Chapter 58 Heparin-Induced Thrombocytopenia
Table 58-1 Risk Factors for Heparin-Induced Thrombocytopenia
| Heparin type: | Unfractionated > low-molecular-weight heparin > fondaparinux |
| Patient type: | Postoperative (major > minor surgery) > medical > obstetric/pediatric |
| Dose*: | Prophylactic dose > therapeutic dose > flushes |
| Duration: | 11-14 days† > 5-10 days > 4 days or fewer |
| Sex: | Female > male |
*Importance of heparin dose is uncertain because of confounding effect of patient type (e.g., postoperative patients tend to receive prophylactic-dose heparin whereas medical patients [e.g., with venous thromboembolism] are more likely to receive therapeutic-dose heparin); nevertheless, reported frequencies of heparin-induced thrombocytopenia (HIT) are relatively high in patients given postoperative prophylactic-dose heparin.
†Heparin exposure beyond 14 days does not usually increase the risk of HIT beyond that of an 11- to 14-day exposure.
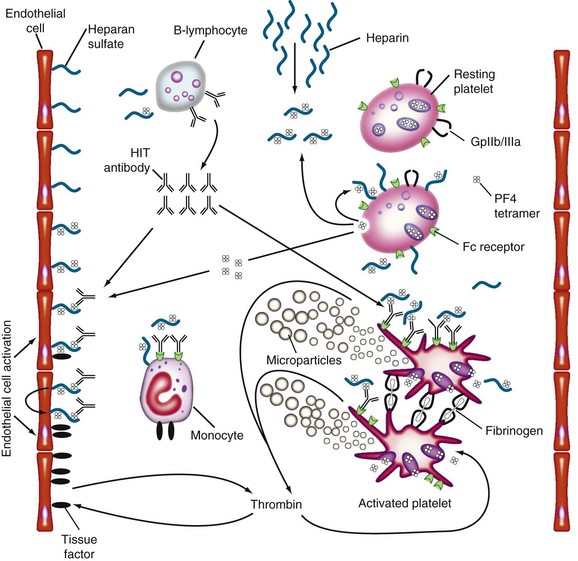
Figure 58-1 PATHOGENESIS OF HEPARIN-INDUCED THROMBOCYTOPENIA (HIT).
(Reprinted with permission from Greinacher A, Warkentin TE: Treatment of heparin-induced thrombocytopenia: An overview. In Warkentin TE, Greinacher A, editors: Heparin-induced thrombocytopenia, ed 4, New York, 2007, Informa Healthcare USA, p 287.)
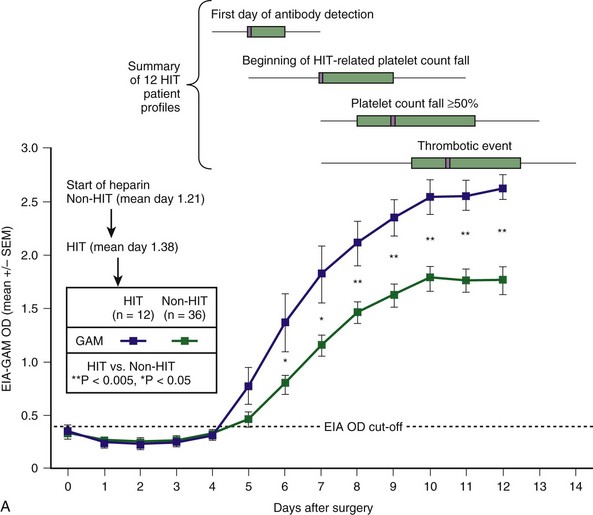
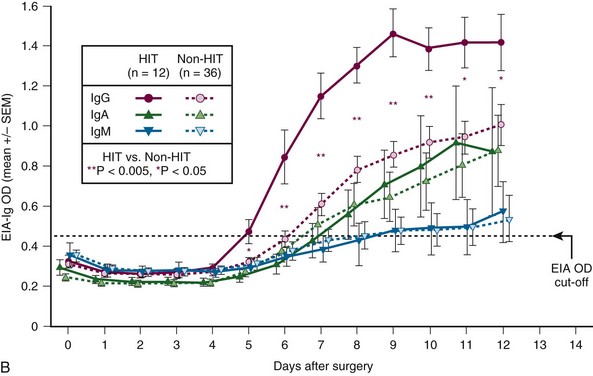
Figure 58-2 CHARACTERISTIC TIMELINE OF HEPARIN-INDUCED THROMBOCYTOPENIA (HIT).
