CHAPTER 119 Hemodialysis Fistulas
INTRODUCTION
Hemodialysis, and to a lesser extent peritoneal dialysis, remains the mainstay of treatment of patients with CKD. In acute situations, such as in patients requiring immediate, short-term (less than 6 months) dialysis access, double-lumen catheters are often used. These catheters are inserted in the femoral, internal jugular, or subclavian vein. Long-term hemodialysis requires regular vascular access (e.g., two to five times a week), necessitating the surgical creation of an arteriovenous fistula for more robust vascular access, typically in an upper extremity. This can be performed using the patient’s native artery and vein (arteriovenous fistula or AVF; Fig. 119-1) or with use of prosthetic graft material (arteriovenous graft or AVG; also called a prosthetic AVF; see Fig. 119-1).
The main concern for a dialysis fistula is its patency and duration of patency. In general, a native AVF is believed to have longer problem-free patency rates than a prosthetic AVG and AVF has been the recommended first choice for long-term hemodialysis access by the National Kidney Foundation that was recommended in its 2000 Dialysis Outcomes Quality Initiative Clinical Practical Guidelines for Vascular Access (NKF/DOQI).1 Most often, a hemodialysis fistula is created in the upper extremity by anastomosis of the cephalic vein to the radial artery, close to or in the tabatière anatomique or the anatomical snuffbox, or just proximal to the wrist. This latter type of hemodialysis fistula is also known as the radiocephalic or Brescia-Cimino (BC) fistula or shunt (see Fig. 119-1A). In patients in whom a BC shunt, an AVF, cannot be created, transposition of the basilic vein of the forearm to a ventral position with end-to-side radial-basilic anastomosis is a viable alternative option.2 In patients in whom it is not possible to use autologous arteries and veins, a prosthetic AVG can be placed between an antecubital vein and the brachial artery (see Fig. 119-1B). The AVG is not the hemodialysis fistula of first choice, because the AVGs typically require more frequent (up to five times more frequent) therapeutic intervention compared with native AVFs to maintain their proper function.3 The most common cause of AVG failure is development of stenosis at the arterial and venous anastomoses, which can cause critical flow decline and subsequent thrombosis of the graft. Alternatively, although the AVF fistula has a better patency rate compared to the AVG, an AVF requires much longer period of time to ‘mature,’ typically approximately 6 to 8 weeks. Maturation of the fistula is necessary for enlargement of the draining vein to accommodate its use for hemodialysis. This process of maturation is not successful in all patients and, therefore, AVF fistulas have a higher rate of early failure, sometimes necessitating catheter insertion to provide adequate dialysis. Maturation of AVGs is generally quicker, allowing their earlier use for hemodialysis.
The use of AVF, although greater than that of AVG, for dialysis initiation has been far from universal, a fact that may be attributable to a variety of factors to include practice differences and lack of timely access to specialty care.4 There also remains a lack of sufficient evidence to support the long-held notion of the supremacy of AVF to AVG.5
There are several special roles for imaging in the management of patients with hemodialysis fistulas. First, cross-sectional imaging techniques such as duplex ultrasonography (DUS), computed tomography (CT), and magnetic resonance angiography (MRA) can provide valuable preoperative information on arterial and venous diameters as well as identify stenotic vascular segments and anatomic variants that may influence the choice of hemodialysis fistula type and location. Second, imaging is essential for surveillance of vascular complications, notably stenosis of the hemodialysis fistula. The creation of hemodialysis fistula results in an unnatural or nonphysiologic flow situation in the upper extremity, which combined with a tendency for accelerated development of atherosclerotic stenoses and obstructions in this specific population, increases the likelihood for occlusion in affected segments, which may require endovascular intervention to maintain graft patency.6
A very important consideration when choosing the optimal imaging strategy in patients who are candidates for or who already have a hemodialysis fistula is the presence of residual renal function. Residual renal function is a strong predictor of survival in patients with CKD,7,8 illustrating the important fact that dialysis clearance is not equivalent to renal clearance. Residual function in the native kidneys retains a role in sodium and water removal, and dialysis (both hemodialysis and peritoneal dialysis) remains inefficient for removing larger and protein-bound uremic toxins. Thus, the preservation of even small amounts of residual renal function in patients on HD is of major clinical importance.9 In general, residual kidney function is better preserved in patients who undergo PD versus HD. Care should be taken to avoid further reduction in residual renal function by use of large volumes of iodinated contrast agents. However, whereas MRA would seem a good alternative, the choice between different imaging modalities is becoming increasingly difficult because of the recent discovery of nephrogenic systemic fibrosis (NSF). NSF is a rare but potentially serious condition, almost exclusively seen in patients with severe CKD and renal failure, which has been linked to the administration of gadolinium-based contrast agents (GBCA) for MRA, and will be discussed in more detail in the section on MRI later. NSF has rekindled interest in nonenhanced MRA techniques, but at present these is no published data on the feasibility and accuracy of noncontrast media enhanced MRA in the preoperative workup of patients due to receive a hemodialysis fistula, nor in patients with hemodialysis fistula dysfunction due to suspected arterial or venous stenoses, although studies are underway to investigate these techniques for this purpose.
