Chapter 90B Hepatic resection for benign disease and for liver and biliary tumors
Overview
Hepatic resection for removal of lesions of the liver may be necessary for a wide variety of conditions (Box 90B.1). Indications for partial hepatectomy include the treatment of primary benign or malignant growths of the liver and biliary tract or metastatic tumors, with the latter being the most common. Hepatic resection also may be necessary in the management of complex or symptomatic cystic disease involving the liver (see Chapter 68, Chapter 69A, Chapter 69B ) and to allow access for repair of selected benign biliary strictures (see Chapter 42A, Chapter 42B ). Liver resection occasionally is necessary in the management of hepatic trauma. More recently, liver resection in living donors has been used in liver transplantation (see Chapter 98B).
Box 90B.1 Most Common Conditions for Which Liver Resection May Be Used
The principles of segmental and subsegmental resection are not new (Nagasue et al, 1985), and they have been developed and refined through the years. Utilization of intraoperative ultrasound (IOUS) (Bismuth & Castaing, 1984; Castaing et al, 1985; Scheele, 1989) to identify intrahepatic structures (see Chapter 21) and a mastery of intrahepatic anatomy are paramount to performing safe resections. This chapter focuses on major resections, including right hepatectomy, left hepatectomy, and extended right (including segment IV) and left hepatectomy (including the right anterior sector). Commonly performed segmental resections, including caudate lobe resection, are briefly described. A more detailed description of segment-oriented resection of the liver is provided in Chapter 92.
Hepatic resection in patients with cirrhosis is further complicated by the presence of portal hypertension and a concomitant functional deficit of the future liver remnant (FLR) (see Chapter 90F). Liver resection for the removal of gallbladder carcinoma and cholangiocarcinoma at the confluence of the bile ducts can be especially troublesome in the postoperative period, because resection is frequently performed in the presence of biliary obstruction, bacterial contamination, and infection.
General Principles
Benign Disease
Partial hepatectomy for the removal of benign tumors or cysts of the liver generally should be performed only for symptomatic patients, in the presence of doubt as to the diagnosis, or for tumors with known malignant potential (see Chapter 79A, Chapter 79B ). Removal of normal liver tissue should be kept to a minimum, thus when intervening for benign lesions, use of techniques for enucleation should be considered. Such enucleative methods may be utilized in the management of hemangioma, adenoma, and fibronodular hyperplasia. Enucleation also may be appropriate for tumor debulking of metastatic neuroendocrine tumors. The technique and principles of enucleation are described in the operation of pericystectomy for the removal of hydatid cysts of the liver (see Chapter 68).
Liver resection may be necessary in the management of some complex benign biliary strictures, especially when associated with ipsilateral liver atrophy (see Chapter 42A, Chapter 42B ). Some benign biliary conditions that sometimes necessitate resection include intrahepatic stones associated with recurrent pyogenic cholangitis (see Chapter 44) and Caroli disease, a congenital cystic disease of the liver (see Chapter 46, Chapter 69A, Chapter 69B ). In these situations, the amount of normal hepatic parenchyma removed should be kept to a minimum in accomplishing the goal of the resection. Liver resection for hydatid disease is discussed in Chapter 68, and resection for hepatic trauma is discussed in Chapter 102.
Malignant Disease
When performing partial hepatectomy for malignant tumors, the guiding principle is to obtain negative margins of resection while maintaining patient safety. Ideally, the closest margin distance from the tumor edge should be approximately 1 cm (Are et al, 2007); however, the margin distance must often be sacrificed, both for patient safety and for preservation of major vascular structures in close proximity to the tumor that are required to sustain the future liver remnant. That said, complete resection with a close margin is still associated with improved survival, and thus the inability to obtain a wide margin based on preoperative imaging should not necessarily be a deterrent to resection (Pawlik et al, 2005; Poultsides et al, 2010). Furthermore, an effort should always be made to perform parenchyma-sparing resections without sacrificing oncologic efficacy.
The difficulty of achieving an adequate margin of clearance when performing wedge resections for peripherally situated tumors should not be underestimated. The deep margin is the most difficult to judge for adequate tumor clearance. Consequently, it has been shown that wedge resections can carry an unacceptable local recurrence rate (DeMatteo et al, 2000; Gall & Scheele, 1986), although this has not been universally observed (Zorzi et al, 2006). Another factor that may compromise wedge resections is the fact that the liver has a tendency to fracture at the interface between tumor and normal parenchyma. This is particularly a problem for hard tumors in a soft liver, as is often the case with metastatic colorectal carcinoma; thus the surgeon must maintain extreme caution during parenchymal transection for wedge resections to avoid a close margin, a problem best averted by transecting along anatomic planes defined by inflow and outflow structures.
Patients undergoing palliative resections for metastatic neuroendocrine tumors that are not amenable to complete resection may be good candidates for enucleation, as is often done for benign lesions (see Chapter 81B). Gallbladder carcinoma and cholangiocarcinoma situated at the confluence of the hepatic bile ducts frequently occlude biliary flow, and resection may be compromised not only by the adverse pathophysiology of obstructive jaundice, but also by infection that may have been introduced before operation as a result of either percutaneous or endoscopic stent placement. In addition, perioperative outcome is complicated by the concomitant partial hepatectomy and resection of the extrahepatic biliary apparatus and subsequent biliary reconstruction (see Chapter 50B).
The presence of cirrhosis also introduces unique challenges for performing liver resection. Preoperatively, appropriate patient selection is paramount to ensure that the FLR is adequate and that the patient will be able to tolerate the procedure. Intraoperatively, a firm, nodular, and enlarged liver poses technical difficulties in mobilization and parenchymal transection that may also contribute to increased blood loss secondary to portal hypertension. Postoperatively, the increased risk of liver insufficiency, hepatic failure, and increased susceptibility to infection are major concerns. Thus, resection should be reserved for patients with cirrhosis who have relatively preserved liver function, and the principles of parenchyma-sparing resection should be strictly followed (see Chapter 90F).
Principal Hazards
The main hazards of hepatic resection are biliary leakage and bleeding (see Chapter 25). Biliary leakage is a particular problem in patients in whom biliary reconstruction is necessary, for example, as in the removal of a hilar cholangiocarcinoma. Bleeding from the hepatic veins and the inferior vena cava (IVC) during parenchymal transection is a major concern. Bleeding is more likely to occur during major resection of superiorly and posteriorly placed tumors, where clearance is minimal between the tumor margin and the passage of the hepatic veins into the vena cava, or when tumors are closely adherent or adjacent to the IVC (Fig. 90B.1).
Future Liver Remnant
A healthy, noncirrhotic liver may tolerate a resection of up to 80% of its volume because the liver’s enormous regenerative capacity enables functional compensation within a few weeks (see Chapter 5; Blumgart et al, 1971); however, such a favorable response cannot be taken for granted for extended hepatic resections. Only if reduction of the functional liver parenchyma is less than 50% can the risk of clinically significant liver insufficiency be virtually disregarded. Postoperative hepatic reserve is of particular concern after an extended left hepatectomy and when all of the parenchyma to the right of the falciform ligament is removed (extended right hepatectomy, which will be discussed later). Minimal to no risk of postoperative liver failure is present if most of the specimen volume has been replaced by an extensive tumor mass. In such patients, compensatory hypertrophy of the unaffected residual liver already has occurred preoperatively, and the loss of functional parenchyma is limited; however, a comparable volume of resection performed for multiple or unfavorably located smaller lesions carries a much greater risk of postoperative liver failure. Small tumors located adjacent to the inflow or outflow vessels of multiple segments may mandate removal of large amounts of functioning parenchyma.
Recently, some have advocated the liberal use of portal vein embolization (PVE) in this situation (see Chapter 93A, Chapter 93B ; Abdalla et al, 2002; Makuuchi et al, 1990; Palavecino et al, 2009); however, evidence is insufficient to suggest that PVE is associated with improved postoperative results in patients with normal parenchyma (Farges et al, 2003). In a series of 1800 liver resections performed at Memorial Sloan-Kettering Cancer Center (MSKCC), significant postoperative liver dysfunction and associated death occurred in only six patients (Jarnagin et al, 2002). In this series, PVE was utilized sparingly, and preoperative measures of liver function were not used routinely, such as the indocyanine green retention test; however, in patients with a damaged or steatotic liver—more common in obese patients, those with diabetes, and patients who have received substantial chemotherapy—the risk of postoperative liver insufficiency and failure is increased (Behrns et al, 1998; Kooby et al, 2003a). PVE may prove to be justified in this group of patients undergoing major resections. Biliary obstruction with jaundice is common in patients with hilar cholangiocarcinoma, who also are at increased risk. PVE in these patients also may be justified in select cases (see Chapter 50B, Chapter 93A, Chapter 93B ; Seyama et al, 2003).
In the cirrhotic liver, liver regeneration is much less effective; impairment of liver function after resection is greater, may last longer, and may result in terminal liver failure. Liver failure is the most common cause of postoperative death after liver resection in patients with cirrhosis. Postoperative liver failure and postoperative mortality are related to the degree of preoperative liver decompensation and to the extent of nontumorous liver parenchyma removed. Liver failure may be precipitated by intraoperative bleeding, and a significant relationship has been found to exist between intraoperative blood loss, postoperative increase in serum bilirubin, and postoperative mortality. The occurrence of other postoperative surgical complications, such as abdominal infection, may also trigger postoperative liver failure. In addition, cirrhosis predisposes to multiorgan failure after hepatectomy. Although evidence supports the safety of major hepatic resection in cirrhotic patients with Child-Turcotte-Pugh (CTP) class A liver function (Fong et al, 1999a; Franco et al, 1990), it also suggests that PVE may improve perioperative outcomes by optimizing the FLR (Palavecino et al, 2009).
Portal Hypertension and Ascites
The formation of ascites is one of the most frequent complications seen after liver resection in cirrhotic patients, occurring in 80% of patients. The etiology is likely twofold, the result of an increase in portal hypertension and of the ligation and division of lymphatics in the hepatic pedicle and liver ligaments. Postoperative ascites is a serious complication. Gross abdominal distension can interfere with ventilatory function and may result in leakage and disruption of the abdominal incision. Leakage of large-volume ascites may also lead to major fluid, protein, and electrolyte losses. In addition, ascitic fluid may become infected, and this may be an irreversible complication that leads to patient demise. Incidentally, ascites formation can also occur after liver resection and after fenestration in patients with polycystic liver disease (see Chapter 69B).
Infection
Despite the nearly universal formation of fluid collections after liver resections, particularly in the resection bed, infection of this fluid is relatively infrequent in most patients; however, a serious risk of infection follows liver resection in patients with biliary obstruction, especially in the presence of preoperative radiologic or endoscopic manipulation, and in cirrhotic patients. Alterations of humoral and cellular immunity are thought to predispose to infection after abdominal surgery in patients with cirrhosis. This risk is particularly high in patients with ascites or jaundice (Rimola et al, 1986). We also have empirically observed a slightly increased rate of infected fluid collections in patients undergoing simultaneous resections of a colorectal primary along with the liver metastases. In these combined resections, we now often leave an intraabdominal drain near the liver resection bed.
Selection of Patients for Liver Resection
When selecting patients for liver resection, the oncologic appropriateness of the resection must be considered first and foremost, followed closely thereafter by the general condition of the patient. Once resection is determined to be reasonable, and the patient appears to be a candidate from a general health perspective, the technical aspects of the resection must then be considered, including the extent of resection and the quantity and quality of the FLR. If the tumor can be completely resected without compromising hepatic arterial and portal venous inflow, biliary drainage, and venous outflow of the remnant, and if the FLR is of sufficient size in a well-functioning liver, nearly all patients are candidates for hepatic resection. Careful consideration must be taken when deciding to perform a major resection to remove multiple or centrally situated small lesions in patients in whom compensatory hypertrophy of the residual liver has not yet occurred. The selection of patients with a benign biliary stricture for hepatic resection is described in Chapter 42A, and the role of liver resection in specific situations, such as for hilar cholangiocarcinoma and hemangioma, are described below.
When considering patients with compromised liver function or cirrhosis for hepatic resection, it is important to understand that, in addition to the disease extent and the general condition of the patient, the degree of hepatic dysfunction is a major prognostic factor. The CTP classification system (Child & Turcotte, 1964; Pugh et al, 1973) is a simple means of assessing liver function preoperatively. Although more sophisticated tests can be used (see Chapter 2), the CTP classification is sufficient for most situations. Also, a low preoperative platelet count is a good surrogate marker for portal hypertension and underlying liver disease that otherwise may not be detectable (Bruix et al, 1996; Maithel et al, 2010). Operative mortality is much higher in CTP class B or C patients compared with those with class A liver function. Liver resection is particularly hazardous in a patient with a Pugh score greater than 8 (CTP class B). Serum bilirubin concentration is also an important prognostic factor, as liver resection is ill advised in patients with a serum bilirubin greater than 2 mg/dL or in the presence of clinically detectable ascites.
The extent of hepatectomy is guided by tumor size and location, but in general, the ideal is to remove as little parenchyma as possible. Small tumors located deep in the right liver are challenging, as it is preferable to avoid performing a right hepatectomy in such cases. If right hepatectomy is necessary, preoperative PVE of the affected lobe may allow hypertrophy of the contralateral side (see Chapter 93A, Chapter 93B ); however, the utilization of alternative methods for liver-directed therapy to obtain tumor control in a cirrhotic patient should be considered, especially in patients with multifocal disease. Alcohol injection, transarterial chemoembolization—bland, conventional, and using drug-eluting beads—cryoablation, radiofrequency ablation, microwave ablation, yttrium-90 therapy, and liver transplantation for managing tumors of the liver are discussed in detail in Chapter 84A, Chapter 84B, Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D, Chapter 86 and in Chapter 98A, Chapter 98B, Chapter 98C .
Anatomy and Classification
Precise knowledge and mastery of the surgical anatomy of the liver, blood vessels, and biliary channels is essential for performing partial hepatectomy; this is described in detail in Chapter 1B. The liver is divided into sectors that comprise liver segments, each supplied by branches of the portal triads and drained by hepatic veins. Partial hepatectomy involves removal of one or more segments by isolation of the relevant portal pedicle, severance of the relevant hepatic veins and biliary drainage channels, and removal of the associated liver tissue.
