Chapter 20 Hemodynamic Instability and Resuscitation
Hemodynamic instability refers to abnormalities of heart rate, blood pressure, filling pressures, or cardiac output that if uncorrected result in organ dysfunction. Hemodynamic instability is common in the intensive care unit (ICU) and encompasses a range of clinical states, from cardiac arrest to subtle tissue hypoperfusion. When hemodynamic instability is obvious and severe, timely intervention may be lifesaving. Equally important is the early recognition and treatment of slowly evolving or subclinical tissue hypoperfusion which, untreated, may progress to organ failure.
This chapter is divided into three sections: (1) postoperative hypertension; (2) hypotension and low cardiac output; (3) cardiac arrest and near cardiac arrest. Relevant physiology and pharmacology are discussed in Chapters 1 and Chapter 3, respectively; echocardiography is reviewed in Chapter 7; and hemodynamic monitoring is discussed in Chapter 8.
POSTOPERATIVE HYPERTENSION
Hypertension occurs because of increased systemic vascular resistance, increased cardiac output, or both (see Eq. 1-5). With increased systemic resistance, diastolic, systolic, and mean arterial pressures (MAP) are all increased. With increased stroke volume, diastolic pressure is usually normal or low, and pulse pressure is high. Postoperative hypertension has a number of causes (Table 20-1), but in most cases the primary problem is increased systemic vascular resistance. Surgical stress, hypothermia, patient anxiety, and inadequate analgesia lead to activation of the sympathetic nervous and renin-angiotensin-aldosterone systems. Essential hypertension is common and routine antihypertensive medications may have been withheld during the perioperative period.
Table 20-1 Etiology of Hypertension Following Cardiac Surgery
| Common Causes of Hypertension Following Cardiac Surgery |
| Pain |
| Anxiety (including paralysis combined with inadequate sedation) |
| Withdrawal of usual oral antihypertensive treatment (particularly β blockers) |
| Inappropriate vasopressor therapy |
| Hypervolemia |
| Hypothermia |
| Shivering |
| Patient-ventilator dysynchrony |
| Poorly controlled essential hypertension |
| Uncommon Causes of Hypertension Associated With Cardiac Surgery |
| Myocardial ischemia or infarction |
| Acute left ventricular failure |
| Drug treatment (corticosteroids, cyclosporine) |
| Coarctation of the aorta |
| Aortic dissection |
| Intracranial catastrophe |
| Uncommon Causes of Hypertension not Associated With Cardiac Surgery |
| Renal disease (including renal artery stenosis, end-stage renal disease, glomerulonephritis, etc.) |
| Endocrine dysfunction (including primary hyperaldosteronism, Cushing syndrome, pheochromocytoma, renin-producing tumor) |
| Toxemia of pregnancy |
| Arteritis |
Before antihypertensive drugs are given, the common causes of postoperative hypertension (see Table 20-1) should be considered and corrected. Clinicians must also assess the patient’s intravascular volume status and cardiac output (see subsequent discussion).
For intravenous antihypertensive therapy, vasodilators such as nitroglycerin, nicardipine, and nitroprusside are an attractive first choice because in patients with elevated systemic vascular resistance, they maintain or augment cardiac output. However, nitroglycerin may be ineffective in treating severe hypertension, and nitroprusside can cause marked hypotension, reflex tachycardia, and hypoxemia. Nesiritide may be considered when circulating volume is increased and filling pressures are high. If cardiac output is low, an inodilating drug such as milrinone is a good choice. Fluid loading may have to accompany vasodilating and inodilating drugs.
HYPOTENSION AND LOW CARDIAC OUTPUT
Recognizing and Treating Hemodynamic Instability
Hypotension
There are limited data concerning what constitutes an ideal blood pressure in critically ill patients. In one study of patients with septic shock, increasing MAP from 65 to 85 mmHg with norepinephrine did not improve markers of tissue perfusion or renal function.1 In the absence of similar studies in cardiac surgical patients, a target MAP of more than 65 mmHg is a reasonable goal for most patients. Suggested blood pressure targets in different groups of cardiac surgery patients are listed in Table 20-2.
Table 20-2 Blood Pressure Targets in the First 48 Hours Following Cardiac Surgery
| Normal (MAP >65 mmHg) |
| Default blood pressure goal |
| High (MAP >75-85 mmHg) |
| Age >75 years |
| Multiple arterial grafts |
| Preoperative or evolving renal impairment |
| Poorly controlled hypertension |
| History of ischemic stroke |
| New neurologic deficit postoperatively |
| Significant uncorrected carotid stenoses |
| Low (MAP >55-60 mmHg) |
| Age <50 years with no history of hypertension |
| High bleeding risk |
| Low blood pressure preoperatively with normal renal function |
| Valve surgery for chronically regurgitant valve lesions |
MAP, mean arterial pressure.
Low Cardiac Output and Tissue Hypoperfusion
Unlike blood pressure, cardiac output is not routinely measured in all patients, and clinicians often rely on clinical and biochemical markers of tissue hypoperfusion (Table 20-3). Unfortunately, clinical assessment of cardiac output is unreliable in the first few hours after cardiac surgery.2 Cool peripheries are normal findings. Polyuria is common, even with evolving renal dysfunction. Tachycardia may be absent due to the inhibitory effects of cardiopulmonary bypass (CPB) and surgery on the cardiac conducting system. Lactic acidosis is suggestive of tissue hypoperfusion, but other causes (e.g., β2-agonist drugs; see Table 31-3) may be responsible. Not uncommonly, low cardiac output occurs in the absence of lactic acidosis. A useful screening tool for low cardiac output is the oxygen saturation of blood drawn from the proximal port of a central venous catheter (i.e., from the superior vena cava; SSVCO2), which provides a close approximation of a true mixed venous oxygen saturation SVO2.3 Values above 65% are reassuring; values below 55% warrant further investigation. Other findings that demand further investigations include escalating inotropic support, a central venous pressure (CVP) above 15 mmHg, and unexplained metabolic acidosis (lactate >5 mmol/l, base deficit >6).
Table 20-3 Clinical and Biochemical Signs Consistent with Inadequate Cardiac Output
| Clinical |
| Cool, clammy peripheries |
| Sweating |
| Central hyperthermia |
| Oliguria with concentrated urine |
| Sinus tachycardia and atrial fibrillation |
| Narrow pulse pressure |
| Biochemical |
| Metabolic acidosis |
| Elevated lactate |
| Hyperkalemia |
| Low SVO2 or SSVCO2 |
SSVCO2, superior vena cava oxygen saturation; SVO2, mixed venous oxygen saturation.
If low cardiac output is suspected—and the cause is not readily apparent on the basis of routine clinical assessment—cardiac output should be measured. Numerous devices may be used (see Chapter 8), but in cardiac surgery units, the pulmonary artery catheter (PAC) is the device most commonly employed. A PAC also allows measurement of pulmonary arterial pressure, pulmonary artery wedge pressure (PAWP), and SVO2. As with blood pressure, the normal value for cardiac output is ill defined. By convention, a lower limit of 2.2 l/min/m2 is widely used; however, many “well” cardiac surgery patients have values below this figure.2 Also, the appropriate cardiac output depends on the patient’s metabolic state. Thus, in a patient with a marked systemic inflammatory response to CPB, a cardiac output of 4 l/min/m2 may be appropriate, whereas in a sedated, mildly hypothermic patient, a value of 2 l/min/m2 may be satisfactory. As a simple guide, if filling pressures are normal (PAWP <15 mmHg, CVP <12 mmHg), and venous oxygen saturation is satisfactory (>65%), a cardiac output as low as 1.8 l/min/m2 is probably acceptable.