DISEASE
Definition
CKD is defined as either kidney damage or decreased kidney function for 3 or more months. The level of kidney function, regardless of diagnosis, determines the stage of CKD according to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI) CKD classification into five stages (Table 119-1). It is important to realize that the prevalence of earlier stages of disease is more than 100 times greater than the prevalence of kidney failure. Kidney failure, also known as CKD stage 5, is defined as a patient with glomerular filtration rate (GFR) of less than 15 mL/kg/1.73 m2, or in practical terms, a patient requiring renal dialysis therapy. It is important to note that kidney failure is not actually synonymous with the term “end-stage renal disease (ESRD).” End-stage renal disease is an administrative term used in the United States to indicate that a patient is undergoing renal dialysis in anticipation of renal transplantation, and is a condition for payment by the Medicare ESRD program.10
| Stage | Description | GFR (mL/min per 1.73 m 2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mild decreased GFR | 60-89 |
| 3 | Moderately decreased GFR | 30-59 |
| 4 | Severely decreased GFR | 15-29 |
| 5 | Kidney failure | <15 or dialysis |
GFR, glomerular filtration rate.
Prevalence and Epidemiology
CKD is highly prevalent and affects approximately 8.5% of the United States adult population (26 million adults in 2008). The number of persons with kidney failure in the United States who are treated with HD and transplantation is rising rapidly and projected to increase from 340,000 in 1999 to 651,000 in 2010.10 Worldwide, an estimated 1.22 million patients were on HD in 2004, representing a 20% increase over 2001.11,12 Because of a shortage in kidney donors, the majority of CKD patients are treated by renal dialysis therapy. With the steady growth of the aging population, the number of CKD patients continues to increase, especially in patients older than 65 years of age. The fraction of patients receiving a renal transplant decreases with age. Conversely, the percentage of patients being treated by HD increases with age. Over the next few years, the number of CKD patients in need of dialysis is expected to increase by approximately 5% per year, reaching over 3 million worldwide by 2010. Because of the chronic nature of the disease, CKD is accompanied by a large increase in healthcare-related costs. In 2008, the annual costs of the Medicare ESRD program were $20 billion, and $42 billion was the amount spent on treating patients with CKD.13
Etiology and Pathophysiology
Up to 10% to 20% of all newly created hemodialysis fistulas thrombose within the first week after creation due to insufficient flow.14 Nonmaturation is defined as a hemodialysis fistula being inadequate for hemodialysis due to insufficient flow or insufficient venous distention within 6 weeks after creation. Causes of hemodialysis fistula nonmaturation are thought to include the use of small-diameter vessels (<1.6 mm in diameter),15 and presence of stenoses or occlusions in arterial inflow and/or venous outflow segments.16 The presence of large caliber side branches may also jeopardize hemodialysis fistula maturation due to altered flow distribution. Hemodialysis fistula nonmaturation rates within the first months after creation range from 5% up to 54%.17
Manifestations of Disease
Clinical Presentation
Patients in need of a hemodialysis fistula usually have a long medical history of renal disease. In fact, because almost all patients with stage 5 CKD are known to have this condition, the creation of a hemodialysis fistula can be anticipated, and sufficient time can be taken to allow for DAG maturation. The K/DOQI guidelines recommend that patients be referred for surgical evaluation when the creatinine clearance falls below 25 mL/min, or a serum creatinine >4 mg/dL (354 micromoles/L), or when creation of a hemodialysis fistula is anticipated within 1 year.18
Patients with functioning hemodialysis fistula should be under continuous surveillance for failure (i.e., stenosis or thrombosis). The K/DOQI guidelines recommend monthly flow measurements using an ultrasound-based dilution technique in every patient on HD. If absolute hemodialysis fistula flow at any time falls below 600 mL/min, or if the patient exhibits a decline >25% between two consecutive measurements in combination with an absolute flow of less than 1000 mL/min, the hemodialysis fistula is considered at risk for thrombosis.18 If this is the case, imaging is indicated to investigate the presence of inflow and/or outflow stenoses.
Imaging Indications and Algorithm
Any presurgical workup should start with a thorough history and physical examination. Women, elderly patients, and patients suffering from diabetes mellitus, obesity, cardiovascular morbidity, and patients with a history of previous vascular access procedures as well as previous limb and thoracic surgery or radiation therapy are at increased risk for hemodialysis fistula nonmaturation.19 Physical examination is an important and valuable tool in the workup of patients awaiting access surgery. Skin lesions, local infections, generalized dermatological problems and scars may indicate poor chance of successful hemodialysis fistula creation at standard locations and should be addressed. All patients should undergo bilateral blood pressure measurements of the upper extremity. A difference of more than 20 mm Hg or an arm-to-arm index of <0.9 are indicative for the presence of arterial pathology.
The anatomy of the venous system is also important to determine because it can be highly variable. The presence of small caliber veins, venous obstructions, low compliance segments, and large accessory veins is associated with higher hemodialysis fistula nonmaturation rates.18 Physical examination is useful to identify factors influencing hemodialysis fistula maturation, but it is typically not sufficient to rely on by itself. For instance, Malovrh and colleagues examined 116 patients due to undergo access creation and found that physical examination failed to identify suitable vessels for hemodialysis fistula creation in more than half of the patients.20 Venous imaging is critical for preprocedural planning for hemodialysis fistula placement.