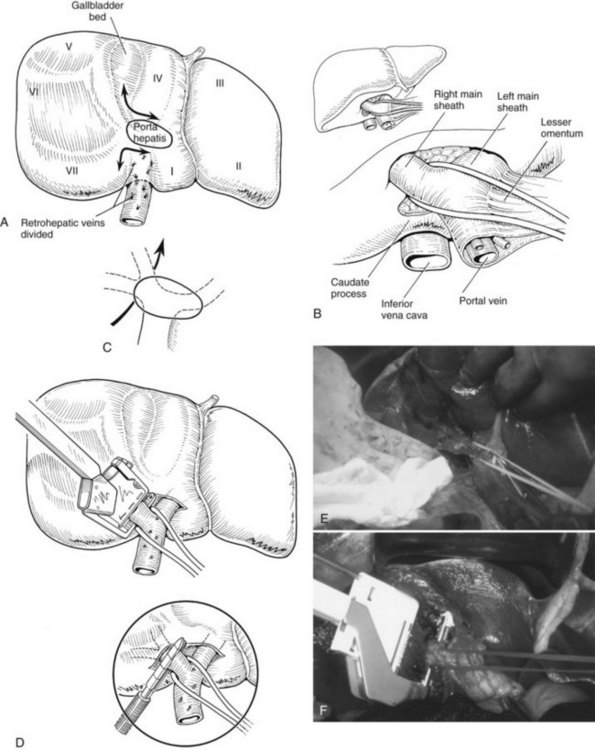
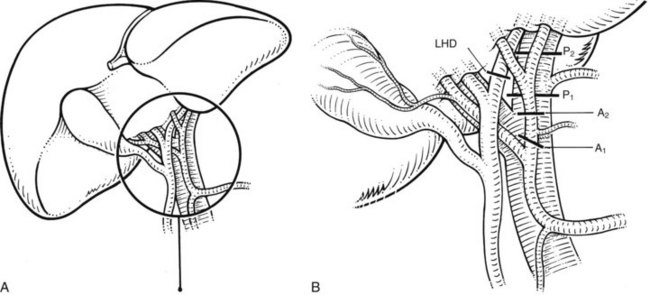
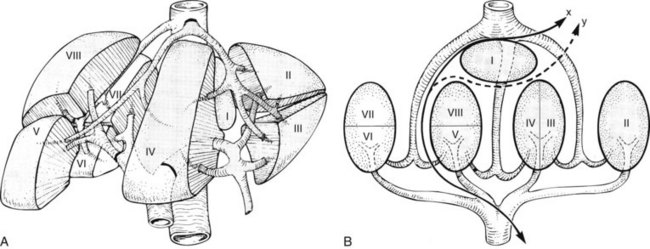
The anatomic division between the right and left liver is not at the falciform ligament, but rather follows a line projected through a plane—the principal plane or Cantlie’s line—running from the medial margin of the gallbladder bed to the left of the IVC posteriorly (Fig. 90B.2; Couinaud, 1954, 1957; Goldsmith & Woodburne, 1957; Healey & Schroy, 1953; McIndoe & Counseller, 1927). Each of these major right and left portions of the liver is divided into sectors and segments.
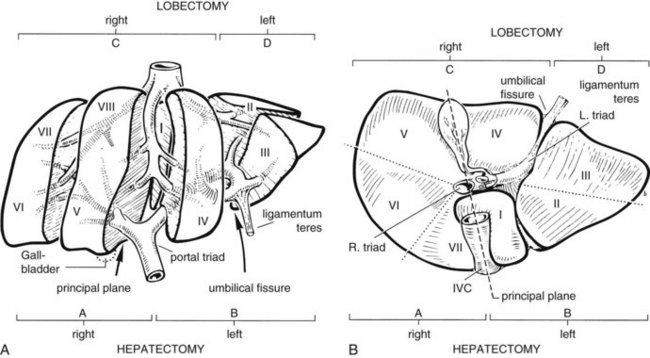
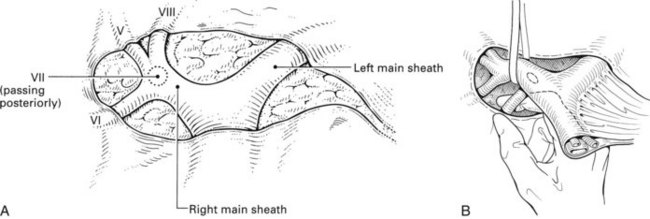
The Brisbane terminology dictates that the hepatic veins form the boundaries between the various sectors of the liver (Strasberg et al, 2000). Specifically, the medial margin of the right posterior sector is the right hepatic vein and comprises segments VI and VII. The right anterior sector is bordered by the right hepatic vein and middle hepatic vein, coursing along the principal plane, and it comprises segments V and VIII. Based on the Brisbane terminology, the area of liver that comprises segments IVa, IVb, and III forms the left medial sector; segment II forms the left lateral sector, because the left hepatic vein actually divides segment II from III. From a clinical perspective, resections of segments III, IVa, and IVb are rarely performed; furthermore, segment IVa/b is often referred to as the left medial sector, or the left medial section in Brisbane terminology, and it is bordered by the middle hepatic vein and umbilical fissure. The left lateral sector, or section, is comprised of segments II and III and represents the portion of liver to the left of the ligamentum teres and falciform ligament; thus in essence, the left liver is divided into a medial and lateral sector along the line of insertion of the ligamentum teres and the falciform ligament. This is the terminology that will be utilized for the remainder of this chapter. The portal venous and hepatic arterial branches conform to the segmental organization and run within the segments, and the draining hepatic veins converge posteriorly toward the inferior vena cava and mark the main scissurae of the liver (see Fig. 90B.2), within which they run (Couinaud, 1954, 1957).
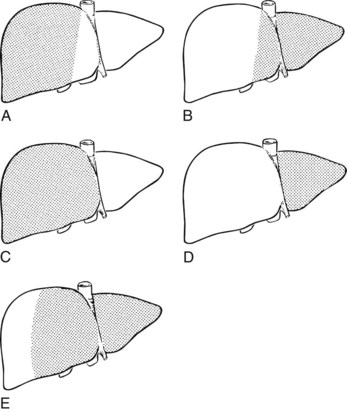
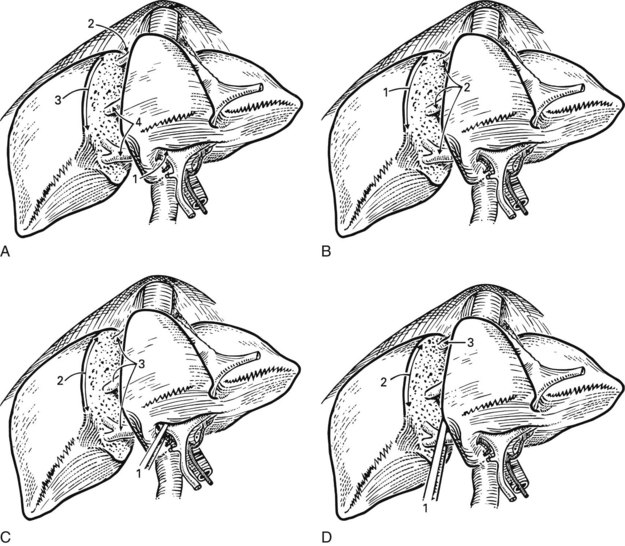
Practically speaking, five types of major anatomic resections are practiced (Fig. 90B.3; see also Fig. 90B.2). The nomenclature of these operations is based on the anatomic descriptions of Couinaud (1954, 1957) and of Bismuth (1982; Table 90B.1). This classification emphasizes the importance of the scissurae of the liver and the umbilical fissure. The alternative, more commonly used terminology of Goldsmith and Woodburne (1957) is also listed in Table 90B.1. A newer terminology proposed by the International Hepato-Pancreatico-Biliary Association in Brisbane (Strasberg et al, 2000) is also referred to in Chapter 1A, Chapter 1B and is discussed above. The five different resections depicted in Figure 90B.3 can also be referred to as 1) right hepatectomy, 2) left hepatectomy, 3) extended right hepatectomy, 4) left lateral sectorectomy, and 5) extended left hepatectomy.
| Couinaud (1957) | Goldsmith & Woodburne (1957) |
|---|---|
| Right hepatectomy (segments V, VI, VII, VIII; see Fig. 90B.3A) | Right hepatic lobectomy |
| Left hepatectomy (segments II, III, IV; see Fig. 90B.3B) | Left hepatic lobectomy |
| Right lobectomy (segments IV, V, VI, VII, VIII; sometimes includes segment I; see Fig. 90B.3C) | Extended right hepatic lobectomy* |
| Left lobectomy (segments II and III; see Fig. 90B.3D) | Left lateral segmentectomy |
| Extended left hepatectomy (segments II, III, IV, V, VIII; sometimes also segment I†; see Fig. 90B.3E) | Extended left lobectomy† |
* Right lobectomy (extended right hepatic lobectomy) represents right hepatectomy extended to segment IV and has been referred to by Starzl and colleagues (1975, 1980) as right trisegmentectomy; this term is commonly found in the literature.
† Also referred to as left trisegmentectomy (Starzl et al, 1982).
The portal vein and hepatic artery divide into major right and left branches outside the liver substance below the hilus (see Fig. 90B.2A). Here, when the overlying peritoneum is incised, it is possible to dissect each major branch beyond its bifurcation. The confluence of the right and left hepatic ducts also occurs outside the liver. At the hilum of the portal vein, the right branch is short, and its first branch often arises posteroinferiorly, close to the bifurcation, where it is easily damaged during dissection. By contrast, the left branch of the portal vein and the left hepatic duct pursue a longer, more horizontal extrahepatic course beneath segment IV and are more easily dissected free along their length.
The ligamentum teres (round ligament) runs sharply into the umbilical fissure of the liver, at the base of which the main vascular and biliary pedicle branches to the segments of the left liver (II, III, IVa, IVb). After entering the umbilical fissure, the left branch of the portal vein curves caudally and gives branches not only to the left lateral sector, segments II and III, but together with branches of the left hepatic artery, it gives rise to feedback vessels to the left medial sector—segment IVa and IVb of the left liver, also known as the quadrate lobe (see Fig. 90B.2A; Goldsmith & Woodburne, 1957; Starzl et al, 1975).
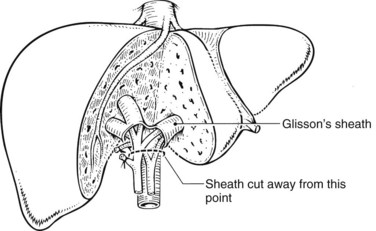
Outside of the liver substance, there is not a distinct sheath around the portal triad structures, and they must be dissected independently. The structures of the portal triad—the portal vein, hepatic artery, and bile duct—are covered with the Glisson capsule as they enter the liver parenchyma such that within the liver, they are contained in well-formed branching fibrous sheaths often referred to as pedicles. Thus, dissection outside of the liver substance mandates individual isolation of each portal triad structure, where there is considerable variation in anatomy. Pedicles are strong structures that contain the constituents of the portal triad, and intrahepatic dissection of these pedicles enables isolation and control of vascular and biliary elements without the need to individually expose each structure (Launois & Jamieson, 1992b).
The liver straddles the IVC immediately below the diaphragm, and the hepatic veins have a short extrahepatic course to empty into the vena cava (see Fig. 90B.2). This superior portion of the vena cava with the draining hepatic veins is masked behind a fold of peritoneum that extends from the cephalad aspect of the falciform ligament to the right and left triangular ligaments. The right hepatic vein emerges from the right scissura and usually enters the vena cava separately. By contrast, the left hepatic vein, which runs in the left scissura between segments II and III, is often joined by the middle hepatic vein, which occupies the principal scissura, before entering the vena cava. Several smaller veins from the posterior surface of the liver and the caudate lobe drain directly into the IVC.
An additional but important hepatic vein, the umbilical vein, runs beneath the falciform ligament and empties into the left hepatic vein. This vein is important in resections that remove segment IVa, because it provides drainage of segment IVb in the presence of ligation of the middle hepatic vein (see Chapter 92).
Preoperative Considerations
Preoperative Investigations
Preoperative investigations should seek to determine the nature of the lesion and give an index of resectability and the ideal surgical approach. Preoperative transcutaneous needle biopsy of liver tumor masses is not routinely necessary and is not always advisable (see Chapter 20). Preoperative biopsy should be pursued only if the information gained will alter the treatment plan. In the presence of a strong presumptive and clinical diagnosis of a potentially resectable liver tumor, we prefer to avoid the possibility of tumor dissemination, rupture, or hemorrhage, which may occur using percutaneous methods (Thompson et al, 1985). Laparoscopic exploration and visualization, particularly when used in combination with laparoscopic US, may provide valuable information regarding the presence of additional lesions not appreciated on preoperative imaging, and it may also reveal the presence of extrahepatic disease. By minimizing unnecessary laparotomy, this approach has been shown to increase the resectability rate of patients submitted to laparotomy (Jarnagin et al, 2000; John et al, 1994; Li Destri et al, 2008).
There are three major risks of hepatic resection: 1) hemorrhage, which may arise at the porta hepatis from branches of the hepatic artery or portal vein or posteriorly from hepatic venous radicles or the IVC; 2) biliary fistula or stricture as a result of damage to the biliary apparatus; and 3) postoperative liver insufficiency or failure. Intraoperative bleeding complications are most likely to occur when resecting tumors or other lesions that are large and in close proximity to the IVC and/or major hepatic veins (Hawkins et al, 2005). Similarly, tumors near the hilus at the confluence of the hepatic ducts and division of major vessels as they enter the liver substance can be challenging to remove. Given the numerous anatomic variations in the vascular and biliary anatomy of the liver, preoperative cross-sectional imaging should be routinely obtained and evaluated for important spatial relationships between tumor and these structures.
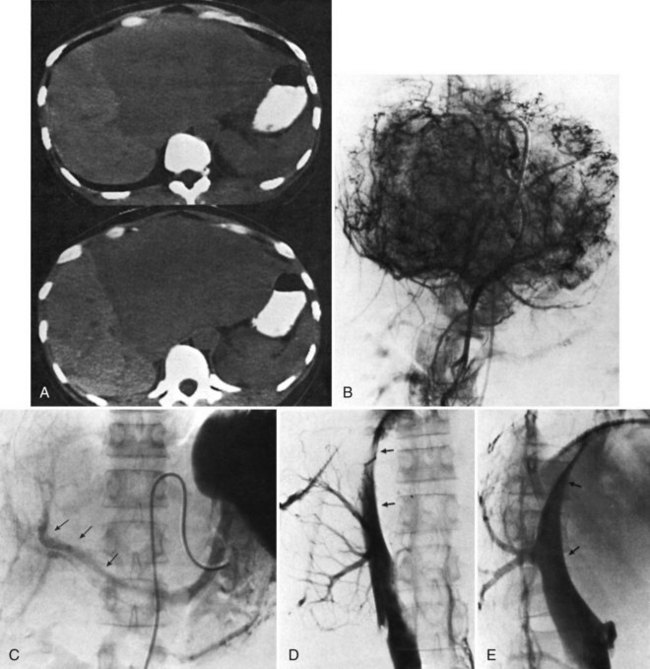
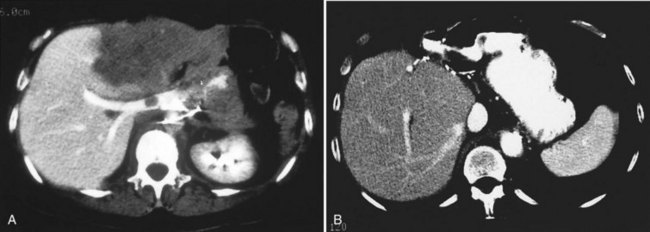
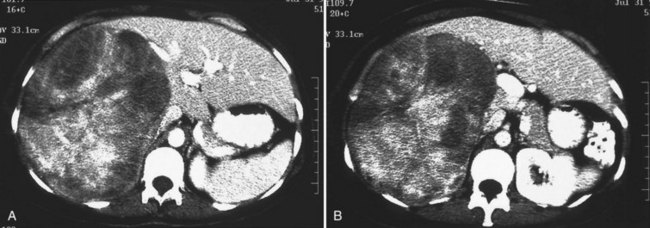
One such modality that provides valuable information in this regard is computed tomography (CT) (see Chapter 16). Peripherally placed lesions, unless substantially large, rarely involve the hilar structures or vena cava; however, more centrally situated lesions may involve major vessels close to the hilus, and appropriate CT scanning (see Chapter 16) can be most helpful in creating an image of the extent of disease and degree of vascular compromise by the tumor (Fig. 90B.4). Liver tumors can sometimes respect intersegmental planes and push structures away rather than invade them directly. This can be demonstrated not only by CT scan (Figs. 90B.5 and 90B.6), but also can be confirmed by angiographic methods (see Fig. 90B.1) or by duplex ultrasound (US). However, with the advancement in technology and the high-quality images obtained with cross-sectional imaging, direct angiography is rarely necessary. Other tumors may hang from the liver in a pedunculated fashion (Baer et al, 1989), and they may be attached to the liver by a relatively small base, even when very large. Such “hanging” tumors are readily depicted on CT scan (Fig. 90B.7). Tumor involvement of the diaphragm also may be suggested by CT, but likewise, this should not deter the surgeon from attempted resection, because such apparent diaphragmatic involvement from metastatic tumors is frequently secondary to an adhesion to a site of previous necrosis of the tumor and not actually from direct tumor invasion (Weinbren & Blumgart, 1986). Primary tumors may still be approached for resection as well, even if they involve the diaphragm (Foster & Berman, 1977).