Diagnosis and Treatment
Causes of hemodynamic instability in the early postoperative period are listed in Table 20-4. A stepwise approach to the diagnosis and initial treatment of hemodynamic instability is provided in Table 20-5. Important diagnostic clues can be obtained from the patient’s history and intraoperative course. The operation notes, angiograms, and echocardiograms should be reviewed. Examination should focus on the cardiovascular system, in particular the presence of any new murmurs. Specific diagnoses may be suggested on the basis of the electrocardiogram (ECG), CVP, and arterial pressure waveforms (see Chapter 8). If the ECG trace is abnormal, 12-lead and atrial ECGs should be obtained. Respiratory problems can cause hemodynamic instability, and a careful respiratory system examination, including checking the ventilator circuit and settings, should be performed. Chest drain bottles should be inspected for blood loss and bubbling. Important trends may be identified on the 24-hour ICU chart. Further blood tests, such as arterial blood gases, complete blood count, coagulation status, and troponin may be indicated, depending on the circumstances. Chest radiographs should be reviewed.
Table 20-4 Causes of Hemodynamic Instability in the Early Period Following Cardiac Surgery
| Common | Uncommon |
|---|---|
| Patient-ventilator dysynchrony | Severe mitral regurgitation |
| Hypovolemia | Other valvular pathology |
| Low systemic vascular resistance | Dynamic LVOT obstruction |
| Left ventricular systolic dysfunction | Dynamic lung hyperinflation |
| Left ventricular diastolic dysfunction | Tension pneumothorax |
| Right ventricular dysfunction | Massive hemothorax |
| Pericardial compression (tamponade) | |
| Rhythm disturbance |
LVOT, left ventricular outflow tract.
Table 20-5 A Stepwise Approach to the Diagnosis and Initial Treatment of the Hemodynamically Unstable Postoperative Cardiac Patient
| Step 1. Confirm the Presence of Hemodynamic Instability. |
| Check the level and zero all transducers. Relevel and rezero all pressure transducers if necessary. |
| Check all infusion pumps and the integrity of all infusion lines. |
| Step 2. Does the Patient Have an Immediately Life-threatening Problem (i.e., MAP < 50 mmHg)? |
| If so, go to Fig. 20-3. |
| Step 3. Clinically Assess the Patient (Steps 3 and 4 Should Occur Simultaneously). |
| Perform a targeted physical exam, concentrating on the cardiac and respiratory systems, the ventilator, and the chest drains. |
| Review the 24-hour chart. |
| Obtain relevant investigations: blood gases, SSVCO2, ECG (atrial and 12-lead), and chest radiograph. |
| Review the old notes and the intraoperative course. |
| Inform the surgeon. |
| Step 4. Consider the Following Interventions. |
| Paralyze and sedate the patient and ventilate with 100% oxygen. If indicated, disconnect the patient from the ventilator and hand ventilate with a manual resuscitator. |
| Pace the heart at 90 beats/min using DDD or DOO mode at maximum output (see Chapter 21). |
| Administer a fluid challenge of 500 ml of a crystalloid (e.g., normal saline or Plasma-Lyte). |
| Commence or increase inotropic support. |
| Step 5. If the Diagnosis Remains Uncertain, Insert a PAC and/or Perform an Echocardiogram. |
| If there is clinical suspicion of low or high cardiac output, a PAC should be inserted. |
| If a PAC is in place and cardiac output is low, an echocardiogram should be performed. |
| If the patient remains hypotensive and the cause is unclear, an echocardiogram should be performed. |
| If a specific diagnosis (e.g., tamponade) is suspected clinically, an echocardiogram should be performed. |
ECG, electrocardiogram; MAP, mean arterial pressure; PAC, pulmonary artery catheter.
If the cause of hypotension or low cardiac output is not rapidly apparent, an echocardiogram should be performed. Pulmonary artery catheterization and echocardiography are complementary techniques: a PAC is preferred for measuring cardiac output, whereas an echocardiogram is preferred for diagnosing the cause of low cardiac output and hypotension. In the ICU, transesophageal echocardiography (TEE) offers significant advantages over transthoracic echocardiography (TTE) (see Chapter 7).
Causes of Early Hemodynamic Instability
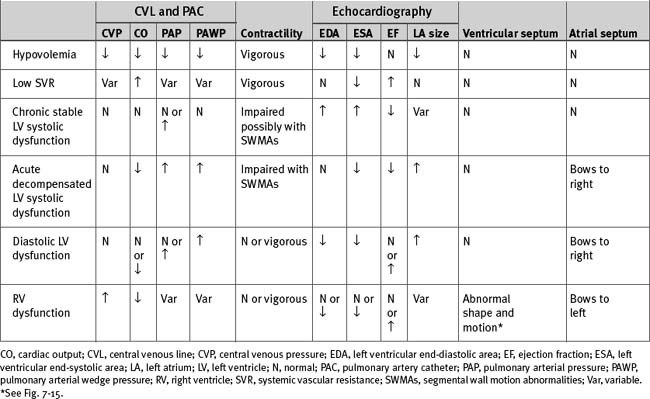
The causes of hemodynamic instability that occur within the first 48 hours after cardiac surgery are listed in Table 20-4; important hemodynamic and echocardiographic findings are summarized in Table 20-6.
Hypovolemia
Hypovolemia is a reduction in circulating blood volume such that left ventricular preload is inadequate to optimize cardiac output (see Chapter 1). Preload is usually equated with left ventricular end-diastolic volume (LVEDV), but surrogates such as left ventricular end-diastolic area (LVEDA), PAWP, and CVP are also used.
In the general ICU environment, approximately 50% of hypotensive patients are fluid responsive.4 Hypovolemia is common following cardiac surgery, as a consequence of blood, urinary, and third-space fluid losses. Blood loss may be visible (seen in the chest drains) or occult (unseen in the chest, retroperitoneum, or gastrointestinal tract). Urinary losses may be substantial in the first few hours following surgery, particularly if hypothermic CPB has been employed or mannitol has been used in the circuit prime. Third-space losses are variable but may involve several liters. In one study, the average weight gain after cardiac surgery was 7.2% of body weight on the first postoperative day.5 Thus, hypovolemia can occur despite a substantial increase in total extracellular volume. In critically unwell patients, hypovolemia and systemic edema often coexist.
Diagnosis
Hypovolemia is usually obvious on the basis of clinical signs and invasive hemodynamic monitoring. However, in certain situations the clinical picture is confusing. Young patients with intact cardiovascular reflexes can have important hypovolemia without showing clinical indicators, whereas modest hypovolemia can cause profound hypotension in elderly patients who are mechanically ventilated and have blunted sympathetic tone. Hypovolemia frequently coexists with other causes of hemodynamic instability, complicating the diagnosis. The surrogates of preload, CVP and pawp, are often misleading (see Chapters 1 and Chapter 8).
Hemodynamic Monitoring.
Hypovolemia is normally characterized by hypotension in association with low atrial pressures. A patient with a CVP below 8 mmHg or a PAWP below 10 mmHg, in the presence of hypotension, is invariably fluid responsive. However, there are no absolute values for CVP and PAWP that predict fluid responsiveness,6,7 and in many circumstances patients with much higher filling pressures also benefit from fluid administration. High CVP may be caused by right ventricular dysfunction or high intrathoracic or intrapericardial pressure. A CVP above 15 mmHg is occasionally required so as to optimize cardiac output in patients with right ventricular dysfunction.4,6 Conversely, in patients with left ventricular dysfunction, CVP may be low despite circulatory overload. In cases of severe left ventricular hypertrophy, PAWP may have to be above 18 mmHg to optimize preload.4 The causes of high CVP and PAWP are listed in Tables 8-3 and 8-4, respectively.
In contrast to absolute values of CVP and PAWP, respiratory fluctuations in CVP and the arterial pressure waveform (pulsus paradoxus) are predictive of fluid responsiveness (see Chapter 8).4 A patient with a respiratory swing on the arterial waveform is invariably fluid responsive, irrespective of the atrial pressure.
Echocardiography.