Imaging Techniques and Findings
Ultrasound
Duplex ultrasonography (DUS) is the imaging modality of first choice in patients due to undergo graft creation as well as in patients with a failing DAG. DUS enables assessment of vessel patency, diameter, flow volume and velocities. The application of DUS enables proper depiction and determination of suitable vessels for hemodialysis fistula creation that may not be detected by physical examination, especially in obese patients.21–24
Preoperative DUS examination should include assessment of the arteries and veins from the subclavian artery down to the radial and ulnar vessels at the wrist. The exact course and continuity as well as the presence of stenoses should be addressed because patients with arterial stenosis are thought to be at increased risk for developing hand and finger ischemia after AVF creation due to steal phenomena. For detection of relevant stenoses (defined as >50% luminal reduction) in the upper extremity arterial system, DUS has a sensitivity and specificity of 90.9% and 100% for the subclavian artery, 93.3% and 100% for upper arm arteries, 88.6% and 98.7% for forearm arteries, and for the arteries of the hand 70% and 100%, respectively.25,26
Another important morphologic parameter apart from the presence of arterial stenosis is arterial diameter. Arteries with diameters smaller than 1.5 to 3.0 mm have been associated with increased AVF nonmaturation rates.14 Additional parameters such as radial artery flow volume and peak systolic velocities before or during reactive hyperemia have also been reported to be predictors of AVF maturation. In Figure 119-2 radial artery flow velocity changes due to fist clenching and reactive hyperemia are shown. Lockhart and associates, in contrast, found that arterial diameters, resistance indices, and peak systolic velocities had little if any predictive value for AVF outcome.16
The superficial venous system of the upper extremity is also easily assessable by DUS and—not surprisingly—results in detection of more veins compared to physical examination alone. DUS also allows for assessment of local hemodynamics, such as the determination of subclavian venous flow. A typical example of Doppler signal changes of the subclavian vein during deep inspiration in a healthy volunteer is shown in Figure 119-3. The absence of changes in venous Doppler signal due to deep inspiration or loss of venous compressibility suggests the presence of a local venous stenosis or occlusion. Preoperative detection of stenoses and obstructions is important to avoid unsuccessful hemodialysis access surgeries. Nack and colleagues reported a DUS sensitivity, specificity, positive predictive value, and negative predictive value of 81%, 90%, 90%, and 78%, respectively, for detection of venous stenosis, thrombi, and occlusions when compared to DSA.27 The clinical value of upper extremity DUS for detection of venous abnormalities is lower for proximal compared to distal veins. Nack and colleagues also reported progressively decreasing DUS sensitivities for detection of abnormalities in the subclavian vein (79%), innominate vein (75%), and superior vena cava (33%), when compared to DSA.27 This can be explained by the fact that these veins course beneath bony structures such as the clavicle and ribs over a substantial length and/or are relatively distant from the skin surface and inaccessible by DUS.
As is the case for arteries, DUS-derived venous diameter is an important parameter for prediction of vascular access outcome. For assessment of venous diameter, a proximally applied cuff should be used to induce venous dilation for better appreciation of maximum or true venous diameter.28 Reported venous cut-off diameters for successful hemodialysis fistula creation range from 1.6 to 2.6 mm.6,28 This range may be partially explained by differences in vein mapping protocols because only few authors reported the measurement conditions and methods to achieve venous dilation. DUS venous diameter measurements, furthermore, are observer-dependent with an interobserver variation reported to be 0.5 mm.29 Recently, Planken and associates have demonstrated that superficial forearm vein diameter measurements vary over time with a coefficient of variation of 27%.28 Forearm superficial venous diameter measurement reproducibility also depends on the applied venous congestion pressure and best reproducibility is achieved at venous congestion pressures >40 mm Hg.30
The preoperative length of nondiseased contiguous vein >10 cm used for AVF in addition to venous diameter was predictive for the success of AVF creation.31,32 Apart from venous diameter, some authors have found an association between the presence and size of venous side branches and AVF nonmaturation. Wong and colleagues suggested that a side branch <5 cm away from the planned anastomosis may impair AVF function, whereas Beathard and associates have stressed the importance of the size of the venous side branches. In the aforementioned studies nonmaturation was more likely in the event of a large venous side branch.33,34 Turmel-Rodrigues and colleagues, in contrast, state that venous side branches are of no importance and come into play only in the presence of a venous outflow stenosis.35
Dynamic parameters to characterize upper extremity veins include flow volume and velocity measurements as well as assessment of flow wave changes due to respiratory maneuvers.36 The capacity of superficial veins to dilate due to venous congestion (also known as venous compliance) has been reported to be higher in patients with successful AVF creation compared to patients with AVF failure or nonmaturation with venous diameter increase 48% and 11.8%, respectively, at a congestion pressure of 50 mm Hg.24 Forearm superficial venous compliance measurements, however, have been reported to be poorly reproducible due to great variations in venous diameters at low venous congestion pressures.30 The clinical value of forearm superficial venous compliance measurements is, therefore, considered of little if any use.