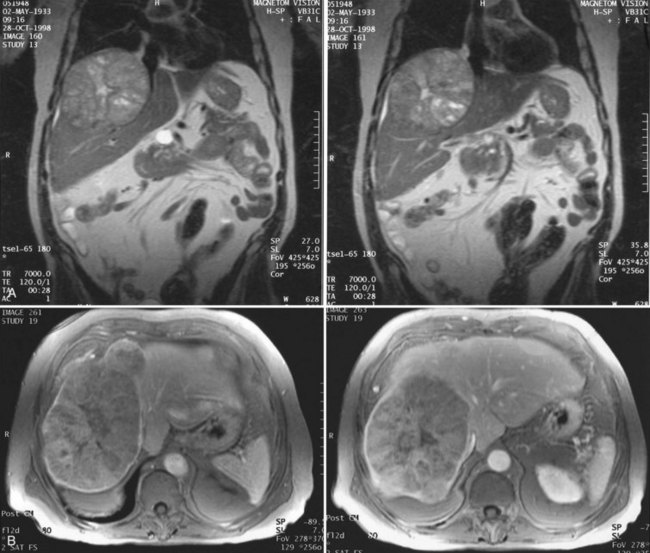
Magnetic resonance imaging (MRI; see Chapter 17) has become increasingly valuable in the imaging of the liver before resection. Masses are well characterized, as are the presence of additional lesions sometimes not visualized on CT scan. MRI depicts major blood vessels accurately and is particularly valuable in the demonstration of the major hepatic veins and vena cava and their relationship to tumor masses (Fig. 90B.8). Compared to CT, MRI also may be more accurate in differentiating primary and secondary malignancies of the liver from benign lesions such as adenoma, focal nodular hyperplasia (FNH), or cysts.
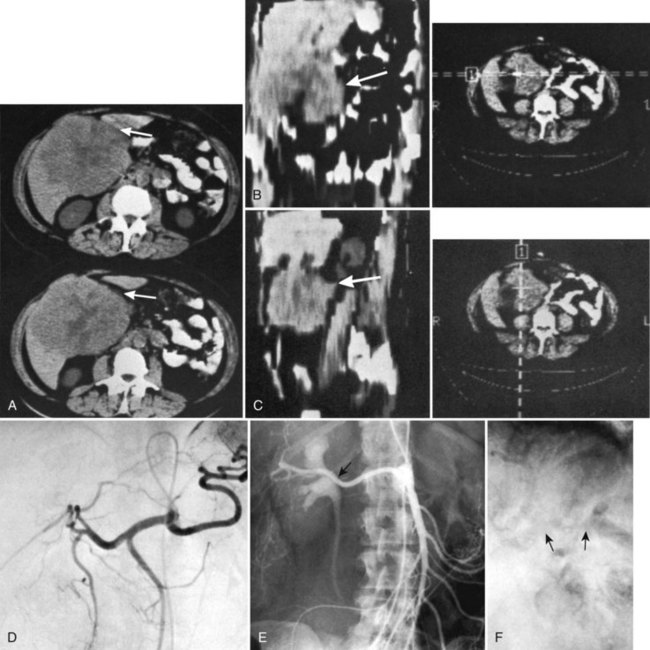
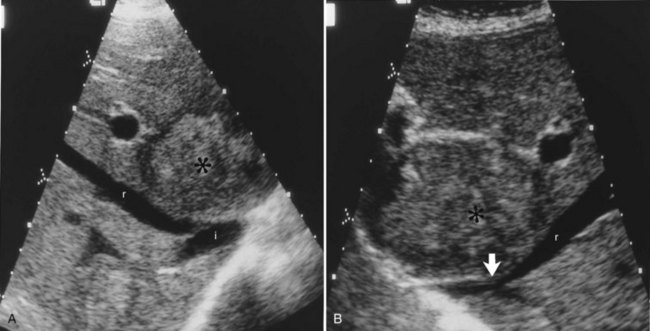
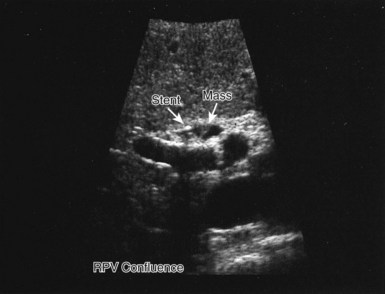
US performed by an experienced operator is also of value in the preoperative assessment of multiple tumors, as it gives information regarding the size of the tumor and extent of liver involvement. US also may help distinguish cysts from solid tumors, and duplex US demonstrates tumor location with respect to important vascular structures, including the hepatic veins and vena cava (Gibson et al, 1986), and it is a valuable technique to further define blood vessels after initial CT scanning (Fig. 90B.9; Hann et al, 1998). US can be of particular importance in the preoperative evaluation of hilar cholangiocarcinoma by defining the extent of involvement of the portal vein and hepatic artery (Fig. 90B.10; Hann et al, 1997); however, as MRI and CT scan continue to produce higher quality images, the need for preoperative US evaluation has declined.
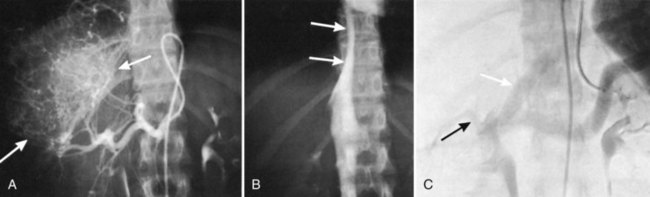
Hepatic angiography and inferior venacavography (see Chapter 19) usually are not necessary and should not be used except perhaps in the instance of large hemangiomata, for which precise information as to the arterial supply of the lesion may assist at surgery, although modern advances in MRI and duplex US have all but rendered these studies obsolete. In the event that adequate US or MRI is unavailable, angiography still has a role in imaging (Blumgart, 1982; Voyles et al, 1983). Some figures of angiographic techniques are provided for illustrative purposes (Fig. 90B.11; see also Figs. 90B.1 and 90B.7), but it should be emphasized that they are unnecessary in most patients. In our practice, hepatic angiography is rarely obtained for the preoperative demonstration of extrahepatic arterial anatomy in patients being considered for placement of a hepatic arterial infusion (HAI) pump for postoperative adjunctive chemotherapy (see Chapter 86).
Other Investigations
To determine the oncologic appropriateness of hepatic resection, a thorough evaluation for the presence of extrahepatic disease should be performed by means of chest radiography, CT, or MRI before major hepatic resection is undertaken. When evaluating the chest, subcentimeter pulmonary nodules are frequently encountered that should not necessarily preclude hepatic resection, particularly for colorectal cancer liver metastases (Maithel et al, 2010). Positron-emission tomography (PET) scan may also be an important tool in the evaluation of the extent of spread of malignant disease (see Chapter 15; Beets et al, 1994; Fong et al, 1999a; Lai et al, 1996; Schüssler-Fiorenza et al, 2004).
If jaundice is present, and biliary involvement by the tumor is suspected, direct cholangiography may be carried out, either by endoscopic retrograde cholangiography (ERCP) or by percutaneous transhepatic cholangiography, to delineate the biliary anatomy (see Chapter 18); however, US and MRI cholangiography are noninvasive studies in many ways superior to direct cholangiography, and they have all but replaced preoperative invasive cholangiography for assessing tumor involvement of the bile ducts, such as in hilar cholangiocarcinoma (see Fig. 90B.10; see also Chapter 52). Of course, if preoperative biliary drainage is necessary, direct cholangiography, either endoscopic or percutaneous, will be required.
Staging Laparoscopy
Staging laparoscopy and laparoscopic US are widely used to prevent unnecessary laparotomy in patients with hepatobiliary malignancy. Staging laparoscopy can significantly improve resectability rates at laparotomy, and laparoscopic evaluation has its greatest yield in detecting peritoneal metastases not detectable on cross-sectional imaging rather than identifying additional hepatic lesions. Furthermore, because of their location, laparoscopic evaluation often fails to identify lymph node metastases (Jarnagin et al, 2000; John et al, 1994; Lo et al, 1998). In one study at MSKCC (Jarnagin et al, 2000), 83% of patients subjected to laparotomy after laparoscopy underwent potentially curative resection, compared with only 66% of patients not staged laparoscopically (see Chapter 21).
Assessment of Resectability
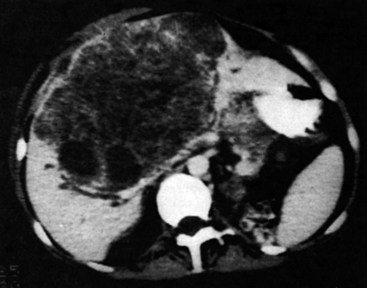
Major difficulties with accurate preoperative assessment of resectability can arise in two situations: First, tumors situated near the major hilar structures (see Fig. 90B.7) or those markedly compressing (see Fig. 90B.11) or involving the vena cava can be difficult to assess preoperatively. Involvement of major structures, such as invasion of bile ducts or portal venous branches, or apparent involvement of the IVC does not necessarily preclude resection (see Fig. 90B.1; Soreide et al, 1985). Second, very large tumors, which push structures aside and have been slowly growing over a long time, are difficult to define precisely, because pressure changes may mimic invasion on radiologic images. Thus, considerable caution must be exercised before declaring such cases unresectable. In patients with tumors of this nature, it is often difficult at initial exploration to be certain of resectability, and considerable dissection and mobilization is necessary before a clear-cut decision can be made. The morphologic configuration of tumors on imaging studies has been shown to have a clear relationship to subsequent resectability. Tumors that expand and compress surrounding tissues (pushing tumors) and tumors that are pedunculated and attached to the liver by a base of narrow width (hanging tumors) are nearly always resectable, and they can be distinguished from tumors that are irregular at the margins and clearly invasive (Baer et al, 1989).
In the early years of hepatic resection, the editor emeritus (Leslie H. Blumgart) adopted and promoted a deliberate policy of full investigation at laparotomy to assess resectability in all patients with solitary large liver tumors, provided no positive evidence of extrahepatic spread was apparent, patients were otherwise fit for major surgery, and the residual liver would be adequate. Of 22 patients with primary liver cancer evaluated in an early study, 13 patients were explored, and resection was carried out in all 13. Only three (23%) of the 13 underwent either a right or left hepatectomy or lesser procedure; the rest were submitted to extensive resections crossing the principal scissura (Cantlie’s line) and necessitated extended resection (see Fig. 90B.11). Two of these 13 patients had tumor embolization before surgery to reduce vascularity and tumor size. Eight of these tumors were found to involve the right and left liver on CT scan; involvement of the main portal vein by tumor extension was found in two patients, one patient had involvement of the right branch of the portal vein, and six patients had severe compression or involvement of the IVC (see Fig. 90B.11). Although extrahepatic involvement was suspected in 3 patients, the resections carried out were potentially curative in 12 of the 13 patients with macroscopic clearance of all tumor; but in 1 patient with fibrolamellar hepatocellular carcinoma (HCC), a deliberate extended resection was performed in the presence of regional lymph node metastases. No intraoperative or in-hospital (30-day) deaths were reported (Soreide et al, 1985). This early experience has been replicated in subsequent studies (Vauthey et al, 1993) and in studies by many other investigators.
The resectability rate within a specialized hepatobiliary surgery unit does not necessarily indicate the overall resectability rate for a particular tumor, because the patient population is highly selected; however, it does seem that an aggressive surgical policy is justified, even in patients with large solitary tumors. In particular, the size of the tumor alone is not a contraindication to resection, although for HCC, large tumor size is associated with earlier recurrence and reduced survival (Pawlik et al, 2005; Nathan et al, 2009). Other authors agree with this view and advocate an aggressive approach to resection (Adson & Weiland, 1981; Balasegaram & Joishy, 1981; Okamoto et al, 1984; Starzl et al, 1982; Steele et al, 1984).
As discussed earlier, radiologic demonstration of apparent involvement of major inflow and outflow vessels does not constitute an absolute contraindication to exploration for resection. Intraoperative techniques are now available to deal with portal vein and IVC involvement. This is particularly applicable to the fibrolamellar variant of HCC (Berman et al, 1980; Craig et al, 1980; Hemming et al, 1997; Nagorney et al, 1985; Soreide et al, 1986), as it tends to be less invasive to surrounding structures and is thus more readily removable. Furthermore, it may have a more favorable prognosis than standard HCC, and its presence should be suspected in patients who have a nonelevated α-fetoprotein with a large tumor in the background of a normal liver; other markers—particularly plasma neurotensin levels (Collier et al, 1984), vitamin B12 binding capacity (Paradinas et al, 1982), or both are increased with this tumor.
Some patients with primary HCC present with biliary tract obstruction secondary to tumor emboli within the bile ducts. These emboli may be shown by direct cholangiography, MRI cholangiography, or US. This presentation should not necessarily preclude an attempt at resection (Blumgart, 1978, 1980).
It has been stated that young patients with large, unresectable HCC are suitable candidates for transplantation, and many physicians and surgeons see liver transplantation as the only therapeutic alternative in such cases. This view seemed to be supported earlier by the National Institutes of Health (NIH) Consensus Development Conference Statement on Liver Transplantation (NIH, 1983); however, subsequent experience did not support this broad approach, and controversy emerged regarding the place of liver transplantation for the management of liver tumors. More recently, transplantation has emerged as an effective and viable treatment option in patients with cirrhosis and limited neoplastic disease, with the Milan criteria now firmly established for guiding patient selection (Mazzaferro et al, 1996). This issue is discussed further in Chapter 97D.
Finally, portal venous involvement in cholangiocarcinoma affecting the confluence of the bile ducts does not always preclude resection (Blumgart et al, 1984; Burke et al, 1998). This topic is fully discussed in this chapter and also in Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D .