However, because ventricular volume is also determined by systolic and diastolic function, there is no absolute value for LVEDA that predicts fluid responsiveness.8 In patients with normal ventricular function, euvolemia is associated with an LVEDA of 12 to 17 cm2. However, with systolic dysfunction, much higher values may be required so as to optimize stroke volume; with diastolic dysfunction, preload may be optimal at a lower end-diastolic area.
Changes in Hematocrit.
A rising hematocrit suggests that urinary losses or third-space losses are not being matched by adequate fluid resuscitation. Sudden blood loss does not lead to an immediate fall in hematocrit. However, over minutes to hours, as fluid is redistributed from the interstitium and cells, hematocrit will fall even without fluid resuscitation. Conversely, vigorous fluid administration will result in a fall in hematocrit without blood loss.
Treatment
Hypovolemia may be treated with crystalloid or colloid solutions (see Chapter 32). Neither fluid has a protective benefit against pulmonary edema or is associated with improved patient outcome.9 With crystalloid fluid, approximately one and a half times the volume of a colloid must be administered to achieve the same clinical effect.9
In critically unwell patients, the PAWP associated with optimal stroke volume may cause pulmonary edema. Therefore a balance must be struck between gas exchange and cardiac output. In mechanically ventilated patients, modest increases in positive end-expiratory pressure (PEEP) (e.g., from 5 to 10 cm H2O) may protect against pulmonary edema and allow a higher PAWP to be tolerated. Conversely, in the presence of intrapulmonary shunting, low cardiac output is associated with impaired gas exchange (see Chapter 27). Thus, in hypovolemic patients, fluid administration may improve oxygenation.
Low Systemic Vascular Resistance
Pathologic vasodilatation after cardiac surgery is most commonly a manifestation of severe inflammatory response to CPB and surgical stress (see Chapter 2). Other causes include vasodilator drug therapy, anaphylaxis, sepsis, and adrenal failure. Low systemic resistance occurs in 20% to 45% of patients after cardiac surgery,10,11 and severe vasodilatory shock occurs in 5% to 10%.11,12 Risk factors for pathologic vasodilatation include low ejection fraction, prolonged CPB, and prior treatment with ACE inhibitors.
Diagnosis
The cardinal features of systemic vasodilation are hypotension and high cardiac output. Calculated systemic vascular resistance is low (see Eq. 1-5). Atrial pressures are normal or low, but enthusiastic fluid administration may lead to high filling pressures. Diastolic blood pressure is usually low (<50 mmHg) and pulse pressure is usually high (>50 mmHg).
Warm, dilatated peripheries are not a consistent finding in vasodilatated patients in the first few hours after surgery. If the cause of the vasodilatation is systemic inflammation, there may be signs of multiorgan involvement, particularly impaired gas exchange (with evidence of pulmonary edema on chest radiograph), fever, leukocytosis, abnormal liver function, and oliguria. Serum amylase may be mildly elevated.
Treatment
Concomitant hypovolemia must be corrected, but there is little gain in boosting atrial pressures to supranormal values. Hypotension should be treated with a norepinephrine infusion titrated to an appropriate MAP. If hypotension persists despite high-dose norepinephrine (>0.3 to 0.6 μg/kg/min), vasopressin (0.01 to 0.04 units/min) may be considered. The use of methylene blue (1.5 mg/kg over 5 to 10 minutes) has been reported, and in one small series it appeared to improve survival rates in postcardiac surgery vasoplegic shock.12 As inadequate stress release of cortisol exacerbates vasoplegic shock, treatment with hydrocortisone (100 mg intravenously 8 hourly) may be considered.13 In a patient with a low ejection fraction, it is important to ensure that treatment with vasopressors does not precipitate a major fall in cardiac output.
Left Ventricular Systolic Dysfunction
Left ventricular systolic dysfunction may be acute, chronic, or a combination of both.
Chronic
Chronic left ventricular systolic dysfunction results in remodeling (see Chapter 1), usually as a consequence of myocardial infarction, chronic ischemia (hibernating myocardium), or valvular heart disease. Ejection fraction is reduced and ventricular volumes are increased. A patient with compensated heart failure may have a normal cardiac output and left atrial pressure and, despite a low ejection fraction, may have good functional capacity. Such a patient often has an uneventful postoperative course. However, decompensation may occur if: (1) there is a further myocardial insult (e.g., myocardial stunning); (2) ventricular afterload is abruptly increased (e.g., after tracheal extubation or the withholding of usual vasodilator therapy); (3) there is a requirement for increased cardiac output (e.g., a severe inflammatory response to CPB).
Acute
Acute left ventricular systolic dysfunction occurs due to myocardial ischemia, acute infarction, myocardial stunning, drugs (e.g., β blockers), or systemic inflammation. Some degree of myocardial stunning occurs in all patients after cardiac surgery.14 However, severe postoperative stunning is likely when: (1) CPB is complicated or greatly prolonged; (2) severe myocardial ischemia was present preoperatively. With acute dysfunction, ejection fraction is reduced but ventricular volumes may be relatively normal. Cardiac output is reduced, left atrial pressure is increased, and there may be signs of pulmonary congestion and tissue hypoperfusion.
Myocardial ischemia must always be considered in any patient who develops new left ventricular systolic dysfunction. The patients at the highest risk for myocardial ischemia are those undergoing coronary revascularization (see Chapter 9), but ischemia can also occur in other situations, namely, the obstruction of a coronary ostium during aortic valve replacement; the kinking or obstruction of a coronary artery following its reimplantation during aortic root replacement; damage to the circumflex coronary artery during mitral valve surgery; and damage to the septal branch of the left anterior descending coronary artery as it runs in front of the pulmonary valve during the Ross procedure (see Chapter 10).
Diagnosis
Echocardiography.
With acute dysfunction, ventricular volumes may be relatively normal. Changes in wall motion are typically regional rather than global. The left atrium may appear tense and enlarged and may show rightward bowing of the interatrial septum. With tissue Doppler, the E deceleration time is likely to be short (<140 ms) and the E:Em ratio is likely to be high (<15; see Chapter 7).
The diagnosis of postoperative myocardial ischemia rests on clinical suspicion and characteristic abnormalities on the ECG, echocardiogram, and biochemical markers (see Chapters 9 and Chapter 18).
Treatment
Preload, heart rate, and rhythm should be optimized. A heart rate of 80 to 100 beats per minute, using pacing if necessary, is appropriate for most patients. Inotropic support should then be commenced and titrated to effect. The choice of drug depends on the hemodynamic state. If cardiac output is low but blood pressure is satisfactory, an inodilator such as dobutamine or milrinone may be used. If both blood pressure and cardiac output are low, epinephrine or a combination of norepinephrine and milrinone (or dobutamine) may be used. If hemodynamic instability persists despite modest inotropic support (epinephrine 0.1 to 0.2 μ/kg/min or equivalent), insertion of an intraaortic balloon pump (IABP) should be considered (see Chapter 22). In patients with systolic ventricular dysfunction, high-dose norepinephrine can cause a precipitous fall in cardiac output and should be avoided unless cardiac output is being measured.
Combinations of inotropic drugs at high dosages (e.g., norepinephrine plus milrinone; or epinephrine/dopamine plus milrinone, and vasopressin) in conjunction with an IABP are sometimes required for severe postoperative myocardial stunning, and biventricular failure. If the hemodynamic state remains inadequate despite an IABP and combination high-dosage inotropic pharmacotherapy, and cardiac dysfunction is potentially reversible, placement of a ventricular assist device should be considered (see Chapter 22).