Multiple studies have shown the value of DUS in preoperative assessment of patients undergoing hemodialysis fistula creation. The performance of DUS prior to hemodialysis access fistula creation has been shown to result in changes in surgical procedure (31%), changes in site of exploration (9.6%), a decrease in unsuccessful explorations (11% to 0%), an increase in the relative number of AVFs created (as opposed to other types of access; 64% vs. 34%), and a decrease in AVF nonmaturation rates (e.g., 38% to 8.3%; and 66% to 46%), when compared to the use of physical examination alone.14,19,20,23,37 However, the recommended threshold values of various DUS-derived parameters (i.e., diameter, flow-volume, velocities, compliance and resistive index) vary and no single parameter appears to be superior as a sole predictor for AVF success.16,38 It remains to be established which combination of parameters might enable better prediction of vascular access function and minimize early failure and nonmaturation rates.
In patients with failing hemodialysis fistulas, DUS is highly sensitive and highly specific for the detection of stenoses and occlusions in the supplying arteries, as well as the draining veins, up to the shoulder region.39 DUS is not reliable for imaging central to the subclavian region, and other techniques should be used instead. However, although regular hemodialysis fistula surveillance has been associated with a much lower rate of graft thrombosis, a randomized controlled trial in 101 patients with AVGs by Sam and coworkers found that 2-year graft survival was similar in patients when they were referred for intervention on the basis of clinical criteria, ultrasound dilution measurements, or DUS stenosis measurements.40
CT
There are only a few studies41–44 that have reported the use of computed tomography angiography (CTA) for both the preoperative workup of patients with CKD scheduled for hemodialysis access fistula surgery and for patients with dysfunctional hemodialysis fistulas. The largest study evaluated only 30 patients.42 Not surprisingly, CTA was found capable of detecting vascular pathology in the hemodialysis fistula and the supplying as well as draining vessels with high accuracy (Figs. 119-4, 119-5, and 119-6).
One of the most important issues standing in the way of universal acceptance of CTA for the assessment of the upper extremity vasculature is the lack of large prospective studies that address the long-term effects of iodinated contrast agents on residual renal function in the CKD patient population. Iodinated contrast agents have long been known to have nephrotoxic effects.45 In any case, measures to prevent contrast-induced nephropathy such as adequate prehydration, use of N-acetylcysteine and or bicarbonate should be taken when patients with a preexisting decline in renal function are sent for contrast-enhanced CT examinations.
Most of the studies on residual renal function in HD patients are older, and were not performed using both modern generation low- or iso-osmolar contrast agents, and the newest generation of biocompatible dialysis membranes, which are known to have a positive effect on contrast agent clearance and to ameliorate contrast nephrotoxicity. It is already known that residual renal function is conserved to a greater extent in patients on PD versus patients on HD, and two recent—but very small—studies have found that despite a limited transient decline in renal function, there was no accelerated decline after 2 weeks to 1 month after administration of iodinated contrast agent in PD patients when they were adequately prehydrated, and small volumes of contrast media (around 100 mL) were used.46,47
MRA
The enthusiasm for contrast-enhanced MRA (CE-MRA) for the depiction of upper extremity vasculature in patients due to undergo hemodialysis fistula creation, and in patients with failing grafts has waned acutely following the reported association of GBCA and NSF in 2006.48 The safety of GBCA in patients with stage 4 and 5 CKD is a hotly contested issue and in continuous flux. An emerging consensus is that macrocyclic Gd-chelate contrast agents, which have not been unequivocally associated with NSF in even a single case, may indeed be safe when administered in small volumes (up to 0.1 mmol/kg) in patients with stage 4 and 5 CKD. Indeed, even the class of linear Gd-chelate contrast agents seem to have a much lower risk for NSF when used in low doses.49 As with CTA, however, the risks of administration of the contrast agents for MRA should be carefully weighed against the diagnostic benefits that will be obtained.
Some widely used Gd-chelate contrast agents for CE-MRA purposes are gadopentetate dimeglumine (Magnevist, Schering, Berlin, Germany), gadoterate dimeglumine (Dotarem, Guerbet, Aulnay, France), gadodiamide (Omniscan, GE Health, Oslo, Norway), gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Mo) and gadoteridol (ProHance, Bracco Diagnostics, Milan, Italy). The total incidence of adverse events related to gadolinium use for CE-MRA appears to be less than 5%. The incidence of any single adverse event is approximately 1%, or lower. By far the most common events are nausea, headache, and emesis.50 When used intravenously, no detectable nephrotoxicity has been reported and the rates of adverse events are extremely low at the usual recommended doses.51
During the last decade, approximately 350 cases of nephrogenic systemic fibrosis (NSF), previously known as nephrogenic fibrosing dermopathy, have been reported worldwide. NSF is thought to be a chronic inflammatory reaction in response to the accumulation of free gadolinium in patients with severe renal failure. The reported clinical signs and symptoms of NSF are subacute progressive swelling of extremities followed by more proximal involvement and severe skin induration, pain, muscle restlessness, and loss of skin flexibility. NSF can lead to serious physical disability and wheelchair requirement, and even death.52 The incidence of NSF is low—especially when low doses of GBCA are used—and the actual pathophysiology remains unknown.49,52 To date, all linear Gd-chelate contrast agents, notably gadodiamide (Omniscan), are believed to be associated with NSF.53,54 Because of these reported adverse events and the suspected causative relationship, many experts discourage the use of GBCA in CKD stage 4 and 5 patients until more data on safety are available.53,54 A recent study by Chrysochou addressed the positive reporting bias regarding the link between gadolinium exposure and NSF and found that no patients with CKD stage 4 developed NSF, regardless of the agent used.55 Because there are no data published on the feasibility and accuracy of the newer, nonenhanced MRA techniques for evaluation of patients with hemodialysis fistulas, the subsequent discussion will focus on CE-MRA.