Anesthetic Considerations (See Chapter 22)
To minimize bleeding during parenchymal transection, the anesthesiologist must maintain a low and controlled central venous pressure (CVP) below 5 mm Hg. For patients with relatively good health status, central venous access is not mandatory, as long as large-bore peripheral intravenous access is obtained, and the patient is not given much fluid until parenchymal transection is complete. To prevent air embolism, the dissection can be performed with the patient in a 15-degree Trendelenburg position. Low CVP is maintained at the desired level using a combination of minimal fluid resuscitation and agents that promote venodilation. A minimal intraoperative urine output (20 to 25 mL/h) is tolerated until parenchymal transection is complete (see Chapter 22; Cunningham et al, 1994; Melendez et al, 1998). Once the specimen is removed, hemostasis is achieved, and the patient is resuscitated.
Every effort should be made to avoid perioperative blood transfusion, as allogeneic red blood cell transfusion has been associated with worse perioperative outcome and possibly earlier cancer recurrence and shortened survival, although the latter remains controversial (Amato & Pescatori, 1994, 1998, 2006; Kooby et al, 2003b). The previous dictum of maintaining the hemoglobin at 10 gm/dL or hematocrit at 30% is no longer standard practice (Hebert et al, 1999). Allogeneic blood transfusion should be reserved for excessive blood loss and hemodynamic instability. A technique of acute normovolemic hemodilution, in which the patient’s blood is removed intraoperatively and replaced with a combination of crystalloid and colloid fluids, can also reduce the need for allogeneic blood transfusion (Matot et al, 2002; Jarnagin et al, 2008; Maithel & Jarnagin, 2009); the blood that is lost is hemodiluted and contains less red blood cell mass. Once the dissection is complete, the blood removed from the patient earlier is reinfused, which minimizes the need for allogeneic transfusion. The body temperature should be carefully monitored, and normothermia is maintained with warmed fluids and external warming devices.
Should vascular isolation of the liver be necessary, the anesthetic management is slightly altered, because it requires the maintenance of cardiac output during cross clamping of the vena cava. We rarely use vascular isolation of the liver, even during the removal of large tumors close to or involving the hepatic veins and/or the IVC. Even tumors directly involving the vena cava may be removed without vascular isolation. Nevertheless, some authors (Bismuth et al, 1989; Huguet & Gavelli, 1994; Huguet et al, 1978, 1992a, 1992b) have advocated vascular isolation techniques during hepatic resection, especially for large tumors. Other authors use this method for even limited resections (Kelly et al, 1996), but it is often unnecessary. Techniques for vascular isolation are discussed fully in Chapter 91A.
Postoperative analgesia can be delivered in the form of intravenous or epidural analgesia. The ability to metabolize narcotics after major liver resections may be reduced, thus patients must be monitored carefully for oversedation and respiratory depression. Our preference is to avoid using epidurals for major liver resections because of the potential for hemodynamic compromise, from the reduction in sympathetic tone and the rise in international normalized ratio (INR) that prevents epidural removal, and its association with increased risk of blood transfusion (Page et al, 2008).
Operative Procedures
Patient Positioning
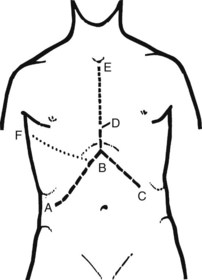
The patient should be positioned supine with the right arm extended at a right angle to the body. It is unnecessary to place the patient in a lateral decubitus position, as tilting the operating table allows for adequate exposure during operation. Electrocardiogram (ECG) leads should be kept clear of the right chest wall and the presternal area, and draping should expose the lower chest up to the nipple line and the entire upper abdomen to below the umbilicus (Fig. 90B.12). This exposure is adequate for most hepatic resections. If preliminary laparoscopy is to be performed, it is done at this stage.
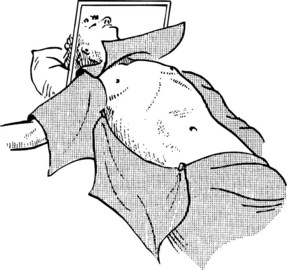
FIGURE 90B.12 Position of the patient on the operating table. Note the wide exposure of the abdomen and chest. The cross bar later holds a large retractor, used to elevate the costal margin (see also Fig. 90B.14).
For large, posteriorly lying tumors, a thoracoabdominal incision may be necessary, but this can usually be avoided with right lateral extension of the incision with good costal margin retraction. The abdomen should be widely exposed from midaxillary line to midaxillary line to allow adequate space for a transverse incision. A cross bar or similar device should be fitted to the table to hold a self-retaining retractor later to elevate the costal margin (see Fig. 90B.12). In patients in whom extracorporeal venovenous bypass is to be performed in conjunction with complete vascular isolation of the liver, or in those whose back-table resection is to be done in association with autotransplantation, or for cases of regional hepatic perfusion (see Chapter 89), the right and left groins, left axilla, and/or left neck should be prepared to allow access to the femoral vein, axillary vein, or internal jugular vein respectively.
Incisions
The incision depends on circumstances (Fig. 90B.13), and the surgeon should not hesitate to extend or enlarge the wound to gain adequate access. For most hepatic resections, the initial incision should be an extended right subcostal incision (ABD in Fig. 90B.13) or a bilateral subcostal incision with a vertical extension. A slight variation of this incision that affords excellent exposure for most resections is an upper midline incision from the xiphoid process to approximately 2 to 3 cm above the umbilicus with right lateral extension toward a point midway between the inferior costal margin and anterior superior iliac spine. A limited upper midline incision may be made first to allow preliminary manual and visual exploration to exclude obviously unresectable disease or the presence of extrahepatic spread. In some situations, particularly for left-sided resections in thin patients, a midline incision is adequate. Large tumors of the right side of the liver, especially those lying posteriorly and involving the IVC, may require a right thoracoabdominal approach, but this is rarely necessary. Our experience in more than 2000 cases of liver resection necessitated a thoracoabdominal incision in only 3%. A median sternotomy gives good access, is rapid, and spares disruption of either pleural cavity, but it is also seldom required. If a median sternotomy is used, the diaphragm may be split through the central tendon to allow excellent exposure of the suprahepatic IVC and the major hepatic veins, and the open pericardium can be used to allow direct access to the intrapericardial IVC.
Exposure, Mobilization, and Assessment
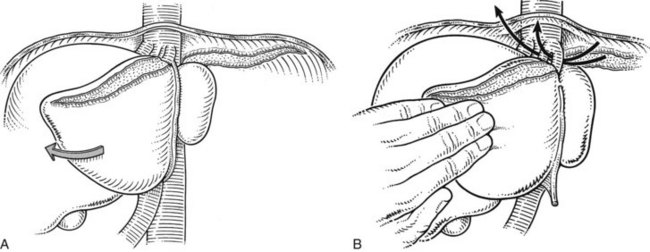
Prior to exploration of the liver, the ligamentum teres is divided, and the falciform ligament is incised and separated from the anterior abdominal wall (Fig. 90B.14A). The falciform ligament is divided as far cephalad as the subdiaphragmatic IVC (see Fig. 90B.14, B and C). Vessels running within the falciform ligament are usually controlled with bovie electrocautery. A long suture should be left on the ligamentum teres to act as a retractor during subsequent exploration and dissection for manipulating the liver.
Mobilization of the right liver begins with complete division of the falciform ligament and identification of the right hepatic vein. As the falciform ligament fibers begin to splay at the back, the surgeon must exercise caution, as the hepatic veins are in close proximity. Early identification of the right hepatic vein prevents subsequent injury during the lateral and posterior mobilization. Once the right vein is visualized, the peritoneal reflection at the edge of the right triangular ligament is incised laterally, and the incision is developed medially between the diaphragm and the liver, until the entire bare area of the liver on the right is exposed (Fig. 90B.15); this allows the liver to be turned to the left. During this dissection, the table is rotated a little to the left, so it is helpful for the surgeon to be standing to the patient’s right.
Next, the short retrohepatic veins draining directly from the caudate lobe to the IVC are serially divided, progressing from the inferior aspect toward the hepatic veins. Complete exposure of the IVC and right hepatic vein requires division of the fibrous tissue, the vena caval ligament, which usually obscures the right side or the upper part of the retrohepatic vena cava (see Fig. 90B.15A). This division can be done with scissors (see Fig. 90B.15A), or an endoscopic gastrointestinal anastomosis vascular stapler (Endo GIA; US Surgical, Norwalk, CT) can be used (see Fig. 90B.15B) to expose the vena cava completely (see Fig. 90B.15C). The vena caval ligament also extends behind the vena cava posteriorly, embracing it and joining a similar fibrous extension from the caudate lobe on the left (see Chapter 1B). Rarely, this posterior part of the ligament is replaced by a thin rim of liver tissue, so that at this point, the vena cava runs through a tunnel of liver parenchyma. This can be a source of difficulty, but this thin plate of liver tissue can similarly be divided to expose the IVC completely. The vena caval dissection is particularly facilitated with low CVP anesthesia.
If the tumor is found attached to the diaphragm during mobilization of the right liver, this is usually only across a limited distance, and the affected area of diaphragm is separated, or a segment of the diaphragm is excised and subsequently repaired. An anterior approach with initial transection of the parenchyma without preliminary mobilization of the right liver is necessary and valuable in some cases (Lai et al, 1996b); this will be discussed later.
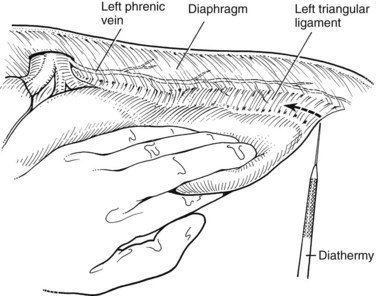
On the left side, the left lateral sector is mobilized by division of the apex of the left triangular ligament. The table is rotated a little to the right to allow good visualization. Care must be taken not to damage the stomach and spleen at the far left of the triangular ligament, and placing a laparotomy pad under the ligament helps to protect these structures while incising the ligament. The ligament is opened, and between its anterior and posterior leaves, the upper part of the IVC and the left hepatic vein become visible (Fig. 90B.16).
Intraoperative Ultrasound
We use IOUS to complete the assessment of the number and location of tumors along with their exact anatomic relationship with the major vascular and biliary structures within the liver parenchyma. US guidance is also utilized to perform a needle biopsy of small, deeply placed lesions and for ablative procedures, should such be necessary. Transhepatic cannulation of biliary or vascular elements to perform contrast studies (Hasegawa et al, 1991b) is rarely used; a detailed description of the use of IOUS in liver resection is provided in Chapter 21. Essentially, examination is carried out with a B-mode, real-time system. The probes utilized for IOUS are waterproof and sterile; a T-shaped probe or a finger probe is used that enables direct application of the probe to the liver surface, and the moisture of the liver allows good contact. Examination should be carried out in a systematic fashion, starting in the umbilical fissure, progressing to examination of the hilar structures, followed by a careful examination of the hepatic veins and finally of the hepatic parenchyma. In case of doubt, further examination may be done after mobilization of the right or left liver, particularly for posteriorly located tumors. The probe also may be used from the inferior surface of the liver.
The information obtained at IOUS is frequently valuable in assessing the liver and in planning resection. We have found that with modern high-quality preoperative imaging studies, more frequently than not, IOUS plays a confirmatory role in diagnosis rather than revealing features not suspected before laparotomy (Jarnagin et al, 2001b). This is particularly true in the noncirrhotic liver. High-quality laparoscopy and laparoscopic US are now available, and studies at MSKCC show that the findings at IOUS changed the operative plan in only a few patients; however, IOUS can help guide large segmental resections traversing narrow planes between the tumor edge and major vascular structures. Thus it is paramount that the surgeon have the ability to interpret IOUS in order to safely maneuver inside the liver.
Major Anatomic Hepatic Resections
All major hepatic resections mandate control of the inflow vasculature to the portion of liver to be resected, with preservation of adequate hepatic arterial and portal venous blood supply to the remnant. This may be done by dissection of the relevant portal pedicle at the hilus and outside the liver substance (Blumgart, 1982). Alternatively, the major branches may be secured within the liver by division of the liver tissue by finger fracture (Foster & Berman, 1977; Ton That Tung & Quang, 1963) or by crushing the liver substance with a Kelly clamp, as described by Ton That Tung (1979). More recently, Launois and Jamieson (1992a, 1992b) have advocated control of the intrahepatic pedicles if oncologically feasible; this is detailed in this chapter. In practice, most surgeons use a combination of techniques in hepatic resection (Fig. 90B.17). Biliary drainage from the remnant must be preserved without biliary leakage and without damage to the biliary tract. The hepatic venous outflow should be controlled after inflow division, and the liver parenchyma must be transected to provide tumor clearance in cases of malignancy.
Inflow Control and Preservation of the Integrity of the Biliary Tree
Control of the hepatic arterial and portal venous blood supply to the portion of liver to be removed can be obtained by extrahepatic dissection or alternatively by transecting the relevant pedicles within the liver substance. Extrahepatic vascular dissection may be accompanied by extrahepatic dissection and control of the relevant biliary radicles, but this is associated with a higher risk of biliary injury, especially during dissection on the right side, where anatomic variations of the major right sectoral ducts at their junction with the main biliary channel are frequent (see Chapter 1A, Chapter 1B ). Thus, when performing extrahepatic dissection for vascular control of right-sided lesions, we typically divide the right hepatic artery and the right portal vein but leave the biliary structures for division within the pedicles at the time of parenchymal transection; however, extrahepatic biliary dissection must be performed for tumors that are close to the hilar structures, making an intrahepatic approach to the portal pedicles impossible without compromising tumor clearance. Extrahepatic dissection of the biliary system is, of course, mandatory in cases of hilar cholangiocarcinoma. For left-sided resections, although a pedicle approach is possible, we usually pursue dissection within or at the base of the umbilical fissure and well to the left of the hilar bifurcation. Since the work of Launois and Jamieson (1992b, 1993), we have used pedicular control.
Outflow Control
The major danger in controlling hepatic venous outflow is the risk of hemorrhage. Some authors prefer total vascular isolation techniques to overcome this problem, especially for large tumors abutting or involving the vena cava or major hepatic venous structures (Delva et al, 1989; Emond et al, 1995; Emre et al, 1993; Hannoun et al, 1993), but this is often unnecessary; for example, at MSKCC, total vascular isolation was not used in more than 1800 consecutive liver resections (Jarnagin et al, 2002).
Intrahepatic control of the hepatic veins, carried out during parenchymal transection, is feasible and acceptable for tumors that allow clearance superiorly with adequate tumor margins and with safe intrahepatic access to these vessels. Extrahepatic dissection and control of the major hepatic veins is possible in nearly all cases, and for the difficult tumor lying high and posteriorly, it enables adequate clearance together with reliable control of hemorrhage during right or left hepatectomy. Because the middle hepatic vein usually merges with the left hepatic vein prior to draining to the IVC, the middle and left hepatic veins are often controlled as a single unit. If isolated left hepatic vein control is desired, a small hepatotomy is usually needed to dissect the plane between the two veins. Extrahepatic venous control necessitates two essential requisites that are applicable for all hepatic resections: 1) maintainence of CVP below 5 mm Hg (see earlier and in Chapter 22) and 2) precise extrahepatic dissection of the major hepatic veins. Dissection is performed with the patient in a 15-degree Trendelenburg position to minimize the risk of air embolism. Using this technique, no case of clinically significant air embolism has been encountered in 496 resected cases (Melendez et al, 1998). The techniques of vascular control described above are associated with an acceptable blood loss and a blood transfusion rate comparable with any series reported using total vascular isolation (Table 90B.2; Cunningham et al, 1994; Jones et al, 1998; Melendez et al, 1998). Furthermore, it avoids the need for fluid loading and for the use of pressor agents during periods of vascular isolation, and it prevents the adverse biochemical and hemodynamic effects that ensue when the clamps maintaining the vascular isolation are released.