Severe acidemia (pH ≤7.0) exacerbates ventricular dysfunction and inhibits the actions of inotropic drugs15,16; early institution of renal replacement therapy should be considered (see Chapter 33). Correction of the pH with sodium bicarbonate does not improve the hemodynamic state.17,18
Weaning Therapy
Following a period of hemodynamic stability, weaning from cardiovascular support can commence. This should be done in a staged manner. Rapid, contemporaneous weaning of inotropic support, IABP, and mechanical ventilation can precipitate acute heart failure. Once inotropic support has been weaned to a modest level (epinephrine <0.1 μg/kg/min or the equivalent), it is appropriate to wean either ventilation or the IABP. If gas exchange is good and the patient is relatively intolerant of the endotracheal tube, mechanical ventilation may be weaned first. The disadvantage of this approach is the patient must be extubated lying relatively flat. If the patient cannot be weaned rapidly from mechanical ventilation or is agitated, he or she should be kept sedated and weaned from the IABP first (see Chapter 22). If the IABP can be removed and the patient extubated within 2 to 4 days, it is useful to leave the PAC in place until these events have occurred. Assuming renal function is intact and blood pressure is satisfactory, as inodilators are stopped, low-dosage ACE inhibitor therapy may be commenced to maintain a low afterload state.
Right Ventricular Dysfunction
Right ventricular dysfunction has a number of causes (Table 20-7) and accounts for about 20% of cases of circulatory failure that occur following cardiac surgery.19 There are three underlying mechanisms: (1) systolic dysfunction; (2) volume overload; and (3) pressure overload, usually caused by increased pulmonary vascular resistance. Systolic dysfunction occurs due to myocardial stunning or coronary insufficiency. Stunning may be the result of inadequate myocardial protection of the right ventricle during aortic cross clamping, such as that which may occur with a critical right coronary stenosis or when only retrograde cardioplegia is used. Right ventricular systolic dysfunction may occur due to gas or particulate emboli in the anteriorly placed right coronary artery during open-heart (i.e., valvular)surgery. Right ventricular volume overload may be chronic (e.g., due to tricuspid valve disease) or may be precipitated by iatrogenic fluid overload. Elevated pulmonary vascular resistance is common following cardiac surgery and is an important cause of right ventricular dysfunction; it is discussed in Chapter 24. In many circumstances, the cause of right ventricular dysfunction is multifactorial.
| Systolic Dysfunction |
| Myocardial stunning |
| Myocardial infarction or ischemia |
| Right Ventricular Volume Overload |
| Acute |
| Excess fluid administration |
| Chronic |
| Pulmonary valve regurgitation (e.g., secondary to repaired tetralogy of Fallot) |
| Structural tricuspid valve regurgitation (Epstein anomaly, endocarditis, rheumatic valve disease) |
| Atrial septal defect |
| Ruptured sinus of Valsalva aneurysm |
| Right Ventricular Pressure Overload* |
| Acute |
| Perioperative events (mechanical ventilation and PEEP, ventilator dysynchrony, SIRS, hypoxemia, hypercarbia, acidemia) |
| Massive pulmonary embolus |
| Chronic |
| Chronic pulmonary disease (cor pulmonale) |
| Left heart disease (mitral stenosis, mitral regurgitation, end-stage aortic valve disease) |
| Pulmonary vascular occlusive disease (Eisenmenger syndrome, chronic pulmonary embolism, primary pulmonary hypertension) |
| Pulmonary valve stenosis |
PEEP, positive end-expiratory pressure; SIRS, systemic inflammatory response syndrome.
Diagnosis
Right ventricular failure is manifested as a high CVP (with V waves) in association with a falling blood pressure and cardiac output. Pulmonary arterial pressure may be normal, high, or low, depending on the cause and on cardiac output. (Assuming pulmonary vascular resistance and left atrial pressure remain constant, a fall in cardiac output results in a fall in pulmonary arterial pressure; refer to Eq. 1-6.) With primary volume overload and right ventricular systolic dysfunction, pulmonary vascular resistance is not elevated; therefore, pulmonary arterial pressure is not increased. There is a small step-up in pressure between the CVP and mean pulmonary arterial pressure (<10 mmHg). By contrast, pulmonary arterial pressure is increased with pressure overload, reflecting increased pulmonary vascular resistance. The transpulmonary pressure gradient is elevated but PAWP may be normal or low.
Echocardiographic features include right ventricular dilation, impaired contractility, tricuspid regurgitation, leftward bowing of the interatrial septum, and characteristic changes in the shape and motion of the interventricular septum (see Fig. 7-15). With chronic pressure overload, right ventricular hypertrophy may be evident. With severe right ventricular dilation, the left ventricle may appear small and underfilled due to leftward displacement of the interventricular septum. In this situation, PAWP may be increased.
Treatment
Traditionally, fluid loading has been advocated for patients with right ventricular infarction,20,21 even though there is little evidence that this strategy improves cardiac output or blood pressure.22,23 Furthermore, aggressive fluid administration reduces right ventricular perfusion pressure (by increasing right ventricular end-diastolic pressure); causes leftward displacement of the interventricular septum; and exacerbates tricuspid regurgitation. Only rarely does the CVP need to be greater than 15 mmHg.
Inotropes and vasopressors help to maintain right ventricular perfusion pressure and to augment contractility. Milrinone plus norepinephrine or dobutamine plus norepinephrine are good choices. For decompensated right ventricular pressure overload, maintaining right ventricular perfusion pressure, usually with norepinephrine, is critically important (see Chapter 24).
Afterload reduction is potentially beneficial. Simple measures such as avoidance of hypoxemia, hypercarbia, and acidemia are important. Airway pressure and PEEP should be kept as low as possible. The use of pulmonary vasodilators, such as inhaled nitric oxide, inhaled or intravenous prostacyclin, and milrinone (see Chapter 24), may reduce pulmonary vascular resistance and improve right ventricular function. However, they should be used with caution for the following reasons:
Gross Thoracic Edema
After prolonged cardiac surgery in which there has been massive blood product transfusion, severe edema of the thoracic structures (heart, lungs, mediastinum) can occur. Chest closure can result in severe hypotension, due primarily to right ventricular compression. If this occurs, the sternum may be left open at the end of surgery, the wound sealed with a dressing, and formal chest closure performed a few days later, once the edema has settled.24 Similarly, sternal opening should be considered for intractable early postoperative right ventricular dysfunction.
Left Ventricular Diastolic Dysfunction
In about 35% of patients with congestive heart failure, left ventricular systolic function has been preserved.25 In most cases, impaired diastolic function is thought to be the underlying pathologic process. Risk factors for diastolic dysfunction include female gender, advanced age, diabetes, hypertension, left ventricular hypertrophy, coronary artery disease, and aortic stenosis.26 Preoperative factors that suggest diastolic dysfunction include a history of acute pulmonary edema, ECG evidence of left ventricular hypertrophy, and a normal (or high) ejection fraction in association with small ventricular volumes.
With mild diastolic dysfunction, left atrial pressure is normal. As the disease progresses, left atrial pressure increases, first with exercise and then at rest. Following cardiac surgery, diastolic function acutely deteriorates and then recovers over 24 to 48 hours.27 In postoperative cardiac surgery patients, diastolic dysfunction is manifested as raised left atrial pressure, low or inadequate cardiac output, and preserved systolic function. The left atrial pressure at which cardiac output is optimal is higher than normal and, in fact, may be at a level at which pulmonary edema develops.
Diagnosis
With echocardiography, systolic function is typically vigorous, with a normal or high ejection fraction and a low LVEDV. These findings are similar to those in hypovolemia, but in contrast to hypovolemia, the left atrium may be large and tense with abnormal rightward bowing of the interatrial septum, which is indicative of raised left atrial pressure. The transmitral Doppler waveform may show impaired relaxation and pseudonormal or restrictive filling (see Fig. 7-7). The E/Em ratio is usually higher than 15 (Chapter 7). PAWP is elevated and cardiac output is normal or low.