(From Warkentin TE, Sheppard JI, Moore JC, et al: Studies of the immune response in heparin-induced thrombocytopenia. Blood 113:4693, 2009.)
Diagnostic Considerations in the Patient With Limb Ischemia and Thrombocytopenia
• Heparin-induced thrombocytopenia (HIT). Occlusion of large lower-limb arteries by platelet-rich “white clots” is characteristic of HIT. The major clue is an otherwise unexplained platelet count fall that begins 5 or more days after initiation of heparin. Urgent thromboembolectomy may be limb sparing. Sensitive assays for HIT antibodies give strongly positive results. See subsequent entry for warfarin.
• Adenocarcinoma-associated disseminated intravascular coagulation (DIC). Severe venous or arterial thrombosis can develop in patients with metastatic adenocarcinoma who have DIC. This often occurs within hours after stopping heparin. A clinical clue is an otherwise unexplained rise in platelet count that occurs with initial or repeated heparin therapy. See next entry for warfarin.
• Warfarin-induced phlegmasia cerulea dolens/venous limb gangrene. Coumarin anticoagulants, such as warfarin, can lead to venous ischemia (phlegmasia cerulea dolens) or venous limb gangrene in patients with DIC caused by HIT or adenocarcinoma. Limb loss can occur even though the limb pulses are palpable.
• Sepsis-associated microvascular thrombosis. Acquired natural anticoagulant depletion (e.g., markedly reduced antithrombin or protein C levels) can complicate DIC associated with sepsis, leading to acral limb ischemia or necrosis.
• Septic embolism. Rarely, infective endocarditis or aneurysmal thrombosis leads to the constellation of thrombocytopenia and acute limb ischemia.
• Antiphospholipid syndrome. Autoimmune thrombocytopenia and hypercoagulability can interact to produce acute limb ischemia and thrombocytopenia in patients with antiphospholipid syndrome.
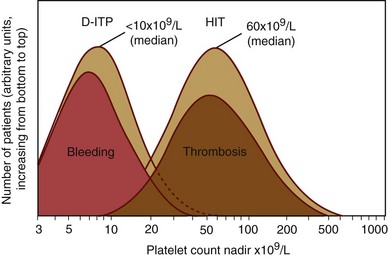
Figure 58-3 SEVERITY OF THROMBOCYTOPENIA IN DRUG-INDUCED IMMUNE THROMBOCYTOPENIA: A COMPARISON OF HEPARIN-INDUCED THROMBOCYTOPENIA (HIT) VERSUS OTHER DRUG-INDUCED IMMUNE THROMBOCYTOPENIA (DITP) DISORDERS.
(From Warkentin TE: Drug-induced immune-mediated thrombocytopenia—from purpura to thrombosis. N Engl J Med 356:891, 2007.)
Table 58-2 4Ts Scoring System: Estimating the Pretest Probability of Heparin-Induced Thrombocytopenia
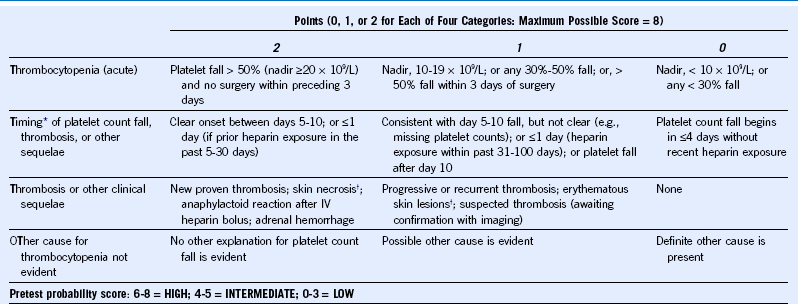
IV, Intravenous.