Parenchymal Transection
Many surgeons place excessive importance on the technique of parenchymal transection, when in fact this aspect is not nearly as critical as proper exposure and vascular control, as outlined above. A number of methods may be used to transect the liver parenchyma. Our preference has been to use a simple crushing technique: the Glisson capsule is scored with diathermy along the line of proposed transection, and a Kelly clamp is used to crush the liver tissue and expose small vessels, biliary channels, and larger pedicles; once exposed, these structures can be secured and divided using a variety of techniques. The most simple and economic method is to use clips and suture ligation; however, a variety of devices can be used that include 1) computer-controlled bipolar cautery (LigaSure; Valleylab, Boulder, CO); 2) a saline-linked radiofrequency ablation monopolar device (TissueLink; TissueLink Medical, Dover, NH), or a bipolar variation (Aquamantys, TissueLink Medical); 3) a Harmonic Scalpel (SonoSurg; Olympus Key Med, New York, NY). All of these devices have been used with success by a variety of investigators (Clavien et al, 2004; Sakamoto et al, 2004; Strasberg et al, 2002). They also have been used successfully in the performance of laparoscopic partial hepatectomy, which will be discussed later. Larger structures can be controlled with sutures or vascular staplers (Fong & Blumgart, 1997).
The technique of using a Kelly clamp to crush the tissue is rapid and efficient, although the surgeon may choose to apply intermittent inflow occlusion (Pringle, 1908) to control blood flow to the remnant, usually maintaining inflow occlusion for periods of 15 minutes, interspersed by 5-minute periods of relief to allow perfusion of the remnant and decompression of the bowel. We rarely use prolonged periods of inflow occlusion, although this may done safely (Berney et al, 1998; Hannoun et al, 1993; Huguet et al, 1978, 1992a, 1992b), and we especially avoid prolonged occlusion during hepatic resection in patients with jaundice or cirrhosis. Prolonged portal inflow occlusion is unnecessary, and for major hepatic resections, complete parenchymal transection is usually accomplished in 20 to 30 minutes. During the short breaks between applications of the Pringle maneuver, the argon beam coagulator (Force GCU System; Valleylab) can be used to control small vessel oozing on the side of the remnant. Avitene (Davol, Warwick, RI), Surgicel (Ethicon, Somerville, NJ), or fibrin sealants also may be applied to the cut surface of the liver to achieve final hemostasis after transection is complete.
As a surrogate for the Kelly clamp to expose the intrahepatic vascular and biliary anatomy, an ultrasonic dissector may be used (Hodgson & DelGuercio, 1984) or a water-jet dissector (Baer et al, 1992b). The ultrasonic dissector (Hanna et al, 1996) has become popular and has some advantages in displaying vessels, particularly in the performance of segmental and subsegmental resections, and especially in the cirrhotic liver; but it is tedious and offers no apparent advantage in the performance of major resections. No published data are available to indicate that the technique is associated with improved control of blood loss. The ultrasonic machine is expensive, and reliance on its use can leave the operator insecure when the instrument is not available. The new version of the water-jet dissector (Helix Hydro-Jet ERBE; USA Inc., Marietta, GA) is much more efficient than the original version, and it may be of considerable value in the exposure of major pedicles.
Drainage of the Abdominal Cavity
Franco and colleagues (1989) suggested early on that hepatic resection should be performed without drainage. After performing a controlled clinical trial (Fong et al, 1996), the authors no longer use drainage routinely after major hepatic resection, unless there is ongoing biliary leakage from the cut liver surface that cannot be controlled with sutures. The placement of drains is associated with a prolonged hospital stay, an increased possibility of infection, and in our experience no decrease in the need for postoperative drainage catheters placed using interventional radiology; however, exceptions do exist, and sometimes a postoperative drain may need to be utilized. In our experience with simultaneous liver and colorectal resections, we have empirically noticed an increased incidence of infected fluid collections in the liver resection bed colonized with colonic flora. Thus for simultaneous resections, we now tend to leave a drain near the liver resection bed. In patients with cirrhosis who undergo resection, placement of a drain must be considered carefully, given the risk of significant ascites formation. Although a drain may protect the incision by preventing an ascitic leak and allowing the fascial closure to heal, the drain may become the source of protracted fluid loss with no clear end point of removal. Finally, we advocate drainage in patients undergoing resection, accompanied by biliary reconstruction. When drainage catheters are utilized, we maintain them on gravity-bag dependent drainage, as the amount of negative pressure generated by closed-suction drains is grossly underestimated (Fig. 90B.18; Grobmyer et al, 2002). For patients who undergo biliary reconstruction, the drain is removed usually within 4 to 5 days provided no bile leakage is evident.
Right Hepatectomy
Inflow and Biliary Control
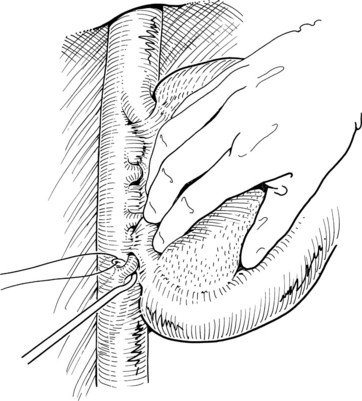
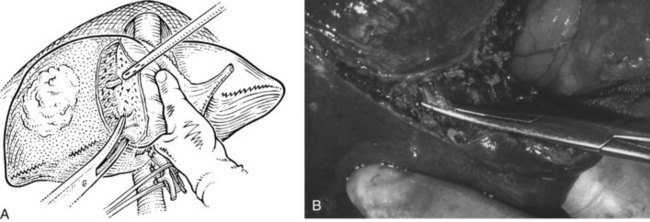
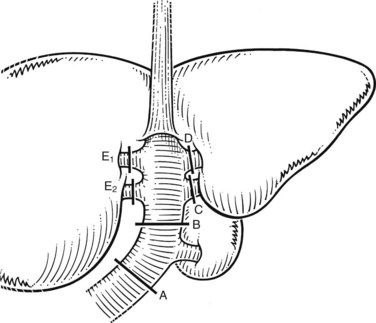
The liver is elevated to display its inferior surface and the portal structures. We generally lower the hilar plate to expose the left hepatic duct and the confluence of the bile ducts (see Chapter 1B). Division of the peritoneal reflection along the free edge of the lesser omentum exposes the lateral side of the portal vein and the common bile duct. The peritoneum overlying the common bile duct is incised on the free edge of the lesser omentum and extending up into Calot’s triangle, and the cystic duct and the cystic artery are exposed, ligated, and divided (Fig. 90B.19). The gallbladder can also be dissected in the anterograde fundus-down technique to expose the cystic duct and artery (see Chapter 33). The cystic duct is affixed with a suture ligature before being divided, and a tie is left on the cystic duct for later retraction.
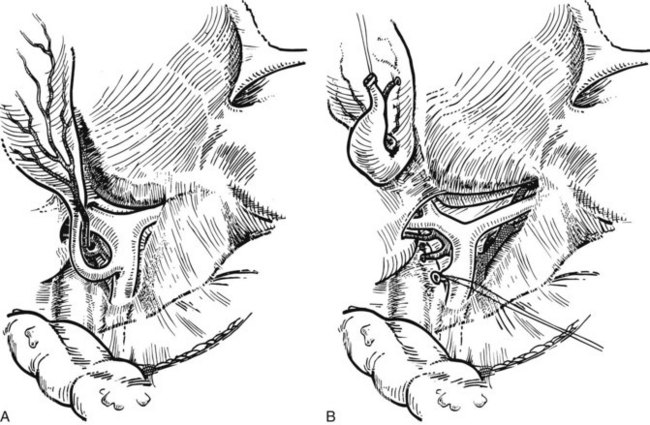
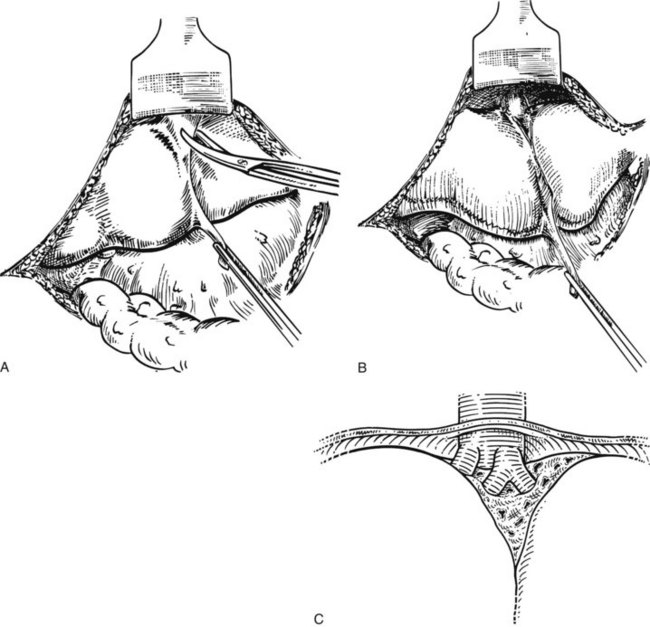
FIGURE 90B.19 A, The peritoneum overlying the common bile duct is incised on the free edge of the lesser omentum and extending up into Calot’s triangle, and the common bile duct, common hepatic duct, and Calot’s triangle are exposed. B, The cystic duct and cystic artery are secured, and a tie is left on the cystic duct for later retraction (see also Fig. 90B.20). The hilar plate is lowered to expose the left hepatic duct and the confluence of the bile ducts, and the right hepatic duct is dissected.
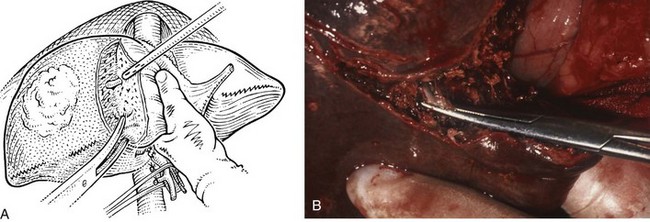
The right hepatic artery is dissected, ligated, and divided (Fig. 90B.20). It originates from the main hepatic arterial trunk and passes posterior to the common bile duct in approximately 80% of patients (see Chapter 1B). It is usually ligated to the right of the common hepatic duct to protect the left hepatic artery from inadvertent ligation or injury and to facilitate access to the underlying portal vein. It is important to be certain that the left hepatic artery is preserved by palpation at the umbilical fissure after temporary occlusion of the right hepatic artery. Although the right hepatic artery can be ligated medial to the bile duct, we prefer to ligate it to the right of the common hepatic duct. We deliberately double-suture–ligate the proximal end and hold this after division for light traction to the left, which together with traction of the cystic duct stump is of considerable benefit in exposing the right portal vein.
The portal vein is approached laterally and posteriorly and is best exposed by dividing its peritoneal covering. The main portal trunk is exposed and dissected superiorly, until the left branch is revealed and preserved. Sometimes, the right anterior and posterior sectoral branches of the portal vein arise individually, and these origins may be separated by 2 cm (see Fig. 90B.20E). It is important not to misidentify these branches during dissection. The right branch of the portal vein may be delivered from the hilus by dividing the surrounding areolar tissue, and a good length amenable for division can usually be obtained. A renal pedicle clamp is passed gently around the right portal vein under direct vision, with special care taken not to damage the first posterior branch of the right portal vein, which comes off early and posteroinferiorly to supply the right portion of the caudate lobe. This branch should be deliberately identified to prevent inadvertent avulsion. If any difficulty is encountered in encircling the right portal vein, this branch should be individually ligated and divided before any instrument is passed around the right portal trunk.
Application of a straight-bladed vascular clamp is a safe method of transecting the right portal vein (see Fig. 90B.20). It is good practice to place two retaining prolene sutures to secure the vein before dividing it to prevent retraction. These sutures should be left with the needle in place and are used to oversew the stump after the vein has been divided (see Fig. 90B.20). If the vein is short, it is inadvisable to simply suture ligate the right portal vein and transect it, because the ligatures have a tendency to slip unless secured. The proximal stump should be doubly ligated. Another option to divide the portal vein, used with increasing frequency, is an Endo GIA (Covidien, Mansfield, MA) gastrointestinal anastamosis endovascular stapler (Fong & Blumgart, 1997). If the right anterior and posterior sectoral branches arise independently (see Fig. 90B.20E), they require individual control. If stapler transection of the right portal vein trunk or the anterior and posterior sectoral branches is contemplated, it is imperative to avoid compromise of the flow through the left vein.
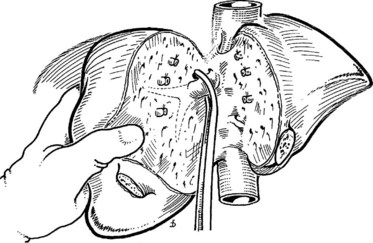
If extrahepatic control of the right hepatic duct is necessary, the duct is now dissected. The confluence of the bile ducts and the origin of the extrahepatic course of the left hepatic duct should be seen clearly after lowering the hilar plate (see Fig. 90B.19B). Occasionally, and particularly if segment IV is large, it may help to divide the liver tissue that occupies the lower limits of the umbilical fissure and bridges the gap between the quadrate lobe (segment IV) and segment III of the left liver. This maneuver opens the umbilical fissure and allows better exposure of the subhepatic space.
When the right hepatic duct is dissected, it should be affixed, ligated, and divided (see Fig. 90B.20). Absorbable suture material should be used to prevent permanent suture acting as a nidus for future stone formation or infection. It may be that difficulty is encountered in passing a ligature around the right hepatic duct, in which case it may be divided under direct vision and subsequently oversewn with a 4-0 absorbable suture (e.g., polydioxanone suture [PDS]) on an atraumatic needle. Alternatively, an Endo GIA stapler can be used to divide and ligate the bile duct.
In many instances, the ducts draining the right anterior and posterior sectors are found entering the confluence separately, or the posterior sectoral duct may join the left hepatic duct (see Chapter 1B). In such cases, both of these major sectoral ducts should be individually identified and secured. It may be difficult to obtain a clear view of the right hepatic duct for dissection and ligation, in which case these structures are best left intact and divided intrahepatically. The duct or its major sectoral tributaries may be secured in the pedicles further laterally at the time of parenchymal dissection. We usually use this approach, because it is safe and obviates precise dissection of the ducts extrahepatically. Deliberate dissection of the hepatic duct must be carried out for tumors that approach the hilus of the liver and engulf the pedicles down to the point of entry of the portal triads into the liver substance.