Treatment
There is no truly effective way to induce ventricular relaxation. The two goals of treatment are optimizing preload—while minimizing pulmonary congestion—and avoiding actions that worsen left ventricular filling. With a restrictive filling pattern (see Fig. 7-7), the majority of ventricular filling occurs in early diastole. Thus, modest tachycardia may improve hemodynamics. In contrast, with impaired relaxation a greater proportion of ventricular filling occurs late in diastole, and filling is more dependent than usual on atrial systole. Thus, tachycardia and loss of atrioventricular synchrony are poorly tolerated. A heart rate of 70 to 80 beats per minute may produce more favorable hemodynamics than a heart rate of 90 to 100 beats per minute. A slightly prolonged atrioventricular delay (e.g., 180 ms versus the usual 150 ms) may also augment cardiac output in patients who are sequentially paced. Atrial fibrillation and other tachyarrhythmias should be aggressively treated (see Chapter 21).
β-Adrenoreceptor agonists, by virtue of their chronotropic effect, may have an adverse effect on cardiac output. Phosphodiesterase inhibitors such as milrinone enhance early diastolic relaxation (lusitropy) by a direct myocardial action and by causing afterload reduction.28 Thus, at least theoretically, they may improve early diastolic filling in patients with impaired relaxation; whether this effect is clinically important in the setting of normal systolic function and raised left atrial pressure is unknown. Probably the major benefit of milrinone in patients with impaired relaxation is that it does not cause tachycardia.
Pericardial Tamponade
Pericardial tamponade is a common cause of hemodynamic instability after cardiac surgery.29 The clinical and echocardiographic findings of tamponade in cardiac surgery patients commonly differ from tamponade associated with medical conditions. This is because tamponade after cardiac surgery has usually developed rapidly, without time for hemodynamic compensation to develop, and even small fluid collections can cause marked hemodynamic compromise. Additionally, clotted blood may cause a localized compressive effect (regional tamponade).
Diagnosis
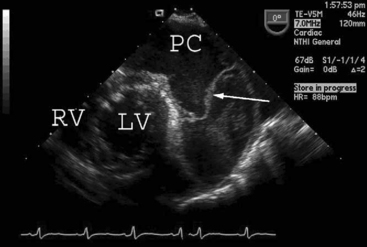
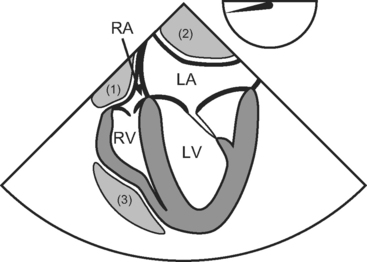
Tamponade classically presents with hypotension, pulsus paradoxus, narrow arterial pulse pressure, and equally increased left and right atrial pressures. This constellation of clinical signs may develop in a patient who has a circumferential collection of unclotted blood. With echocardiography, unclotted blood appears as an echo-free (black) space around the heart (Fig. 20-1). The ventricular cavity typically appears small and underfilled—an echocardiographic sign termed “pseudohypertrophy”30—and the venae cavae appear distended.
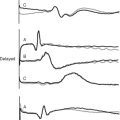
In contrast to circumferential tamponade, regional tamponade is easily misdiagnosed. When clot overlies the right atrium or ventricle, CVP is usually high and PAWP usually low, mimicking right ventricular dysfunction; when clot lies behind the left atrium, PAWP is usually high and CVP is usually low, mimicking left ventricular dysfunction. Typical echocardiographic appearances of regional tamponade are shown in Figure 20-2. As clotted blood is echogenic, appearing gray or white, it may be difficult to delineate clot from myocardium, particularly in anterior collections. TEE usually provides far superior imaging than TTE.
Treatment
The decision to reoperate for tamponade can be difficult to make. The degree to which a pericardial collection is responsible for hemodynamic instability depends not only on the size of the collection but also on the speed with which it has developed, the underlying ventricular function, the vascular tone, and the volume status. In patients with severely impaired ventricular function, even small collections can cause profound hemodynamic compromise. The decision to surgically drain a pericardial collection must be balanced against the increased risk for mediastinitis associated with reopening sternal wounds.31
The definitive treatment for tamponade is chest reopening. Less invasive procedures such as opening the lower end of the sternal wound or percutaneous placement of a pigtail catheter are not recommended for tamponade that develops within the first few hours after surgery. There are two reasons for this. First, an active source of bleeding must be sought and controlled. Second, regional tamponade may not be treated reliably unless the pericardial space is fully explored.
Medical treatment of tamponade is supportive. Fluid and inotropes should be given to optimize cardiac function and maintain blood pressure. CVP and PAWP may need to be high. PEEP should be kept to a minimum to avoid further obstruction of systemic venous return.32 Patients should be sedated and paralyzed. A common practice is “stripping” the drain tubing, either with a roller or manually, to remove clot from within the drains and to augment the negative intrapericardial pressure. Although there are few data supporting this practice,33 it is occasionally dramatically effective in relieving pericardial tamponade.
Left Ventricular Outflow Tract Obstruction
Dynamic LVOT obstruction is a process whereby the anterior mitral leaflet is displaced into the LVOT (systolic anterior motion of the anterior mitral leaflet; SAM), leading to obstruction of left ventricular ejection and creating a mitral coaptation defect causing mitral regurgitation (see Fig. 7-12). It most commonly occurs in three situations: (1) following mitral valve repair; (2) in association with hypertrophic obstructive cardiomyopathy; (3) following aortic valve replacement for aortic stenosis. However, it can occur in any patient with left ventricular (particularly septal) hypertrophy who becomes hypovolemic, in whom vasodilation occurs, or who receives excessive β-adrenoreceptor agonist therapy. LVOT obstruction is made worse by interventions that reduce left ventricular systolic dimensions (hypovolemia, inotropic drugs) and afterload (vasodilators, IABPs) because they increase LVOT velocity, causing further entrainment of the anterior mitral leaflet.
Diagnosis and Treatment
With echocardiography the pathognomonic finding is systolic displacement of the anterior mitral leaflet into the LVOT (see Fig. 7-12). Additional echocardiographic findings include:
Arrhythmia
Careful inspection of 12-lead and atrial ECGs (without pacing) usually allows the arrhythmia to be detected and diagnosed. Occasionally, an arrhythmia is identified by the presence of cannon A waves on the CVP trace (see Chapter 8) or is based on abnormalities of the pulsed-wave Doppler transmitral E and A waves during an echocardiogram (see Chapter 7).
Obstructed Venous Return
Tension Pneumothorax
Tension pneumothorax causes an obstruction of venous return because of high intrathoracic pressure and mediastinal shift. It may present with hemodynamic collapse or, rarely, with gradual cardiovascular decline. There may be unilaterally diminished breath sounds with tracheal deviation (away from the side of the pneumothorax) and quiet heart sounds. If the patient is sufficiently stable, an immediate chest radiograph may be obtained to confirm the diagnosis, but in the presence of a cardiac arrest or hypotension immediate needle thoracocentesis followed by tube thoracostomy (see Chapter 40) may be lifesaving.
Miscellaneous
Massive Hemothorax
Massive hemothorax (often involving the left chest because of bleeding from the left internal mammary artery bed after CABG surgery) usually presents with hypovolemia, anemia, and impaired gas exchange. Occasionally, direct compression of the heart can lead to mediastinal shift and impaired venous return, resulting in a tamponade-like clinical picture. The diagnosis is usually clear on the chest radiograph (see Fig. 6-26) or echocardiogram (see Fig. 7-16).
Difficult Diagnosis
Hemodynamic Instability in the Presence of Vigorous Systolic Function
A patient may be hypotensive or have a low cardiac output and yet have vigorous left ventricular function on echocardiography: “The heart looks fine but the patient looks terrible.” It should be clear from the foregoing discussion that normal or supranormal left ventricular systolic function is seen in several causes of hemodynamic instability. They are listed in Table 20-8.
Table 20-8 Causes of Hemodynamic Instability in Which Left Ventricular Function May Appear Vigorous on Echocardiography
| Hypovolemia |
| Low systemic vascular resistance |
| Left ventricular diastolic dysfunction |
| Right ventricular dysfunction |
| Dynamic LVOT obstruction |
| Pericardial tamponade |
| Obstructed venous return |
| Mitral regurgitation |
| Rhythm disturbance |
LVOT, left ventricular outflow tract.