The scoring system shown here has undergone minor modifications from previously published scoring systems.
† Skin lesions occurring at heparin injection sites.
Reprinted, with permission, from Warkentin TE: Clinical picture of heparin-induced thrombocytopenia. In Warkentin TE, Greinacher A, editors: Heparin-induced thrombocytopenia, ed 4, New York, 2007, Informa Healthcare USA, pp 21–66.
Table 58-3 HIT Expert Probability (HEP) Scoring System

ATE, Arterial thromboembolism; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; HIT, heparin-induced thrombocytopenia; IABP, intra-aortic balloon pump; VAD, ventricular assist device; VTE, venous thromboembolism. In its initial evaluation, a HEP score of ≥4 points indicated an approximately 50% probability of HIT, whereas a score of ≤3 points was associated with only a 3% probability of HIT.
Reprinted, with permission, from Cuker A, Arepally G, Crowther MA, et al: The HIT Expert Probability (HEP) Score: A novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion, J Thromb Haemost 8:2642, 2010.
Table 58-4 Frequency of Thrombocytopenia (> 50% Platelet Count Fall) Among EIA+ Patients (Polyspecific or IgG-Specific Assay) Who Received Heparin (UFH or LMWH): A Comparison of SRA+ versus SRA− Status
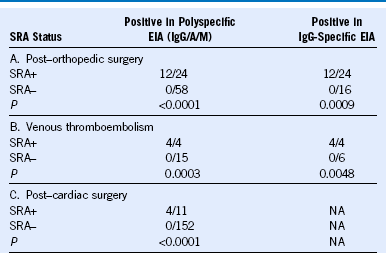
EIA, Enzyme immunoassay; LMWH, low-molecular-weight heparin; NA, not available; SRA+, positive in the serotonin-release assay; SRA−, negative in the serotonin-release assay; UFH, unfractionated heparin.
The data are consistent with the SRA having a high sensitivity for heparin-induced thrombocytopenia (> 95%); the specificity of the SRA depends on the clinical situation but in most circumstances is at least 90%. Patients in studies A and B were tested in both the polyspecific and IgG-specific assays.
Reprinted, with modifications, with permission, from Warkentin TE: How I diagnose and manage HIT, Hematology Am Soc Hematol Educ Program 2011:143, 2011.
Table 58-5 Comparison of the Two Classes of Anticoagulants Used to Treat Heparin-Induced Thrombocytopenia
| Indirect (AT-Dependent) Factor Xa Inhibitors: Danaparoid, Fondaparinux | Direct Thrombin Inhibitors: r-Hirudin (Lepirudin, Desirudin), Argatroban, Bivalirudin | |
|---|---|---|
| Half-life | √ Long (danaparoid, 25 hr*; fondaparinux, 17 hr): reduces risk of rebound hypercoagulability | Short (< 2 hr): potential for rebound hypercoagulability |
| Dosing | √ Both prophylactic- and therapeutic-dose regimens† | Prophylactic-dose regimens are not established (exception: subcutaneous desirudin) |
| Monitoring | √ Direct (anti-Xa levels): accurate drug levels obtained | Indirect (aPTT): risk for DTI underdosing due to aPTT elevation caused by non-DTI factors, including HIT-associated DIC |