Pedicle Ligation
An important alternative to extrahepatic dissection of the hilar structures is the pedicle ligation technique that uses the intrahepatic approach first described in Japan by Takasaki and colleagues (1986a, 1986b) and then in the West by Launois (1994) and Launois and Jamieson (1992b, 1993). This approach is most useful for right-sided tumors located peripherally away from the hilus, which allows its use without compromising tumor clearance. This technique allows the surgeon to dissect and clamp the required sheaths early in the operation to better define the segment or segments to be removed.
Essentially, the method relies on intrahepatic definition and control of the portal triads to the segments to be removed. The structures of the portal triad carry the Glisson capsule with them, as they penetrate the liver and are contained in a well-formed sheath within the parenchyma (Fig. 90B.21). The pedicular sheaths can be exposed after appropriate hepatotomies (Fig. 90B.22) and are tough enough to be encircled (Fig. 90B.23), dissected, and clamped. By eliminating the hilar dissection, pedicle ligation saves time and prevents potential injury to the contralateral vessels and bile ducts.
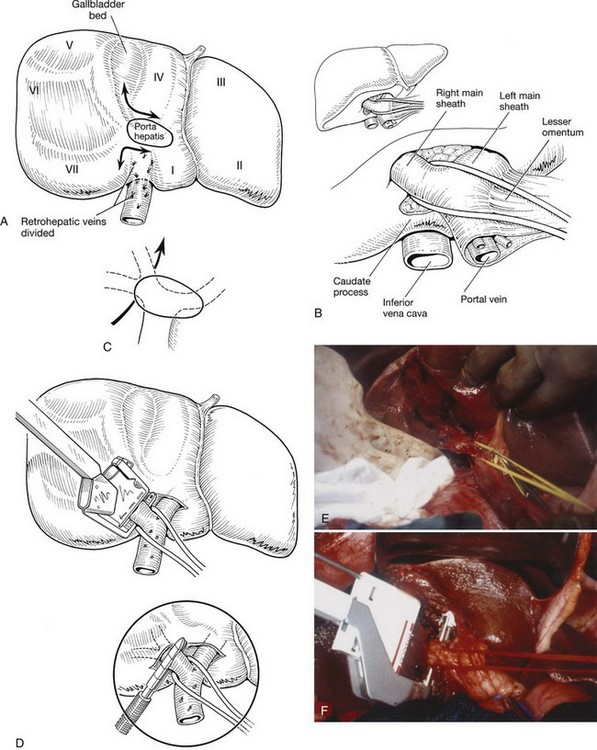
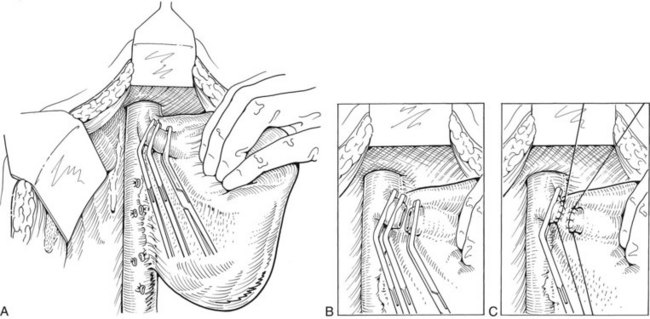
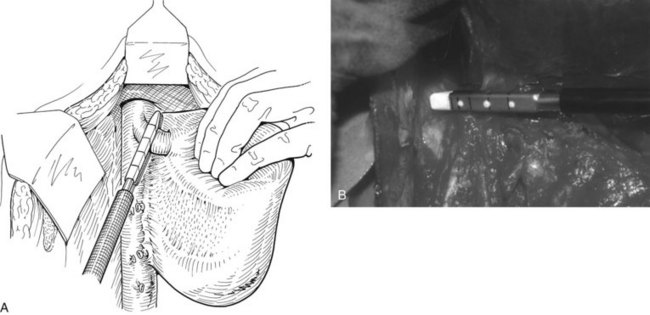
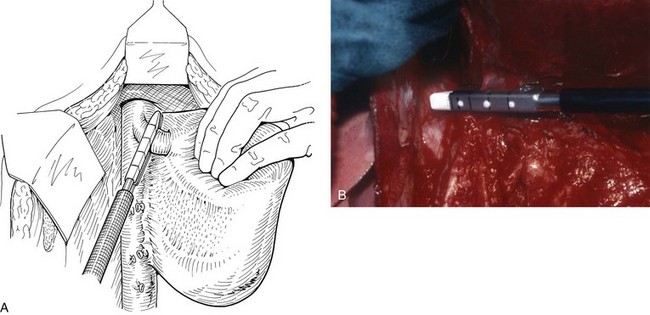
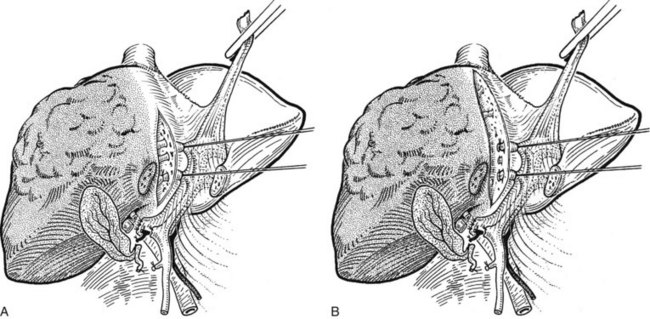
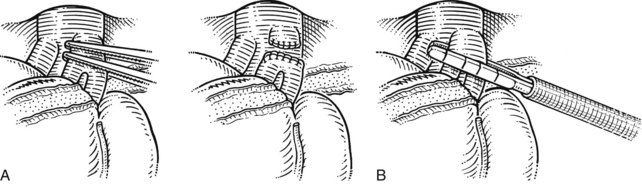
FIGURE 90B.22 A, Approach to the right portal pedicle. The liver has been mobilized from the inferior vena cava by division of the retrohepatic veins lying behind the caudate process. Hepatotomies have been made in the region of the gallbladder fossa and the caudate process. The curved double-headed arrows indicate the sites of the hepatotomies above and below the porta hepatis. The approach in the caudate process is essentially a posterior approach. B, A vessel loop is drawn about the right pedicle and pulled to the left to protect the left pedicular structures during control and transection of the right main sheath. C, The curved arrow indicates the path of the finger (see also Fig. 90B.23) or instrument during dissection of the right pedicle, allowing its isolation. D, The right portal pedicle is controlled and subsequently divided with a vascular stapler, such as the Endo GIA (inset). E, Intraoperative photograph shows the right portal pedicle exposed by finger dissection and surrounded by a yellow vessel loop, which is drawn to the patient’s left (see also B above). The vessel loop is drawn firmly to the left, and the pedicle is divided using the stapler, as shown in D. F, Operative photograph shows division of the right portal vein pedicle, which is encircled by a red vessel loop pulled to the left. The vascular stapler is being used to divide the pedicle as illustrated in D.
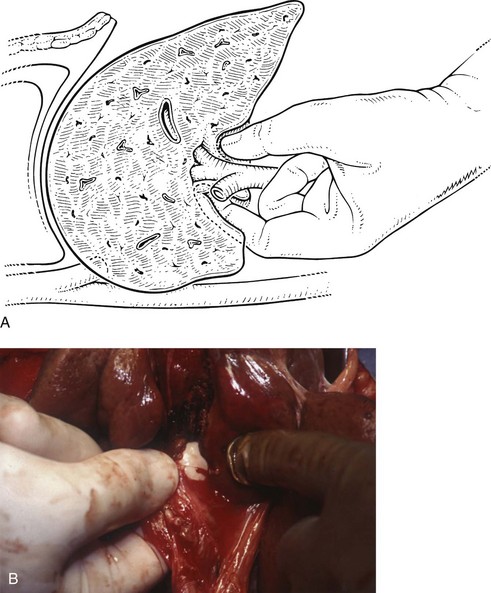
FIGURE 90B.23 A, Illustration shows digital isolation of the right pedicle. B, Photograph illustrates insertion of the index finger through a hepatotomy in the caudate lobe, where it merges with the base of the gallbladder fossa to isolate the right portal pedicle. A vessel loop is drawn about the pedicle as shown in Fig. 90B.22F.
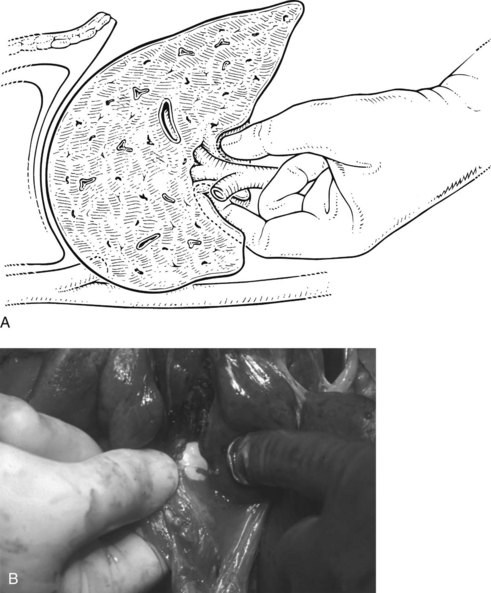
We have found it important first to ligate the most caudal retrohepatic veins draining from the caudate process and inferior part of the liver to the vena cava (see Fig. 90B.22A). Failure to do this has resulted in hemorrhage during passage of a finger or dissector (Fig. 90B.24) around the right portal pedicle, and this preliminary move is essential prior to using the right pedicle approach. Other than this cautionary note, the technique is almost identical to that described by Launois (described later). Care must be taken to respect the anatomy of the pedicles, in particular the pedicle to the right posterior sector (see Figs. 90B.23 and 90B.24). When the right pedicle has been exposed (see Fig. 90B.22B and E), and a trial of clamping reveals the demarcation line along the principal plane, the pedicle can be divided. A second confirmatory test prior to dividing the pedicle can be rapidly performed with real-time US to demonstrate flow with color Doppler in the left portal vein. We usually use a vascular stapling device to divide the pedicle (see Fig. 90B.22D and F; Fong & Blumgart, 1997). This method also can be used to control the left sheath, but we prefer to dissect the structures within the umbilical fissure, which will be discussed later.
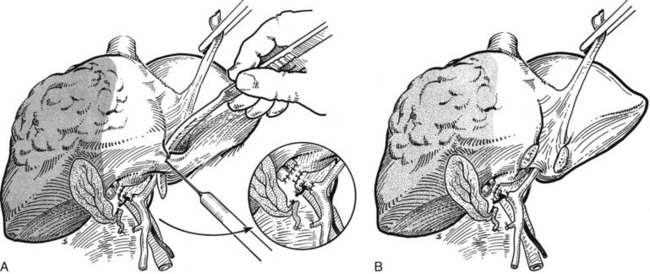
Exposure and Control of the Right Hepatic Vein
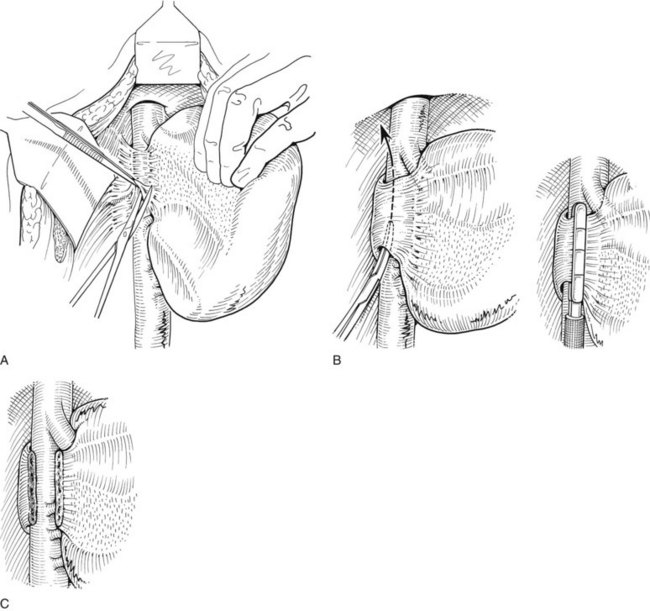
When dissecting the vena cava and the hepatic veins, it is important to maintain the CVP below 5 mm Hg to avoid inadvertent injury and substantial hemorrhage. To prevent air embolism, the patient can be placed in a 15-degree Trendelenburg position (Cunningham et al, 1994). The right hepatic vein is approached starting from the inferior aspect of the liver and working upward from the point where the caudate process crosses the IVC. The retrohepatic veins are sequentially dissected, controlled, and divided. This may be done with fine ligatures passed around the vessels with an aneurysm needle (Fig. 90B.25), but this can be tedious; for smaller vessels, we apply two clips on the vena cava side and one on the liver side of each vessel. These small veins can sometimes also be controlled with a coagulating device, such as a Harmonic Scalpel or the LigaSure device (Valleylab) when appropriate. Larger veins, and especially an inferior right hepatic vein, can be controlled by formal division between clamps or with an Endo GIA vascular stapler.
Complete posterolateral exposure of the right hepatic vein requires division of the IVC ligament, which obscures to a variable extent the right side of the upper part of the retrohepatic vena cava. This dissection may have been done at an earlier stage in the operation, but if not, it should be completed at this stage by the techniques described previously (see Fig. 90B.15). We often divide this caval ligament with an Endo GIA vascular stapler. It is important to perform an extensive retrohepatic dissection and to secure all the short hepatic veins draining from the posterior aspect of the liver; this dissection should be carried well over to the left of the IVC, because this is the only way sufficient access is obtained to visualize and dissect the main right hepatic vein safely.
Before any attempt is made to pass an instrument around the right hepatic vein, full exposure of the vein should be obtained superiorly. This exposure is achieved by dissecting the filamentous fibrous tissue above the liver and anterior to the suprahepatic IVC. This dissection may also have been done as a preliminary procedure during exposure and mobilization of the liver at an early stage (see Fig. 90B.14). In fact, it is advised to have the right hepatic vein in view prior to dividing the lateral attachments to the liver in order to prevent injury to the vein during lateral mobilization. The right phrenic vein emptying into the right hepatic vein provides a nice landmark for its location. This dissection must be performed right against the exposed liver substance, as it is ineffective and dangerous to wander away from the liver surface.