Vasodilatory Shock.
Distributive shock, such as that which occurs with sepsis or severe systemic inflammation, produces a vasodilated, hyperdynamic circulation in which cardiac output may be as high as 6 to 8 l/min/m2. This may complicate the assessment of cardiac function. For instance, a patient with impaired systolic function who develops septic shock will, on echocardiographic examination, show “improved” systolic function due to a fall in left ventricular afterload. However, the increase in cardiac output that occurs as a consequence of septic shock may be inadequate for the patient’s needs. Thus, in the presence of severe septic shock, the findings of a high cardiac output (e.g., 3.5 l/min/m2), a normal ejection fraction (e.g., 60%), and a vigorous wall motion may be consistent with impaired systolic function and inadequate cardiac output. Also, as outlined in Chapter 2, SVO2 is an unreliable marker of tissue oxygen delivery in this situation because of the presence of regional hypoperfusion and impaired oxygen utilization. In contrast, deep sedation and hypothermia, such as may be employed following a cardiac arrest, reduces oxygen requirements and sympathetic nervous system activity, thereby diminishing cardiac output. Cardiac output may be low and wall motion may appear depressed, even in the absence of ventricular dysfunction.
Late Hemodynamic Instability
Much of the preceding discussion relates to the hypotension or low cardiac output that develops early in the postoperative period. Hemodynamic instability that occurs beyond 48 hours after surgery has a different presentation and differential diagnosis. After 48 hours, symptoms and signs are often subtle and of gradual onset. The clinical markers of low cardiac output listed in Table 20-3 are more reliable at this time than in the immediate postoperative period. The following features should also raise the possibility of low cardiac output:
If the suspicion of low cardiac output exists, an echocardiogram should be performed.
The causes of hemodynamic instability that should be considered are:
Postpericardectomy Syndrome
Postpericardectomy syndrome is characterized by malaise, fever, leukocytosis, myalgia, atrial arrhythmias, pleuritic chest pain, dyspnea and, occasionally, pericardial tamponade. The syndrome has been reported to occur in up to 17% of patients after cardiac surgery and is thought to have an autoimmune basis.34 Symptoms typically develop several weeks after surgery and have a variable course. Erythrocyte sedimentation rate and C-reactive protein are elevated. The ECG may demonstrate nonspecific ST-segment changes. Exudative (see Table 35-3) pleural and pericardial effusions are common; pulmonary infiltrates occur in a minority of patients.35 Postpericardectomy syndrome is a diagnosis of exclusion, and it is essential to rule out conditions such as infective endocarditis, pneumonia, and pulmonary embolism.
Multiple Organ Dysfunction Syndrome
MODS is well described as a sequela of cardiac surgery. In one study it occurred in 11% of cardiac surgery patients and was associated with a mortality rate of 41%.36 The best preventive strategy is the early and aggressive treatment of hemodynamic instability. Once MODS has developed, treatment is primarily supportive, but specific strategies that may improve outcome have been identified; they are described in Chapter 2.
CARDIAC ARREST AND NEAR CARDIAC ARREST
The incidence of cardiac arrest after cardiac surgery is about 1% and, of the arrests that occur in the ICU, 33% to 79% of patients survive.37–39 The two most common causes of cardiac arrest are ventricular fibrillation and pericardial tamponade.38,39 Early chest reopening, within 10 minutes of the arrest, is associated with improved survival.37
Specific Considerations for Cardiac Surgery Patients
The approach to resuscitation outlined later follows the recommendations of the American Heart Association40 but includes some important caveats, taking into account the specific needs of cardiac surgery patients. As stated before, early chest reopening should be considered in all patients who have not been effectively resuscitated within a few minutes of an arrest call. Most cardiac surgery patients are intubated and ventilated and have invasive monitoring in situ. This offers many advantages but can also complicate matters because patients may suffer a cardiac arrest as a direct consequence of a problem with the ventilator or the artificial airway. Furthermore, a monitoring error may lead to false reassurance or may precipitate inappropriate intervention. Sedation and paralysis render patients neurologically inaccessible. Thus, loss of consciousness—an important trigger to commence external chest compressions—is not available. Finally, bolus doses of epinephrine can cause marked hypertension, with potentially catastrophic results, in patients with near cardiac arrest or patients in true cardiac arrest who are rapidly defibrillated.
Definitions and Causes
Cardiac arrest is widely identified by the absence of a palpable carotid or femoral pulse. However, if systolic blood pressure falls below 50 to 60 mmHg, carotid pulsations may be absent41 despite the presence of useful cardiac function. Thus, in patients with invasive arterial blood pressure monitoring, cardiac arrest is more usefully defined as the absence of any spontaneous circulation. In practice, this equates with a nonpulsatile waveform and a MAP below about 25 mmHg. Near cardiac arrest may be defined as marked hypotension, that is, a MAP between 30 and 50 mmHg with a pulsatile waveform.
Cardiac arrest may be categorized as: (1) ventricular fibrillation (VF) or pulseless ventricular tachycardia (P-VT); (2) asystole or profound bradycardia; or (3) pulseless electrical activity (PEA). PEA is a state in which there is an electrical rhythm that is not VF/VT or profound bradycardia but there is no palpable pulse. Severe hypotension in which there is electrical rhythm (not VT/VF or profound bradycardia) is sometimes called pseudo-PEA. The causes of cardiac arrest and near cardiac arrest in cardiac surgery patients, along with common exacerbating factors, are listed in Table 20-9.
Table 20-9 Causes of Cardiac Arrest and Near Cardiac Arrest Following Cardiac Surgery
| Ventricular Fibrillation and Ventricular Tachycardia (see Chapter 21) |
| Myocardial infarction or ischemia (incomplete revascularization or graft occlusion) |
| Reperfusion injury (e.g., following coronary revascularization with preoperative severe ischemia) |
| R-on-T phenomenon with asynchronous pacing (see Chapter 21) |
| Acquired (usually secondary to hypokalemia or drug toxicity) or congenital long QT syndrome |
| Cardioversion with low energy or unsynchronized shocks |
| Drug toxicity (e.g., digoxin) |
| Asystole, Heart Block, and Profound Bradycardia (see Chapter 21) |
| Pacing problem (wires, connections, pacing box) |
| Injury to the cardiac conducting system due to the effects of cardioplegia, cardiac surgery, or cardiac pathology (e.g., aortic root abscess) |
| Sudden discontinuation of pacing (overdrive suppression; a temporary effect) |
| Massive hyperkalemia |
| Drug toxicity (e.g., β blockers, diltiazem) |
| Following prolonged ventricular fibrillation |
| Pulseless Electrical Activity and Pseudo-pulseless Electrical Activity |
| Narrow complex tachycardia with rapid ventricular response (see Chapter 21) |
| Hypovolemia |
| Tamponade |
| Massive myocardial infarction |
| Tension pneumothorax |
| Dynamic lung hyperinflation |
| Drug toxicity (vasodilators, myocardial depressants) |
| Anaphylaxis |
| Common Exacerbating Factors |
| Hypoxemia |
| Hypercarbia |
| Acidemia |
| Hypo- and hyperkalemia |
| Hyper- and hypomagnesemia |
| Hypo- and hyperthermia |
Management
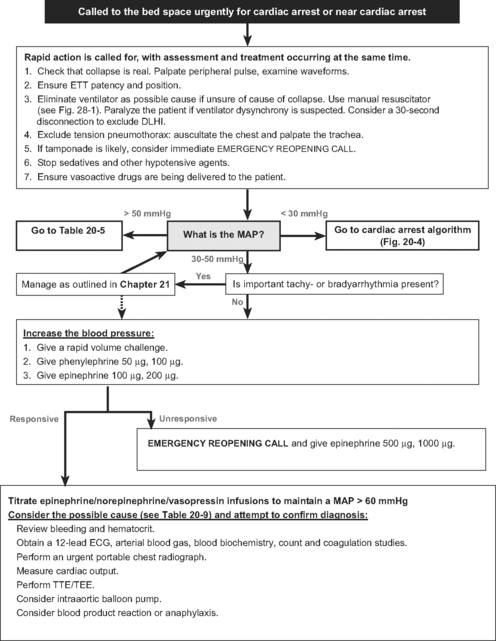
Initial Resuscitation
An algorithm for the initial approach to a cardiac arrest call and the early treatment of near cardiac arrest is shown in Figure 20-3. Initial assessment and intervention center on confirming the presence of cardiac arrest and identifying and eliminating an airway, ventilation, monitoring, or drug-delivery problem. First, it should be established that the hypotension is real by palpating a central pulse. The arterial waveform, ECG, transducer, and infusion devices should be inspected. If necessary, transducers should be rapidly leveled, zeroed, and flushed. If a pulse can be palpated but the arterial waveform suggests cardiac arrest, blood pressure should be measured noninvasively. If the patient is being paced, it should be discontinued briefly to rule out underlying ventricular fibrillation. As soon as cardiac arrest or near cardiac arrest is confirmed, external chest compression should be commenced. An exception to this is in near cardiac arrest when the patient is still conscious and in cardiac arrest when the rhythm is VF/VT (see later discussion). Intubated patients should be disconnected from ventilators and ventilated with a manual resuscitator (see Fig. 28-1) using 100% oxygen. Extubated patients should receive mask-assisted ventilation, and intubation should be considered. Prolonged attempts at intubation must be avoided because they interfere with regular mask ventilation and can delay defibrillation. Chest movements should be inspected, the trachea palpated, and breath sounds auscultated. Infusions of hypotensive drugs should be discontinued. This initial period of diagnosis and treatment should be completed within 30 to 60 seconds.
