver donor and to perform donor operations in experienced centers (Malago et al, 2001). This is the only way to maintain the highest quality and to decrease donor mortality and morbidity.
Abecassis M, et al. Consensus statement on the live organ donor. JAMA. 2000;284:2919-2926.
Azoulay D, et al. Effects of 10 minutes of ischemic preconditioning of the cadaveric liver on the graft’s preservation and function: the ying and the yang. Ann Surg. 2005;242:133-139.
Barr ML, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81:1373-1385.
Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transpl. 2002;8:110-117.
Cescon M, et al. Right liver graft without middle hepatic vein reconstruction from a living donor. Transplantation. 2002;73:1164-1166.
Chan SC, et al. Tailoring donor hepatectomy per segment 4 venous drainage in right lobe live donor liver transplantation. Liver Transpl. 2004;10:755-762.
Chan SC, et al. Applicability of histidine-tryptophan-ketoglutarate solution in right lobe adult-to-adult live donor liver transplantation. Liver Transpl. 2004;10:1415-1421.
Chan SC, et al. Versatility and viability of hepatic venoplasty in live donor liver transplantation using the right lobe with the middle hepatic vein. Hepatobiliary Pancreat Dis Int. 2005;4:618-621.
Chan SC, et al. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217-2222.
Chan SC, et al. Donor quality of life before and after adult-to-adult right liver live donor liver transplantation. Liver Transpl. 2006;12:1529-1536.
Chan SC, et al. Long-term biological consequences of donor right hepatectomy including the middle hepatic vein in adult-to-adult live donor liver transplantation. Liver Transpl. 2006;12:259-263.
Chan SC, et al. Working up donors for high-urgency and elective adult-to-adult live donor liver transplantation. Liver Transpl. 2007;13:509-515.
Chan SC, et al. Effect of side and size of graft on surgical outcomes of adult-to-adult live donor liver transplantation. Liver Transpl. 2007;13:91-98.
Chan SC, et al. Toward current standards of donor right hepatectomy for adult-to-adult live donor liver transplantation through the experience of 200 cases. Ann Surg. 2007;245:110-117.
Clavien PA, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162.
Cronin DC, Millis JM, Siegler M. Transplantation of liver grafts from living donors into adults: too much, too soon. N Engl J Med. 2001;344:1633-1637.
de Villa VH, et al. Right lobe living donor liver transplantation: addressing the middle hepatic vein controversy. Ann Surg. 2003;238:275-282.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213.
Fan ST. Living Donor Liver Transplantation. Shenzhen: Takungpao; 2007. Available at http://www.ldlt.hk
Fan ST, et al. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336-340.
Fan ST, et al. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236:676-683.
Fan ST, et al. Determinants of hospital mortality of adult recipients of right lobe live donor liver transplantation. Ann Surg. 2003;238:864-869.
Fan ST, et al. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003;238:137-148.
Hashikura Y, et al. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994;343:1233-1234.
Hashikura Y, et al. Donor complications associated with living donor liver transplantation in Japan. Transplantation. 2009;88:110-114.
Heymsfield SB, et al. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979;90:185-187.
Imamura H, et al. Pringle’s manoeuvre in living donors. Lancet. 2002;360:2049-2050.
Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058-1060.
Lee S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812-814.
Liu CL, et al. Hepatic venoplasty in right lobe live donor liver transplantation. Liver Transpl. 2003;9:1265-1272.
Liu CL, et al. The right may not be always right: biliary anatomy contraindicates right lobe live donor liver transplantation. Liver Transpl. 2004;10:811-812.
Liu CL, et al. Safety of donor right hepatectomy without abdominal drainage: a prospective evaluation in 100 consecutive liver donors. Liver Transpl. 2005;11:314-319.
Lo CM, et al. Extending the limit on the size of adult recipient in living donor liver transplantation using extended right lobe graft. Transplantation. 1997;63:1524-1528.
Lo CM, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261-269.
Lo CM, et al. Safety and outcome of hepatitis B core antibody–positive donors in right-lobe living donor liver transplantation. Liver Transpl. 2003;9:827-832.
Malago M, et al. Ethical considerations and rationale of adult-to-adult living donor liver transplantation. Liver Transpl. 2001;7:921-927.
Merten GJ, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
Sano K, et al. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. 2002;236:241-247.
Strong RW. Whither living donor liver transplantation? Liver Transpl Surg. 1999;5:536-538.
Surman OS. The ethics of partial-liver donation. N Engl J Med. 2002;346:1038.
Tanaka K, Yamada T. Living donor liver transplantation in Japan and Kyoto University: what can we learn? J Hepatol. 2005;42:25-28.
Trotter JF, et al. Documented deaths of hepatic lobe donors for living donor liver transplantation. Liver Transpl. 2006;12:1485-1488.
Urata K, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321.
World Health Organization. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163.
Yamaoka Y, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation. 1994;57:1127-1130.