| Effect on INR | √ No significant effect: thus simplifies overlap with warfarin | Increases INR: argatroban > bivalirudin > r-hirudin; complicates warfarin overlap |
| Protein C pathway | √ Adverse effect unlikely (with reduced thrombin generation, there will be less thrombin to activate protein C) | Thrombin inhibition could impair thrombin-mediated activation of protein C pathway |
| Reversibility of action | √ Irreversible inhibition: AT forms covalent bond with factor Xa | Irreversible inhibition only with r-hirudin |
| Efficacy and safety in non-HIT settings | √ Treatment and prophylaxis of VTE (danaparoid, fondaparinux) and ACS (fondaparinux) | Not established for most non-HIT settings (exception: bivalirudin established for PCI) |
| Platelet activation | √ Danaparoid inhibits platelet activation by HIT antibodies | No effect |
| Inhibition of clot-bound thrombin | No effect | √ Inhibition of both free and clot-bound thrombin |
| Drug clearance | Predominantly renal | Variable (predominantly hepatobiliary: argatroban; predominantly renal: r-hirudin) |
| Cost | √ Relatively low‡ | Relatively high‡ |
ACS, Acute coronary syndrome; aPTT, activated partial thromboplastin time; AT, antithrombin; DIC, disseminated intravascular coagulation; DTI, direct thrombin inhibitor; HIT, heparin-induced thrombocytopenia; INR, international normalized ratio; PCI, percutaneous coronary intervention; VTE, venous thromboembolism.
Check mark (√) indicates favorable feature in comparison of drug classes (author’s opinion).
*For danaparoid, half-lives of its anti-thrombin (anti-IIa) and its thrombin generation inhibition activities (2-4 hr and 3-7 hr, respectively) are shorter than for its anti-Xa activity (approximately 25 hr).
†Although therapeutic dosing is recommended for HIT, availability of prophylactic-dose regimens increases flexibility when managing potential non-HIT situations.
‡Another cost consideration is that a patient can be discharged to home on subcutaneous danaparoid or fondaparinux, whereas an additional 5-7 in-hospital days may be required for DTI-warfarin overlap before discharge from hospital.
Reprinted, with modifications, with permission, from Warkentin TE: Agents for the treatment of heparin-induced thrombocytopenia, Hematol Oncol Clin North Am 24:755, 2010.
Table 58-6 Fondaparinux for Treatment of Acute Heparin-Induced Thrombocytopenia
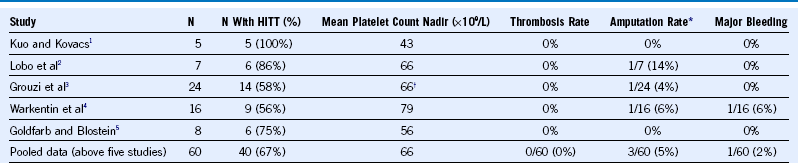
HITT, Heparin-induced thrombocytopenia and thrombosis (in this context, the thrombosis preceded treatment with fondaparinux).
Table 58-7 Treatment Schedules for Danaparoid and Fondaparinux
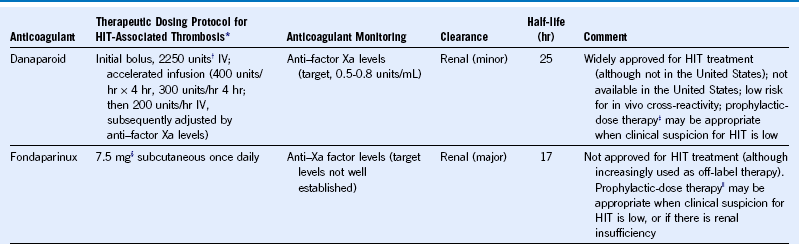
HIT, Heparin-induced thrombocytopenia; IV, intravenous.
|| Prophylactic-dose regimen, 2.5 mg subcutaneous every day (assumes normal renal function).