Because intrathoracic vessels are prone to movement during respiration, patients should hold their breath for about 15 to 20 seconds depending on scan protocol and technical factors related to system performance. Contrast medium is injected at speeds up to 3.0 mL/sec, followed by 25 mL of saline flush. Spatial resolution in recent reports is typically on the order of 1.0 × 1.0 × 1.2 mm3 (craniocaudal/frequency direction × left-right/phase-encoding directions × anteroposterior/slice direction).6 Using this approach for imaging, upper extremity arteries and veins can be visualized with high accuracy (Fig. 119-7). Because upper extremity length of an adult is about 70 to 80 cm, depicting the entire upper extremity requires imaging of at least two fields of view because of the limited MR-bore length. An example of a preoperative contrast-enhanced MR arteriogram and venogram of an ESRD-patient is shown in Figure 119-8.
Planken and colleagues reported a multiphase approach using multiple dynamic scans that resulted in good to excellent subjective image quality images of upper extremity arteries down to the wrist.56 Assessment of the arterial palmar arch and digital arteries remains cumbersome, however, with the currently acquired spatial resolutions, except when using timed arterial compression.57 An important advance in this regard is the recent clinical introduction of blood pool contrast media, which enable ultra-high spatial resolution scans for assessment of the palmar arch and digital arteries (Fig. 119-9). Although CE-MRA is believed to be highly accurate for detection of arterial stenoses and obstructions, there are only limited studies about the accuracy and reproducibility for detection of thoracic and upper extremity arterial stenoses in patients with CKD. A typical example of a subclavian artery stenosis depicted by both CE-MRA and DSA is shown in Fig. 119-10.
Reported contrast-enhanced MR venography techniques use either direct injection of diluted contrast-media (1 : 15 to 1 : 25 mL of gadolinium chelate in saline solution) in the ipsilateral extremity or contralateral intravenous injection of nondiluted contrast media, and acquisition during delayed venous enhancement after initial arterial first pass. Both techniques have their strengths and weaknesses. Direct venography with application of a proximal blood pressure cuff inflated to 60 mm Hg yields better vessel opacification with lower contrast dose as compared to the contralateral injection approach. Of course, this is very helpful in the context of concerns related to NSF risk. Examples of a central venous stenosis and obstruction by direct contrast-enhanced MR venography are shown in Figs. 119-11 and 119-12. Indirect contrast-enhanced MR venography using delayed venous enhancement techniques (i.e., after the first pass arterial phase) show relatively poor performance for detection of central venous stenosis and occlusions and are not recommended. Furthermore, on direct contrast-enhanced MR venography, diameter measurements have been shown to be more accurate compared to duplex ultrasonography when using surgical measurements as the standard of reference.58 Because of the lower contrast dose, better vessel opacification and accuracy, direct contrast-enhanced MR venography seems to be the method of first choice for venous imaging.
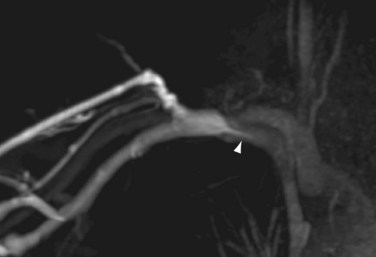
 FIGURE 119-11 Example of a direct MR venogram of a patient with a central venous stenosis (arrowhead).
FIGURE 119-11 Example of a direct MR venogram of a patient with a central venous stenosis (arrowhead).
Although CE-MRA is a promising and attractive modality for imaging the upper extremity arteries and veins, there are only sparse data about its clinical value for hemodialysis fistula planning or surveillance and future studies are needed. Recently, several new techniques for non-contrast enhanced MRA have been introduced. These promising techniques may prove to be important for hemodialysis fistula imaging and noninvasive vascular imaging of CKD patients in general but at present evidence is anecdotal (Fig. 119-13).