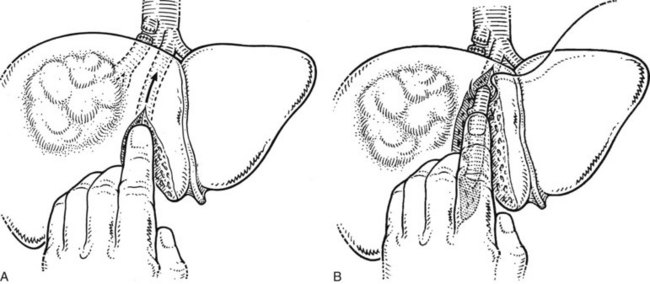
After the right hepatic vein is exposed and encircled, several methods may be used to safely divide it. Traditionally, a vascular clamp is applied on the caval side, usually with space for the application of a second clamp on the caval side and a third on the hepatic side, although this may not always be possible (Fig. 90B.26A). If it is not possible to place a clamp on the hepatic side, division of the vessel does not result in great hemorrhage, provided inflow control has been obtained previously. In any event, the vessel is now divided with a scalpel (see Fig. 90B.26B). One clamp is removed, and the exposed stump is closed with an over-and-over 3-0 polypropylene vascular suture (see Fig. 90B.26C). The proximal (hepatic) side of the right hepatic vein is now closed with a 3-0 polypropylene suture. Alternatively, in well over 1000 cases, we have used the Endo GIA vascular stapler to divide the right hepatic vein and have rarely encountered any problems (Fig. 90B.27). The major concern is for misfiring of the stapler, which can result in a tear of the vein; however, this is an extremely uncommon event. The stapled approach is quick, effective, and safe (Fong & Blumgart, 1997), and we now use it routinely, except for large tumors located where the right hepatic vein drains into the IVC; by their size and rigidity, such tumors often limit the entry of the stapler into the tunnel to the left of the right hepatic vein and anterior to the IVC. Extrahepatic control of the middle hepatic vein is unnecessary during right hepatectomy. An important alternative approach to the right hepatic vein involves exposure by preliminary transection of the parenchyma down to the anterior surface of the IVC (Lai EC et al, 1996b). This so-called anterior approach is described later in this chapter.
Parenchymal Transection
Liver tissue is divided by a tissue-fracture technique, as described earlier. A Pringle maneuver is performed as needed, and the liver tissue is crushed in the line of transection, using a Kelly clamp or similar instrument (Fig. 90B.28) and leaving the vessels intact. During this process, inflow occlusion can be obtained by encircling and compressing the hilar structures in a vessel loop or umbilical tape. It may be helpful for the first assistant to compress the left liver with the hand and to pull it downward gently. Traction can also be applied by placing chromic sutures through the edge of the liver parenchyma on each side of the transection plane. Suction is applied at the blunt end of the sucker, which is used to tease the tissues away from the vessels (see Fig. 90B.28).
Large vessels may be isolated easily by crushing the tissue between the finger and thumb. When visualized, smaller vessels and bile ducts are ligated with metal clips, ties, or a coagulation energy device and divided. Large vessels may be suture ligated or controlled with an Endo GIA vascular stapler. As the tissue is divided, the fissure that is developed in the liver is opened by the operator, whose nondominant hand holds the right liver. With fingers located posteriorly in the region of the vena cava, that hand elevates the right lobe, opening the wound in the liver to expose its depth. As dissection proceeds, the middle hepatic vein is displaced to the left, and the dissection proceeds along its right margin. The branches from segments V and VIII are controlled. If the right sectoral bile ducts have not previously been divided extrahepatically, they are now controlled during division of the portal triads, whereupon the right portal triad is encountered and divided close to the hilus. If the right hepatic vein has not been secured behind the liver, it is now isolated and ligated or divided with the stapler within the liver (Fig. 90B.29).
Once the specimen is removed, final hemostasis is obtained by a variety of techniques. Suturing of large vessels is most appropriate; fibrinogen sealants (Guthy, 1986; Scheele, 1989) may be used for small vessels, but the argon beam coagulator is effective and is associated with very little tissue necrosis (Postema et al, 1993). In a few cases, if oozing should continue, and particularly if it seems to be coming from divided veins, the passage of large sutures (chromic catgut or vicryl) anteroposteriorly in a mattress fashion secures hemostasis. These sutures should not be so tight or so numerous as to cause tissue ischemia (Fig. 90B.30). It should be noted that the majority of bleeding at this stage of the operation is low-pressure venous oozing, which can often be controlled with manual pressure and time. Any bleeding from the exposed undersurface of the diaphragm must be carefully controlled; in this respect, the argon beam coagulator can be of great value.
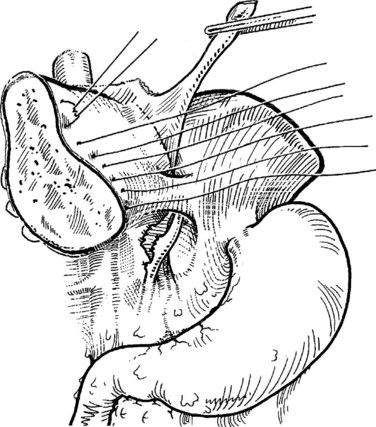
FIGURE 90B.30 Method of suture of the cut surface of the liver to achieve hemostasis. This technique is seldom necessary.
Wound Closure and Drainage
If a diaphragmatic incision has been made, it is closed, and if any defects have been created in the diaphragm, these are similarly sutured. An attempt should be made to remove the pleural air via negative suction applied from the abdomen to obviate the need for a chest tube. There is no need to attempt to close the exposed raw area of the liver with peritoneal flaps, because these could cause compression of liver tissue and distortion of hilar vessels. The ligamentum teres should not be used for this purpose, and similarly, omental grafts are usually unnecessary, although any convenient omentum may be laid against the raw surface. We do not drain the subdiaphragmatic space after routine liver resection, as discussed earlier; however, drainage of the subdiaphragmatic space is carried out with one or two drains if needed (see Fig. 90B.18). A chest drain may be needed in cases of diaphragm resection.
Anterior Approach to Right Hepatectomy
Hanging Maneuver
When performing a right hepatectomy, complete mobilization of the liver before parenchymal transection is considered a basic maneuver for conducting a safe procedure. When a large tumor invades the diaphragm or is attached to the lateral side of the vena cava at a higher level, mobilization may be difficult. In such cases, Lai and colleagues (1996b) reported an anterior approach with initial parenchymal transection from the anterior surface down to the IVC; this will be discussed later. Such an approach may also be useful for resecting large HCCs or adenomas that are soft and may fracture and bleed during the mobilization. Because it may be difficult to control bleeding in the deeper parenchymal plane, Belghiti and colleagues described a new technique of hanging the liver after lifting it with a tape passed between the anterior surface of the IVC and the liver parenchyma (Belghiti et al, 2001; Trotovsek et al, 2005).
The most important step in this maneuver is the definition of the anterior plane of the IVC. Initially, IOUS is performed to confirm the absence of tumor in contact with the anterior surface of the IVC. The upper surface of the liver is exposed up to the anterior surface of the suprahepatic IVC (see Fig. 90B.14). The space between the right and the middle hepatic veins is dissected approximately 2 cm downward; we find it convenient to do this using a right angle or a renal pedicle clamp. The anterior surface of the infraheptic IVC is exposed. The inferior-most veins draining between the caudate process and the vena cava are ligated and divided.
Before parenchymal transection, the right pedicle is divided, devascularizing the right liver. The parenchymal transection is conducted from the anterior surface down to the posterior plane in front of the IVC. During the parenchymal transection, upward traction of the tape helps define the plane of transection and facilitates exposure of the deeper parenchymal plane in front of the IVC. After completing the exposure of the anterior surface of the IVC, the right side of the IVC is dissected with division and ligation of the inferior hepatic veins and IVC ligament. Finally, the trunk of the right hepatic vein is stapled with a vascular stapler or divided between clamps and oversewn. The coronary and right triangular ligaments are transected, and the specimen is removed. This technique can be applied routinely for right hepatectomy and does not necessarily need to be reserved only for large tumors abutting the right hepatic vein and IVC. One of the authors (J.B.) has considerable experience with this technique and has reported an experience of 200 cases performed safely (Ogata et al, 2007).
Anterior Approach
In some patients with very large right-sided hepatic tumors, an alternative is an anterior approach that involves initial parenchymal transection before the right hepatic lobe is mobilized. If this approach is adopted, the hilar inflow vessels are first controlled as described earlier, and the liver parenchyma is transected from the anterior surface of the liver working posteriorly. This parenchymal transection is pursued until the anterior surface of the right hepatic vein and IVC is encountered; the hanging maneuver described earlier can offer useful definition in this approach. Venous tributaries, including the right hepatic vein, are controlled from the front (Lai et al, 1996). The editor emeritus (Leslie H. Blumgart) has used this approach in selected patients and has found it possible even in the presence of diaphragmatic invasion.
Extended Right Hepatectomy
An extended right hepatectomy involves removal of segments IV, V, VI, VII, and VIII; this is in effect a right hepatectomy extended to segment IV. In the nomenclature of Goldsmith and Woodburne (1957), it is referred to as an extended right hepatic lobectomy. Starzl and coworkers (1975, 1980) referred to this procedure as a right trisegmentectomy, and this term is in common use in the United States.
After inflow control to segments V, VI, VII, and VIII by extrahepatic dissection or pedicle control, as described for right hepatectomy, the next essential step is to display precisely the umbilical fissure of the liver (Starzl et al, 1975). A strong suture tied to the ligamentum teres is elevated. In many cases, the lower part of the umbilical fissure is concealed by a bridge of liver tissue fusing segment III of the left liver to the quadrate lobe (segment IV) (see Chapter 1B). This tissue is divided easily by passing a curved dissector beneath it (Fig. 90B.31). No large vessels traverse here, and hemostasis is readily secured by diathermy or with the argon beam coagulator.
After opening the umbilical fissure, the ligamentum teres can be visualized, running down to join the termination of the left portal vein within the umbilical fissure (see Fig. 90B.31B). The triad of the left portal vein, left hepatic artery, and left bile duct enters at the base of the umbilical fissure. Their anatomy is shown, with branching of the pedicles to supply segments II, III, and IV (Fig. 90B.32).
It is important for the safe performance of an extended right hepatectomy that the hilar plate be lowered (Fig. 90B.33; see Chapter 29), separating the main left bile duct from the undersurface of segment IV. Whether arising from the common hepatic artery or from the left gastric artery, the left hepatic artery always enters the base of the umbilical fissure and is not endangered during this operation. The middle hepatic artery supplying segment IV is sometimes identified separately, traveling to the right of the left hepatic artery and entering the umbilical fissure at its base and on its right (see Chapter 1B).
Close to the base of the umbilical fissure, a major branch—often two branches—of the left portal vein passes posterolaterally to the caudate lobe (see Fig. 90B.32). Division of these branches interrupts the portal flow to the caudate lobe, but this needs to be carried out only if the caudate lobe is to be removed together with the rest of the specimen. The vital step in the performance of an extended right hepatectomy is the division of inflow vessels that originate from the umbilical fissure of the liver from the left portal triads and feed back from the main left trunk to segments IVa and IVb, the medial sector of the left liver (see Fig. 90B.32). It is not important to attempt to divide these structures within the umbilical fissure, if no tumor is immediately adjacent to the fissure. It is easier and safer to secure them within the liver substance during parenchymal dissection, just to the right of the umbilical fissure, because there is no chance of damage to the blood supply to or biliary drainage from the left lateral sector; however, when a tumor lies close to the umbilical fissure, it is necessary to control the feedback vessels at the right margin of the umbilical fissure. This control can be obtained by passing a suture around these vessels and ligating them in continuity (Fig. 90B.34). Alternatively, the vessels are dissected and individually ligated and divided.
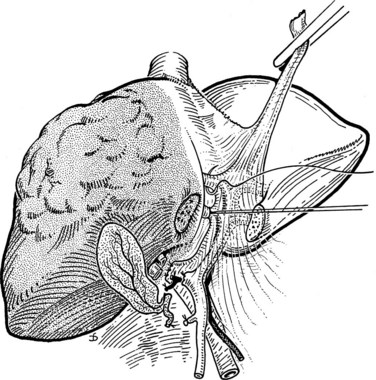
FIGURE 90B.34 Feedback vessels from the left portal triad may be secured in the parenchyma just a little to the right of the falciform ligament (see Fig. 90B.35). This dissection must be within the umbilical fissure in the event of adjacent tumor in segment IV, but this is more safely carried out within the liver tissue just to the right of the falciform ligament (see Fig. 90B.32). Preliminary suture ligation within the umbilical fissure may be performed as illustrated. This procedure deprives segment IV of its blood supply.
The parenchymal dissection is begun anteriorly and proceeds down to the base of the umbilical fissure (Fig. 90B.35A). When the feedback vessels are divided, segment IV is devascularized. Division of the liver substance is developed toward the vena cava just to the right of the falciform ligament (Fig. 90B.35B). The middle hepatic vein is encountered as the dissection is deepened and projected toward the right. When it is identified, it is easily controlled by ligation or division with the Endo GIA stapler. If it is necessary to pursue the middle hepatic vein intrahepatically, toward the vena cava, to obtain tumor clearance, the index finger of the surgeon’s nondominant hand can be slid gently along the upper surface of the vein (Fig. 90B.36). Any minor branches avulsed during this maneuver are controlled by finger pressure, and the vein is ligated distal to the finger (see Fig. 90B.36B). Alternatively, the tissue surrounding the vein can be crushed under direct vision, and the small tributaries that are exposed can be ligated. If the right hepatic vein has not previously been divided extrahepatically, it is now approached from within the liver substance, as described earlier, and appropriately controlled.
One particular difficulty may arise when a large tumor occupies segment IV and displaces the left bile duct at the base of that segment and as it traverses into the umbilical fissure (Fig. 90B.37). In such cases, mobilization of the left bile duct in the hilar area and at the base of the umbilical fissure is difficult, perhaps impossible, without deliberate excision of a portion of the left hepatic duct. In such cases, there should be no hesitation in sacrificing any necessary length of the left bile duct to obtain tumor clearance. After removal of the specimen, biliary repair may be carried out by anastomosing the stump of the left hepatic duct to the upper end of the common hepatic duct. Alternatively, biliary-enteric continuity may be restored by creation of a Roux-en-Y hepaticojejunostomy to the left bile duct, which is the preferred method (Fig. 90B.38).
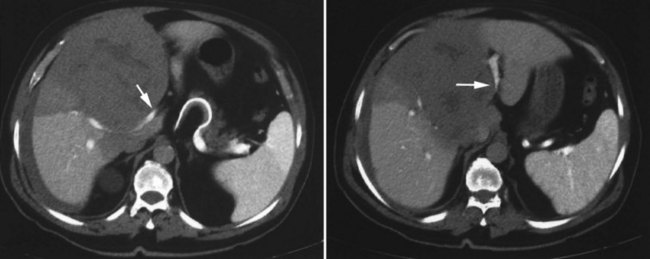
FIGURE 90B.37 Large metastatic carcinoma within segment IV threatens the integrity of the left hepatic duct (arrows). At operation, right lobectomy was performed, and a length of the left hepatic duct was excised and repaired as illustrated in Figure 90B.38.
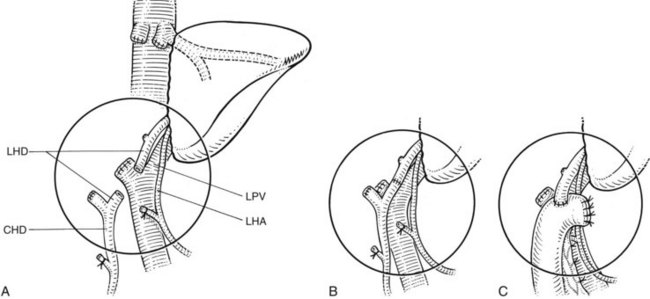
FIGURE 90B.38 The left hepatic duct may be compromised during right lobectomy for tumor extending into the base of segment IV and pushing into the umbilical fissure (see Fig. 90B.37). A, After resection with excision of a portion of the left hepatic duct (LHD). B, Repair of the duct by direct end-to-end anastomosis. C, Repair by hepaticojejunostomy Roux-en-Y. CHD, common hepatic duct; LHA, left hepatic artery; LPV, left portal vein.