Table 58-8 Treatment Schedules for Lepirudin, Desirudin, Bivalirudin, and Argatroban
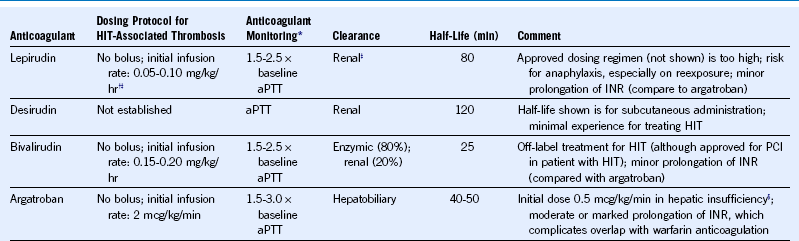
aPTT, Activated partial thromboplastin time; HIT, heparin-induced thrombocytopenia; INR, international normalized ratio; PCI, percutaneous coronary intervention.
Dosing protocols shown are appropriate for most patients with strongly suspected or confirmed HIT whether or not complicated by thrombosis. (Dosing for bivalirudin and argatroban is substantially different when given for PCI.)
1 Kuo KHM, Kovacs MJ. Successful treatment of heparin-induced thrombocytopenia with fondaparinux. Thromb Haemost. 2005;93:999.
2 Lobo B, Finch C, Howard A, et al. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost. 2008;99:208.
3 Grouzi E, Kyriakou E, Panagou I, et al. Fondaparinux for the treatment of acute heparin-induced thrombocytopenia: A single-center experience. Clin Appl Thromb Hemost. 2010;16:663.
4 Warkentin TE, Pai M, Sheppard JI, et al. Fondaparinux treatment of acute heparin-induced thrombocytopenia confirmed by the serotoninrelease assay: A 30-month, 16-patient case series. J Thromb Haemost. 2011;9:2389.
5 Goldfarb MJ, Blostein MD. Fondaparinux in acute heparin-induced thrombocytopenia: A case series. J Thromb Haemost. 2011;9:2501.

 , and seropositive non-HIT controls by
, and seropositive non-HIT controls by  . On each day beginning on postoperative day 6, there is a significant difference in the mean of the OD levels between the patients with HIT and the seropositive non-HIT controls (P < .05 by nonpaired t test). At the top of the figure, summary data for 12 HIT patient profiles are shown for four key events (first day of antibody detection, beginning of HIT-related platelet count fall, platelet count fall ≥50%, and thrombotic event), summarized as median (small purple squares within rectangles), interquartile range (rectangles), and range (ends of thin black lines). B, Mean (±SEM) OD values of anti-PF4/heparin antibodies detected using an in-house immunoassay (EIA-Ig) that detects antibodies of the individual immunoglobulin classes, IgG (red circles), IgA (green triangles), and IgM (blue inverted triangles) for HIT (solid symbols) and non-HIT (open symbols). On each postoperative day beginning on day 5, there is a significant difference in the mean of the OD units for the IgG immunoassay between the patients with HIT and the seropositive non-HIT controls (**P < .005 for days 6 to 10; *P < .05 for days 5, 11, and 12).
. On each day beginning on postoperative day 6, there is a significant difference in the mean of the OD levels between the patients with HIT and the seropositive non-HIT controls (P < .05 by nonpaired t test). At the top of the figure, summary data for 12 HIT patient profiles are shown for four key events (first day of antibody detection, beginning of HIT-related platelet count fall, platelet count fall ≥50%, and thrombotic event), summarized as median (small purple squares within rectangles), interquartile range (rectangles), and range (ends of thin black lines). B, Mean (±SEM) OD values of anti-PF4/heparin antibodies detected using an in-house immunoassay (EIA-Ig) that detects antibodies of the individual immunoglobulin classes, IgG (red circles), IgA (green triangles), and IgM (blue inverted triangles) for HIT (solid symbols) and non-HIT (open symbols). On each postoperative day beginning on day 5, there is a significant difference in the mean of the OD units for the IgG immunoassay between the patients with HIT and the seropositive non-HIT controls (**P < .005 for days 6 to 10; *P < .05 for days 5, 11, and 12).