Rhythm-Specific Management
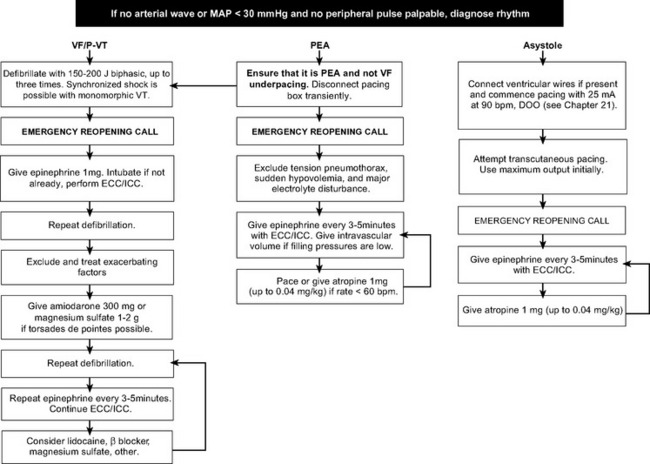
A rhythm-specific algorithm for managing cardiac arrest is shown in Figure 20-4.
Ventricular Fibrillation and Tachycardia.
The primary goal in VF or pulseless VT (see Fig. 20-4) is rapid defibrillation which, in the ICU, should occur within 1 minute of the onset of the rhythm. The technique of defibrillation is described in Chapter 40. Administration of external chest compressions while preparing for defibrillation is controversial. Often, VF/VT responds to the first attempt at defibrillation. By avoiding external chest compressions until after the first shock, potential damage to the sternum and heart is avoided, and defibrillation is not delayed. However, cardiac compressions should not be withheld for longer than 1 to 2 minutes under any circumstances.
Current guidelines42 recommend a single initial biphasic shock of 150 to 200 joules. If this is unsuccessful, the following should occur: (1) commencement of external chest compressions and ventilation by a manual resuscitator; (2) placement of an emergency reopening call; (3) administration of epinephrine 1 mg; (4) a search for reversible contributing factors. A blood sample for arterial blood gas and electrolyte analysis should be obtained and the prearrest ECG inspected to rule out long QT syndrome and myocardial ischemia and infarction. Further doses of epinephrine should be administered every 3 to 5 minutes. Following the first dose of epinephrine, amiodarone 300 mg or, if torsades de pointes is suspected, magnesium sulfate 1 to 2 g (4 to 8 mmol) should be given. Further amiodarone may be administered. Although widely used, lidocaine has not been demonstrated to be effective.43 Once the chest is open, defibrillation should be attempted using internal paddles (10 to 50 joules). For patients with intractable VT/VF, a return to the operating room for institution of CPB should be considered.
Asystole and Severe Bradycardia.
In an unmonitored setting, patients found with asystole have virtually no chance of survival because the rhythm typically represents the end result of untreated VF. However, in the ICU asystole is typically a witnessed event and, depending on the cause, it may be eminently treatable (see Fig. 20-4). Severe bradycardia secondary to complete heart block and a slow ventricular escape is much more common than true asystole. If epicardial pacing wires are in situ, asynchronous ventricular pacing at maximum output should be commenced (see Chapter 21). If this is unsuccessful, alternating the lead polarity and exchanging the pacemaker and leads may be effective. If epicardial pacing is not effective, transcutaneous pacing should be commenced. As soon as asystole is diagnosed, external chest compressions and ventilation by a manual resuscitator should be commenced. If pacing is unsuccessful, epinephrine 1 mg every 3 to 5 minutes and atropine 1 to 3 mg should be administered and an emergency chest reopening call made. A blood sample for arterial blood gas and electrolyte analysis should be obtained. A return to the operating room for institution of CPB may be considered.
Pulseless Electrical Activity.
If a cause can be found rapidly and treated, resuscitation from PEA (see Fig. 20-4) is likely to be successful. Common causes of PEA are cardiac tamponade, dynamic lung hyperinflation, tension pneumothorax, and coronary artery graft occlusion or dehiscence. Severe hypovolemia due to blood loss (e.g., into the chest) may also manifest as PEA (Table 20-9). Severe hyperkalemia can cause PEA, but it more commonly results in a broad-complex bradyarrhythmia. PEA may occur transiently after successful defibrillation.
Specific Issues During Resuscitation
External Chest Compressions and Ventilation.
External chest compressions should be performed at a rate of 100 per minute and should achieve a MAP of at least 50 mmHg. If the patient is not intubated, compressions should be briefly interrupted to deliver two breaths every 30 compressions. If the patient is intubated, 8 to 10 breaths per minute should be delivered without interrupting compressions. Even experienced clinicians have a tendency to hyperventilate patients during a cardiac arrest.44 This increases intrathoracic pressure, reduces systemic venous return, and reduces coronary perfusion pressure.44 Thus, a conscious effort must be made to avoid hypoventilation. The techniques of bag-mask ventilation and endotracheal intubation are outlined in Chapter 40.
Pharmacology.
Only a few drugs are indicated for managing cardiac arrest, and even these have limited evidence supporting their use.45
Sodium bicarbonate and calcium chloride (with nebulized albuterol and glucose/insulin) are indicated specifically for cardiac arrest due to hyperkalemia,46 but they no longer form part of standard cardiac arrest protocols.
Chest Reopening.
Early chest reopening, ideally within 10 minutes of the arrest,37 enhances the survival rates of patients who suffer cardiac arrest after cardiac surgery. Chest reopening affords several benefits. Once the chest is open, cardiac compressions and defibrillation can be performed internally, which is more efficient. Tamponade (and tension pneumothorax) may be identified and relieved. A bleeding site may be recognized and controlled. Inspection of the heart may reveal dehiscence or occlusion of a coronary graft. Pacing wires may be reattached. An operating team is assembled because it may be required for subsequent surgery. If a decision is made to return to the operating room, internal cardiac compressions can continue while the patient is transferred from the ICU. As far as possible, a sterile technique should be maintained during chest reopening in the ICU. Prophylactic antibiotics should be administered.
Role of Cardiopulmonary Bypass and Extracorporeal Support.