Left Hepatectomy
Inflow Control
Inflow control to the left liver is obtained by dissection at the base of the umbilical fissure. The ligamentum teres is elevated, and the hilar plate is lowered as described earlier (see Fig. 90B.33). The left hepatic artery is easily identified by opening the peritoneum covering the left portion of the immediate subhilar area at the base of the umbilical fissure. It is double ligated and divided. The gastrohepatic ligament is inspected, and any accessory or replaced hepatic artery arising from the left gastric artery is similarly ligated.
Dissection at the base of the umbilical fissure exposes the left portal vein, as it traverses into the umbilical fissure; it should be controlled at this point and not at its origin from the main portal venous trunk. This dissection allows identification of the branches to the caudate lobe, which arise just before the left portal vein enters the umbilical fissure. If the caudate lobe is to be removed together with the left lobe, the vein is secured below the takeoff of the caudate branches (see Fig. 90B.32A). If the caudate lobe is to be preserved, which is usually the case, the vein is divided distal to the takeoff of the caudate branches (see Fig. 90B.32B). The left hepatic bile duct is easily identified just above the left portal vein, and it can be encircled with a suture and divided as it curves into the umbilical fissure; these steps are illustrated in Fig. 90B.39. A line of demarcation in the Glissonian plane, extending from the gallbladder fossa to the left of the vena cava, is now clearly visible on the liver surface (Fig. 90B.40) and is marked with a diathermy.
Outflow Control
The left lateral sector of the liver, segments II and III, is now mobilized from the diaphragm by division of the left triangular ligament from its tip up to the lateral margin of the suprahepatic IVC, taking care to avoid injury to the underlying spleen and stomach. As the dissection approaches medially, the surgeon must be careful not to injure the left hepatic vein. The upper surfaces of the left and middle hepatic veins and of the suprahepatic IVC are displayed (Fig. 90B.41; Czerniak et al, 1993). The main trunk of the middle hepatic vein usually joins the left hepatic vein but may enter the vena cava separately. In essence, the veins are cleared below the diaphragm, and their junction with the vena cava is displayed. The left lateral sector of the liver is turned to the right (see Fig. 90B.41), the gastrohepatic ligament is fully divided, and the ligamentum venosum is identified. We divide the ligamentum venosum close to its entry into the left hepatic vein. This division opens up a triangular tunnel, the sides of which are the left hepatic vein anteriorly, the anterior surface of the vena cava posteriorly, and the upper surface of segment II inferiorly. This tunnel is developed carefully, and an instrument is passed beneath the left and middle hepatic veins to emerge anterior to the vena cava, between the middle hepatic vein and the right hepatic vein (see Fig. 90B.41B), and the common trunk is controlled.
If the middle hepatic vein requires resection to obtain adequate tumor clearance, the common trunk can be divided at this time. It can be secured between vascular clamps, divided, and oversewn with a 3-0 polyproprylene suture. Alternatively, the Endo GIA vascular stapler may be used to divide the veins, which is our preferred method (Fig. 90B.42). If the middle hepatic vein can be preserved, the common trunk should not be divided. A small hepatotomy can be made in between the middle and left hepatic veins, which usually allows individual extrahepatic control of the left hepatic vein. Alternatively, the left hepatic vein can be divided intrahepatically during parenchymal dissection, if the tumor is located remotely. Controlling the major hepatic veins draining to the left liver is crucial in minimizing hemorrhage during the removal of large, left-sided tumors.
Extended Left Hepatectomy
In some instances, a large tumor occupying the left half of the liver encroaches across the principal scissura (Cantlie’s line) into segments V and VIII, the anterior sector of the right liver (see Figs. 90B.1 and 90B.4). In other cases, multiple tumors occupy portions of the left liver and segments V and VIII. In such cases, left hepatectomy can be performed with extension of the procedure to also remove segments V and VIII.
The procedure is extensive, and although Couinaud (1957) described the anatomic basis for extension of a left hepatectomy to include removal of the anterior sector of the right liver, there were few reported series of successful operations. Joishy and Balasegaram (1980) reported extended left hepatectomy done in eight patients. The nature of these operations is described as indicating a line of transection to the right of the principal plane, but almost certainly not extending to the right scissura. An early description of this procedure was reported by Blumgart in 1978, but it was not until Starzl and colleagues (1982) reported four cases, which they referred to as left hepatic trisegmentectomies, that the necessary hilar dissection was described precisely. The difficulty in defining the plane of parenchymal transection on the right and in controlling hemorrhage and biliary complications was recognized. Subsequently, reports of small numbers of patients undergoing this procedure continued to populate the literature, but perioperative problems, and biliary complications in particular, were frequent (Al-Hadeedi et al, 1990; Hasegawa et al, 1989).
Preoperative Evaluation
Careful and detailed preoperative anatomic assessment is paramount, and patients should not be excluded from operation based on the size of the tumor alone. Even patients with very large tumors that displace blood vessels and compress bile ducts, so-called pushing tumors, may be selected for laparotomy (Baer et al, 1989). Quality cross-sectional imaging by either CT or MRI is important to delineate the expansile pushing form of a tumor (see Fig. 90B.6). US, including Doppler imaging of the portal and hepatic vessels, is also valuable in this regard. These studies also show impingement of large tumors on the vena cava. Arteriography and inferior venacavography are rarely if ever necessary.
Operative Technique
The operation involves removal of segments II, III, IV, V, and VIII (Blumgart et al, 1993). Segment I can be included in the resection if necessary for tumor clearance (Fig. 90B.43; see also Fig. 90B.32). An important aspect of the operative technique is the early and complete mobilization of the liver, not only by division of the left triangular ligament from its apex to the left of the IVC but also by complete division and mobilization of the ligament on the right. This mobilization not only allows palpation of segment VII for the presence of unsuspected additional tumor but also assists later in the identification of the correct plane of parenchymal dissection. IOUS is performed to confirm the previously defined anatomic configuration of the tumor and major hepatic veins. In particular, an attempt is made to identify the presence of a large inferior right hepatic vein; its presence augments drainage of segments VI and VII and may allow the right main hepatic vein to be sacrificed during operation if required (Baer et al, 1991).
Initial dissection is identical to that for left hepatectomy. The suprahepatic bare area of the liver is fully exposed to display the suprahepatic IVC and the hepatic veins. The left liver is now turned to the right side, and the portal triad is approached from the left (Fig. 90B.44). The point of ligation of the portal vessels to the left liver is dictated by the involvement of the caudate lobe (segment I) by tumor. If segment I requires resection, the left hepatic artery, bile duct, and portal vein must be ligated close to the hilus to interrupt the blood supply to segment I and to the left liver (see Figs. 90B.32 and 90B.44). As described by Starzl and coworkers (1982) and Blumgart and colleagues (1993), however, when segment I is to be preserved, the structures of the left portal triad are secured at the base of the umbilical fissure to leave intact the biliary drainage and blood supply of segment I (see Fig. 90B.44).
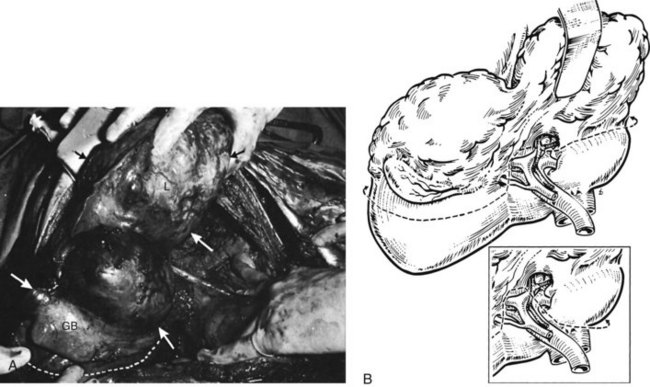
FIGURE 90B.44 A, Operative photograph of a large, left-sided primary hepatocellular carcinoma occupying the left lobe of the liver (segments II, III, and IV) and extending into segments V and VIII beneath the gallbladder (GB). The left lobe (L) has been mobilized and turned toward the right. The extent of the tumor is indicated by the arrows. The line of parenchymal incision in the right liver is indicated by the dotted line. B, Initial approach during extended left hepatectomy allows dissection of the portal triad structures from the left posterolateral aspect. The line of future transection is indicated. If the caudate lobe is to be preserved, the left portal vein is ligated beyond its caudate branch; but if the caudate is to be removed as well, the portal vein can be ligated more proximally (inset). The line of transection runs along the obliterated ligamentum venosum, then across the base of the quadrate lobe and hilus; it curves to the right, extending into the right scissura lateral to the gallbladder fossa. The dissection as illustrated in the main diagram would preserve the caudate lobe; in the inset, the caudate lobe also is removed (see also Fig. 90B.43).
After obtaining vascular control at the hilus, the middle and left hepatic veins and the left of the subdiaphragmatic IVC are dissected and exposed as described earlier for left hepatectomy (Czerniak et al, 1993). If the caudate lobe is to be removed en bloc, it is mobilized from the left side by dividing its direct venous tributaries to the IVC.
The difficult aspect of the operation is the definition of the line of transection of the parenchyma, which lies well to the right of the principal scissura. The plane is horizontal, lateral to the gallbladder fossa, and parallel and just anterior to the right scissura. In an attempt to define this plane, Starzl and coworkers (1982) described finger dissection, beginning from above and anterior to the main right hepatic venous entry into the vena cava. We have avoided this approach, which may be associated with considerable hemorrhage. It is important to define the plane of parenchymal dissection, however; it passes anterior to the right hepatic venous trunk and allows division of the anterior sectoral branch of the right portal triad, while preserving and avoiding damage to the posterior sectoral triad (Fig. 90B.45). Although there are no reliable surface markings, a plane passing from the anterior border of the right hepatic vein above and extending horizontally to emerge well to the right of the gallbladder fossa designates the approximate correct position within the parenchyma. This line can be accurately defined when it is possible to clamp the anterior sectoral pedicle to the right liver, and this is often possible using the pedicular glissonian approach. This approach should be possible in all cases in which the tumor does not extend so close to the right main pedicle as to make access impossible without compromising tumor clearance. The right anterior sectoral pedicle is isolated and clamped, and the right posterior pedicle is left intact; a line of demarcation extending along the right scissura develops rapidly (Fig. 90B.46).
The parenchymal transection phase is now carried out, opening the liver tissue from below, going upward, and keeping the plane of parenchymal dissection horizontal. Frequent use of IOUS facilitates continuing along the correct transection plane and spares injury to the right posterior pedicle and right hepatic vein. If significant bleeding occurs, it usually arises from branches of the right hepatic vein. Dissection carried out under low CVP (see Chapter 22) allows bleeding from the transected branches of the right vein to be visualized and controlled. When the right anterior sectoral pedicle has been identified, it should be test clamped prior to dividing it to ensure adequate perfusion to the posterior sector. The plane of transection is kept anterior to the right posterior pedicular structures. Unlike the hepatic veins, these structures are dense and white and should be readily appreciated.
Many large, expansile (pushing) tumors produce a narrow zone of compressed atrophic liver tissue just anterior to the posterior pedicular triad. In such instances, although tumor clearance is narrow, it can be achieved by peeling the tumor away from the residual parenchyma in this atrophic plane. With large tumors, the plane of dissection is dictated by the tumor (see Fig. 90B.6). For smaller tumors, the plane of dissection can be anatomically defined.
After removal of the resected specimen, the remnant (segments VI and VII) presents an en face raw surface area (Fig. 90B.47). Any bleeding from small branches of the hepatic artery or portal vein is controlled, and any visible open bile ducts are closed with fine, resorbable sutures. Final hemostasis is achieved as described earlier, and the omentum may be laid over the cut surface. An increased risk for biliary complications attends this operation, and three of the four patients initially reported by Starzl and coworkers (1982) had biliary complications: two had fistulae, and one had bile duct stricture.
Experience: Extended Left Hepatectomy
Extended left hepatectomy has been described in some detail, because large tumors arising from the left liver and extending to the right, previously considered unresectable, can be removed successfully with complete tumor clearance and a chance of cure (Blumgart et al, 1993; Soreide et al, 1985). As stated earlier, prediction of resectability is sometimes difficult; but in large tumors with a clear-cut margin on cross-sectional imaging and evidence of displacement, rather than invasion, of right-sided posterior sectoral intrahepatic structures (see Fig. 90B.6), exploration for excision is worthwhile (Soreide et al, 1985). Although high perioperative morbidity and mortality and poor outcome have been reported with this operation (Hasegawa et al, 1989), many small series lend support to its utility (Al-Hadeedi et al, 1990; Bismuth et al, 1989, 1992; Blumgart et al, 1993; Elias et al, 1992; Houssin et al, 1993; Huguet et al, 1994; Iwatsuki & Starzl, 1988; Starzl et al, 1982; Tsuzuki et al, 1990).
In a series of 51 patients who underwent extended left hepatectomy at MSKCC from 1992 through 1998 (Povoski et al, 1999), no intraoperative deaths were reported, and postoperative morbidity was 53%; biliary leak and abdominal abscess were the most commonly encountered complications. Postoperative mortality rate was 8%. Compared with historic controls for hepatic resection at MSKCC from 1985 through 1994 (Fong et al, 1995), in which overall postoperative morbidity was 40% and postoperative mortality rate was 4%, morbidity and mortality rates were greater for extended left hepatectomy, although length of hospital stay was identical. In a more recent study, Cho and colleagues (2010) reported no operative deaths in 15 consecutive patients, emphasizing the importance of adequate venous drainage of the liver remnant. The morbidity in this study was high (40%), underscoring the point that extended left hepatectomy remains one of the most difficult procedures in hepatic resection surgery.
Factors that influence resectability and oncologic efficacy, especially with regard to such extensive anatomic dissections, have long been debated. In the series of patients from MSKCC, the finding of positive microscopic margins or of tumor within one high-power field (HPF) was associated with the largest hepatic lesions (Povoski et al, 1999).
Left Lateral Sectorectomy (Left Lateral Segmentectomy)
The left lateral sector of the liver, which comprises segments II and III, is resected by approaching the portal triads to segments II and III in the umbilical fissure. Any bridge of tissue between segments III and IV is divided as described previously. The left lobe is mobilized, and dissection is carried out within the umbilical fissure to the left of the main triad; the pedicles to segments II and III usually can be dissected and controlled individually (see Fig. 90B.32). This approach is important in allowing tumor clearance for lesions close to the umbilical fissure. Alternatively, the liver may be split anteroposteriorly, just to the left of the ligamentum teres and the falciform ligament; as this is done, the vessels are encountered intrahepatically and divided serially. The latter approach is suitable only for more peripherally placed tumors.
As the liver tissue is divided, the left hepatic vein is encountered posteriorly and can be controlled within the liver substance. Division of the left hepatic vein usually completes the parenchymal transection. When a posteriorly situated tumor lies close to the junction of the left hepatic vein and the middle hepatic vein, it is best to approach the left hepatic vein extrahepatically, as described previously for left hepatectomy (see Figs. 90B.41 and 90B.42).