Angiography
Another alternative to iodinated contrast media and GBCA is carbon dioxide (CO2) gas injection. Heye and colleagues have demonstrated that CO2 venography is an acceptable alternative (sensitivity, 97%; specificity, 85%; accuracy, 95%) for assessment of upper-extremity and central veins in patients with contraindications to iodinated contrast material.59 In Figure 119-14 a CO2 venogram and corresponding conventional venogram using iodinated contrast media are shown. However, CO2 injections may cause pain and the CO2 contrast technique can lead to an overestimation of the degree of stenosis. The use of CO2 contrast may furthermore lead to serious complications such as brain gas embolism, pulmonary embolism, or acute cardiac arrest.59
Upper extremity IA-DSA is traditionally performed by intra-arterial injection of contrast media. Arterial access can be achieved by femoral or brachial puncture. In patients with CKD, the brachial artery approach is generally avoided because it can be painful, it may jeopardize distal perfusion and thereby maturation and function of a (future) hemodialysis fistula created distal to the puncture site. Thrombosis of the brachial artery is a serious complication due to brachial punctures for catheter access in up to 7%.60 The contralateral venous injection of contrast media is a less invasive alternative with fewer complications. However, in order to achieve sufficient arterial enhancement, this technique requires a high contrast dose, which is unacceptable in CKD patients due to the potential further deterioration of residual renal function. A recently described alternative by Duijm and colleagues is to insert the catheter through the access itself or the draining vein. This strategy enabled full depiction of both the outflow (venous) trajectory, as well as the inflow (arterial) trajectory by retrograde catheterization of the arterial inflow in 162/166 (97.7%) of patients.61 The authors concluded, however, that hemodialysis fistula evaluation by noninvasive imaging such as DUS is sufficient in the majority of patients as isolated proximal arterial inflow stenoses were seen in only 4.8% of patients.
Central venous stenosis and obstruction occur frequently after central venous catheter insertion or placement of pacemaker wires; however, often patients remain asymptomatic until after the hemodialysis fistula is created. Imaging of the subclavian and innominate veins and the superior vena cava prior to hemodialysis fistula creation is important because CKD patients typically have had central venous catheters and up to 40% of patients with a history of central venous catheters have central venous stenosis or obstruction.62 Examples of central venous obstructions due to pacemaker wires and central venous catheter use are shown in Figures 119-15 and 119-16. Venography by cannulation of an ipsilateral dorsal hand vein allows imaging of the entire cephalic or basilic veins from the hand up to the confluence of the basilic and brachial veins into the subclavian vein. Although the superficial veins of the upper extremity are connected to each other at multiple levels, the puncture site will limit venous opacification to the draining vein of the puncture site only. It is important to avoid puncturing veins proximal to the distal radius in order to preserve draining veins for future access use. It is the experience of this author that the use of a proximal tourniquet inflated to 60 mm Hg enables depiction of collateral veins and improves assessment of venous diameter because of dilation.
REPORTING: INFORMATION FOR THE REFERRING PHYSICIAN
KEY POINTS
 Long-term hemodialysis requires vascular access and the surgical creation of a hemodialysis fistula, typically in the patient’s distal forearm. Hemodialysis fistulas can be created using the patient’s native arteries and veins (arterial venous fistula or AVF), or by using a prosthetic graft (arteriovenous graft or AVG). Generally, AVF is the preferred type of hemodialysis fistula. Imaging plays a key role for the preoperative planning and postoperative surveillance of patients with hemodialysis fistulas. Residual renal function is a key predictor of survival in patients with chronic kidney disease. The preservation of even small amounts of residual renal function in patients on dialysis is of major clinical importance.
Long-term hemodialysis requires vascular access and the surgical creation of a hemodialysis fistula, typically in the patient’s distal forearm. Hemodialysis fistulas can be created using the patient’s native arteries and veins (arterial venous fistula or AVF), or by using a prosthetic graft (arteriovenous graft or AVG). Generally, AVF is the preferred type of hemodialysis fistula. Imaging plays a key role for the preoperative planning and postoperative surveillance of patients with hemodialysis fistulas. Residual renal function is a key predictor of survival in patients with chronic kidney disease. The preservation of even small amounts of residual renal function in patients on dialysis is of major clinical importance. Duplex ultrasonography is the imaging test of first choice in patients due to undergo hemodialysis fistula creation, as well as in patients with dysfunctional hemodialysis fistulas.
Duplex ultrasonography is the imaging test of first choice in patients due to undergo hemodialysis fistula creation, as well as in patients with dysfunctional hemodialysis fistulas. Although CTA is capable of demonstrating vascular stenoses with high accuracy, there is limited data on the effect of iodinated contrast media administration on residual renal function.
Although CTA is capable of demonstrating vascular stenoses with high accuracy, there is limited data on the effect of iodinated contrast media administration on residual renal function. Because of concern for nephrogenic systemic fibrosis (NSF), contrast-enhanced MR angiography probably should not be performed with linear gadolinium (Gd)-chelate contrast agents in patients with stage 4 and 5 chronic kidney disease. However, preliminary evidence suggests that macrocyclic Gd-chelate contrast agents at a dose of 0.1 mmol/kg or less may be safely used in these patients
Because of concern for nephrogenic systemic fibrosis (NSF), contrast-enhanced MR angiography probably should not be performed with linear gadolinium (Gd)-chelate contrast agents in patients with stage 4 and 5 chronic kidney disease. However, preliminary evidence suggests that macrocyclic Gd-chelate contrast agents at a dose of 0.1 mmol/kg or less may be safely used in these patients, 2006 Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2-90.
Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147.
Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14-22.
Planken RN, Tordoir JH, Duijm LE, et al. Current techniques for assessment of upper extremity vasculature prior to hemodialysis vascular access creation. Eur Radiol. 2007;17:3001-3011.
Tordoir JH, Mickley V. European guidelines for vascular access: clinical algorithms on vascular access for haemodialysis. Edtna Erca J. 2003;29:131-136.