When patients suffer cardiac arrest in the early period after cardiac surgery, and they do not respond to conventional resuscitative measures, including chest reopening, emergency CPB should be considered. In two series, survival rates through the use of this approach were 42% and 56%.47,48 Institution of CPB may be used to rest and reperfuse the heart, control malignant arrhythmias, and correct metabolic derangements. Further surgery, most commonly revision of a coronary graft, may be performed. Following CPB it may be appropriate—in selected patients—to provide a further period of mechanical support (see Chapter 22). In one small study of prolonged cardiac arrest in various patient groups, the use of extracorporeal membrane oxygenation as a rescue therapy resulted in a 30% rate of survival, and arrest after cardiac surgery was associated with improved outcome.49
1 Bourgoin A, Leone M, Delmas A, et al. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33:780-786.
2 Linton RA, Linton NW, Kelly F. Is clinical assessment of the circulation reliable in postoperative cardiac surgical patients ? J Cardiothorac Vasc Anesth. 2002;16:4-7.
3 Reinhart K, Kuhn HJ, Hartog C, et al. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intens Care Med. 2004;30:1572-1578.
4 Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000-2008.
5 Larson SL, Schimmel CH, Shott S, et al. Influence of fast-track anesthetic technique on cardiovascular infusions and weight gain. J Cardiothorac Vasc Anesth. 1999;13:424-430.
6 Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intens Care Med. 2003;29:352-360.
7 Kumar A, Anel R, Bunnell E, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691-699.
8 Tousignant CP, Walsh F, Mazer CD. The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg. 2000;90:351-355.
9 Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
10 Kristof AS, Magder S. Low systemic vascular resistance state in patients undergoing cardiopulmonary bypass. Crit Care Med. 1999;27:1121-1127.
11 Carrel T, Englberger L, Mohacsi P, et al. Low systemic vascular resistance after cardiopulmonary bypass: incidence, etiology, and clinical importance. J Card Surg. 2000;15:347-353.
12 Levin RL, Degrange MA, Bruno GF, et al. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg. 2004;77:496-499.
13 Kilger E, Weis F, Briegel J, et al. Stress doses of hydrocortisone reduce severe systemic inflammatory response syndrome and improve early outcome in a risk group of patients after cardiac surgery. Crit Care Med. 2003;31:1068-1074.
14 Kloner RA, Przyklenk K, Kay GL. Clinical evidence for stunned myocardium after coronary artery bypass surgery. J Card Surg. 1994;9:397-402.
15 Huang YG, Wong KC, Yip WH, et al. Cardiovascular responses to graded doses of three catecholamines during lactic and hydrochloric acidosis in dogs. Br J Anaesth. 1995;74:583-590.
16 Toller W, Wolkart G, Stranz C, et al. Contractile action of levosimendan and epinephrine during acidosis. Eur J Pharmacol. 2005;507:199-209.
17 Mathieu D, Neviere R, Billard V, et al. Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study. Crit Care Med. 1991;19:1352-1356.
18 Cooper DJ, Walley KR, Wiggs BR, et al. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis: a prospective, controlled clinical study. Ann Intern Med. 1990;112:492-498.
19 Reichert CL, Visser CA, Koolen JJ, et al. Transesophageal echocardiography in hypotensive patients after cardiac operations: comparison with hemodynamic parameters. J Thorac Cardiovasc Surg. 1992;104:321-326.
20 Goldstein JA, Vlahakes GJ, Verrier ED, et al. Volume loading improves low cardiac output in experimental right ventricular infarction. J Am Coll Cardiol. 1983;2:270-278.
21 Gewirtz H, Gold HK, Fallon JT, et al. Role of right ventricular infarction in cardiogenic shock associated with inferior myocardial infarction. Br Heart J. 1979;42:719-725.
22 Ferrario M, Poli A, Previtali M, et al. Hemodynamics of volume loading compared with dobutamine in severe right ventricular infarction. Am J Cardiol. 1994;74:329-333.
23 Dell’Italia LJ, Starling MR, Blumhardt R, et al. Comparative effects of volume loading, dobutamine, and nitroprusside in patients with predominant right ventricular infarction. Circulation. 1985;72:1327-1335.
24 Christenson JT, Maurice J, Simonet F, et al. Open chest and delayed sternal closure after cardiac surgery. Eur J Cardiothorac Surg. 1996;10:305-311.
25 Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217-223.
26 Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317-327.
27 Breisblatt WM, Stein KL, Wolfe CJ, et al. Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol. 1990;15:1261-1269.
28 Krams R, McFalls E, van der Giessen WJ, et al. Does intravenous milrinone have a direct effect on diastolic function ? Am Heart J. 1991;121:1951-1955.
29 Ball JB, Morrison WL. Experience with cardiac tamponade following open heart surgery. Heart Vessels. 1996;11:39-43.
30 Bommer WJ, Follette D, Pollock M, et al. Tamponade in patients undergoing cardiac surgery: a clinical-echocardiographic diagnosis. Am Heart J. 1995;130:1216-1223.
31 El Oakley R, Paul E, Wong PS, et al. Mediastinitis in patients undergoing cardiopulmonary bypass: risk analysis and midterm results. J Cardiovasc Surg. 1997;38:595-600.
32 Mattila I, Takkunen O, Mattila P, et al. Cardiac tamponade and different modes of artificial ventilation. Acta Anaesthesiol Scand. 1984;28:236-240.
33 Wallen M, Morrison A, Gillies D, et al. Mediastinal chest drain clearance for cardiac surgery. Cochrane Database of Systematic Reviews. 2004:CD 003042.
34 Engle MA, Gay WAJr, McCabe J, et al. Postpericardiotomy syndrome in adults: incidence, autoimmunity and virology. Circulation. 1981;64:II58-II60.
35 Kaminsky ME, Rodan BA, Osborne DR, et al. Postpericardiotomy syndrome. Am J Roentgenol. 1982;138:503-508.
36 Kollef MH, Wragge T, Pasque C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest. 1995;107:1395-1401.
37 Mackay JH, Powell SJ, Osgathorp J, et al. Six-year prospective audit of chest reopening after cardiac arrest. Eur J Cardiothorac Surg. 2002;22:421-425.
38 Anthi A, Tzelepis GE, Alivizatos P, et al. Unexpected cardiac arrest after cardiac surgery: incidence, predisposing causes, and outcome of open chest cardiopulmonary resuscitation. Chest. 1998;113:15-19.
39 Wahba A, Gotz W, Birnbaum DE. Outcome of cardiopulmonary resuscitation following open heart surgery. Scand Cardiovasc J. 1997;31:147-149.
40 Resuscitation International Committee. 2005 International Consensus Conference on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation (Si). 2005;III:1-163.
41 Deakin CD, Low JL. Accuracy of the advanced trauma life-support guidelines for predicting systolic blood pressure using carotid, femoral, and radial pulses: observational study. Br Med J. 2000;321:673-674.
42 International Liaison Committee on Resuscitation. Part 3: Defibrillation. Circulation. 2005;112:III17-III24.
43 American Heart Association. Part 7.2: Management of cardiac arrest. Circulation. 2005;112:IV57-IV66.
44 Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32:S345-S351.
45 International Liaison Committee on Resuscitation. Part 4: Advanced life support. Circulation. 2005;112:III25-III54.
46 American Heart Association. Part 10.1: Life-threatening electrolyte abnormalities. Circulation. 2005;112:IV121-IV125.
47 Rousou JA, Engelman RM, Flack JE3rd, et al. Emergency cardiopulmonary bypass in the cardiac surgical unit can be a lifesaving measure in postoperative cardiac arrest. Circulation. 1994;90:II280-II284.
48 Birdi I, Chaudhuri N, Lenthall K, et al. Emergency reinstitution of cardiopulmonary bypass following cardiac surgery: outcome justifies the cost. Eur J Cardiothorac Surg. 2000;17:743-746.
49 Chen YS, Chao A, Yu HY, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41:197-203.