United States Renal Data System USRDS Annual data report. 2008. USRDS Coordinating Center. Minneapolis, MN. http://www.usrds.org/adr.htm. Accessed August 1, 2009
1 National Kidney foundation, K/DOQI Clinical Practice Guidelines for Vascular Access, 2000. Am J Kidney Dis. 2001;37:S137-S181.
2 Sidawy AN. Strategies of arteriovenous dialysis access. In Rutherford RB, editor: Vascular Surgery, 6th ed, Philadelphia: Saunders, 2005.
3 Staramos DN, Lazarides MK, Tzilalis VD, et al. Patency of autologous and prosthetic arteriovenous fistulas in elderly patients. Eur J Surg. 2000;166:777-781.
4 Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005-2007: Still “catheter first.”. Hemodial Int. 2009;13(4):533-542.
5 Huber TS, Buhler AG, Seegar JM. Evidence-based data for the hemodialysis access surgeon. Semin Dial. 2004;17(3):217-223.
6 Planken RN, Tordoir JH, Duijm LE, et al. Current techniques for assessment of upper extremity vasculature prior to hemodialysis vascular access creation. Eur Radiol. 2007;17:3001-3011.
7 Termorshuizen F, Dekker FW, van Manen JG, et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061-1070.
8 Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068-1081.
9 Marron B, Remon C, Perez-Fontan M, et al. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl. 2008:S42-S51.
10 Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147.
11 Moeller S, Gioberge S, Brown G. ESRD patients in 2001: global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant. 2002;17:2071-2076.
12 Grassmann A, Gioberge S, Moeller S, Brown G. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant. 2005;20:2587-2593.
13 United States Renal Data System USRDS Annual data report. 2008. USRDS Coordinating Center. Minneapolis, Minn. http://www.usrds.org/adr.htm. Accessed August 1, 2009
14 Robbin ML, Gallichio MH, Deierhoi MH, et al. US vascular mapping before hemodialysis access placement. Radiology. 2000;217:83-88.
15 Lauvao LS, Ihnat DM, Goshima KR, et al. Vein diameter is the major predictor of fistula maturation. J Vasc Surg. 2009;49:1499-1504.
16 Lockhart ME, Robbin ML, Allon M. Preoperative sonographic radial artery evaluation and correlation with subsequent radiocephalic fistula outcome. J Ultrasound Med. 2004;23:161-168.
17 Leblanc M, Saint-Sauveur E, Pichette V. Native arterio-venous fistula for hemodialysis: what to expect early after creation? J Vasc Access. 2003;4:39-44.
18 Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2-90.
19 Allon M, Bailey R, Ballard R, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int. 1998;53:473-479.
20 Silva MBJr, Hobson RW2nd, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27:302-307.
21 Katz ML, Comerota AJ, DeRojas J, et al. B-mode imaging to determine the suitability of arm veins for primary arteriovenous fistulae. J Vasc Technol. 1987;11:172-174.
22 Vassalotti JA, Falk A, Cohl ED, et al. Obese and non-obese hemodialysis patients have a similar prevalence of functioning arteriovenous fistula using pre-operative vein mapping. Clin Nephrol. 2002;58:211-214.
23 Mihmanli I, Besirli K, Kurugoglu S, et al. Cephalic vein and hemodialysis fistula: surgeon’s observation versus color Doppler ultrasonographic findings. J Ultrasound Med. 2001;20:217-222.
24 Malovrh M. Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis. 2002;39:1218-1225.
25 Wittenberg G, Landwehr P, Moll R, et al. [Interobserver variability of dialysis shunt flow measurements using color coated duplex sonography]. Rofo. 1993;159:375-378.
26 Wittenberg G, Schindler T, Tschammler A, et al. [Value of color-coded duplex ultrasound in evaluating arm blood vessels—arteries and hemodialysis shunts]. Ultraschall Med. 1998;19:22-27.
27 Nack TL, Needleman L. Comparison of duplex ultrasound and contrast venography for evaluation of upper extremity venous disease. J Vasc Technol. 1992;16:69-73.
28 Planken RN, Keuter XH, Hoeks AP, et al. Diameter measurements of the forearm cephalic vein prior to vascular access creation in end-stage renal disease patients: graduated pressure cuff versus tourniquet vessel dilatation. Nephrol Dial Transplant. 2006;21:802-806.
29 Lees TA, Manzo R, Strandness DE, Appleton D. Observer variation in the measurement of venous diameters using duplex scanning. J Vasc Technol. 1994;18:177-180.
30 Planken RN, Keuter XH, Kessels AG, et al. Forearm cephalic vein cross-sectional area changes at incremental congestion pressures: towards a standardized and reproducible vein mapping protocol. J Vasc Surg. 2006;44:353-358.
31 Lemson MS, Leunissen KM, Tordoir JH. Does pre-operative duplex examination improve patency rates of Brescia-Cimino fistulas? Nephrol Dial Transplant. 1998;13:1360-1361.
32 Allon M, Lockhart ME, Lilly RZ, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013-2020.
33 Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487-1494.
34 Wong V, Ward R, Taylor J, et al. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg. 1996;12:207-213.
35 Turmel-Rodrigues L, Mouton A, Birmele B, et al. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16:2365-2371.
36 Tordoir JH, Mickley V. European guidelines for vascular access: clinical algorithms on vascular access for haemodialysis. Edtna Erca J. 2003;29:131-136.
37 Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62:1109-1124.
38 Robbin ML, Chamberlain NE, Lockhart ME, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology. 2002;225:59-64.
39 Tordoir JH, de Bruin HG, Hoeneveld H, et al. Duplex ultrasound scanning in the assessment of arteriovenous fistulas created for hemodialysis access: comparison with digital subtraction angiography. J Vasc Surg. 1989;10:122-128.
40 Ram SJ, Work J, Caldito GC, et al. A randomized controlled trial of blood flow and stenosis surveillance of hemodialysis grafts. Kidney Int. 2003;64:272-280.
41 Rooijens PP, Serafino GP, Vroegindeweij D, et al. Multi-slice computed tomographic angiography for stenosis detection in forearm hemodialysis arteriovenous fistulas. J Vasc Access. 2008;9:278-284.
42 Karadeli E, Tarhan NC, Ulu EM, et al. Evaluation of failing hemodialysis fistulas with multidetector CT angiography: comparison of different 3D planes. Eur J Radiol. 2009;69:184-192.
43 Ye C, Mao Z, Rong S, et al. Multislice computed tomographic angiography in evaluating dysfunction of the vascular access in hemodialysis patients. Nephron Clin Pract. 2006;104:c94-100.
44 Lin YP, Wu MH, Ng YY, et al. Spiral computed tomographic angiography—a new technique for evaluation of vascular access in hemodialysis patients. Am J Nephrol. 1998;18:117-122.
45 Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14-22.
46 Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21:1334-1339.
47 Moranne O, Willoteaux S, Pagniez D, et al. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21:1040-1045.
48 Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
49 Prince MR, Zhang H, Morris M, et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008;248:807-816.
50 Shellock FG, Kanal E. Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging. 1999;10:477-484.
51 Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001;176:1385-1388.
52 Thomsen HS. Nephrogenic systemic fibrosis: A serious late adverse reaction to gadodiamide. Eur Radiol. 2006;16:2619-2621.
53 Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
54 Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647-649.
55 Chrysochou C, Buckley DL, Dark P, et al. Gadolinium-enhanced magnetic resonance imaging for renovascular disease and nephrogenic systemic fibrosis: critical review of the literature and UK experience. J Magn Reson Imaging. 2009;29:887-894.
56 Planken RN, Leiner T, Nijenhuis RJ, et al. Contrast-enhanced magnetic resonance angiography findings prior to hemodialysis vascular access creation: a prospective analysis. J Vasc Access. 2008;9:269-277.
57 Wentz KU, Frohlich JM, von Weymarn C, et al. High-resolution magnetic resonance angiography of hands with timed arterial compression (tac-MRA). Lancet. 2003;361:49-50.
58 Planken RN, Tordoir JHM, de Haan MW, et al. Contrast enhanced-MR angiography of upper extremity arteries and veins prior to vascular access surgery. Blood Purif. 2005;23:227-261.
59 Heye S, Maleux G, Marchal GJ. Upper-extremity venography: CO2 versus iodinated contrast material. Radiology. 2006;241:291-297.
60 Heenan SD, Grubnic S, Buckenham TM, Belli AM. Transbrachial arteriography: indications and complications. Clin Radiol. 1996;51:205-209.
61 Duijm LE, Overbosch EH, Liem YS, et al. Retrograde catheterization of haemodialysis fistulae and grafts: angiographic depiction of the entire vascular access tree and stenosis treatment. Nephrol Dial Transplant. 2009;24:539-547.
62 Surratt RS, Picus D, Hicks ME, et al. The importance of preoperative evaluation of the subclavian vein in dialysis access planning. AJR Am J Roentgenol. 1991;156:623-625.

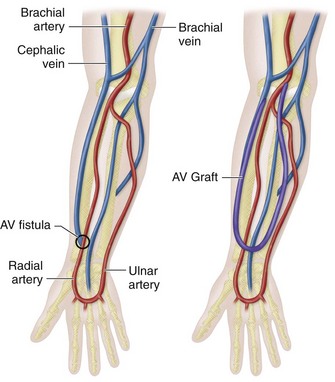
 FIGURE 119-1
FIGURE 119-1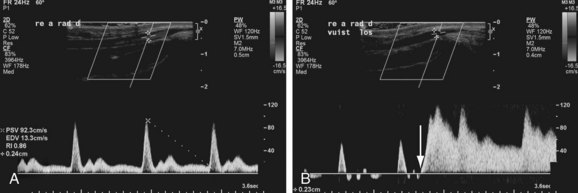
 FIGURE 119-2
FIGURE 119-2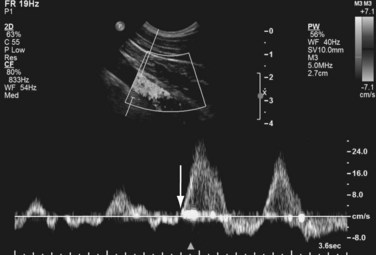
 FIGURE 119-3
FIGURE 119-3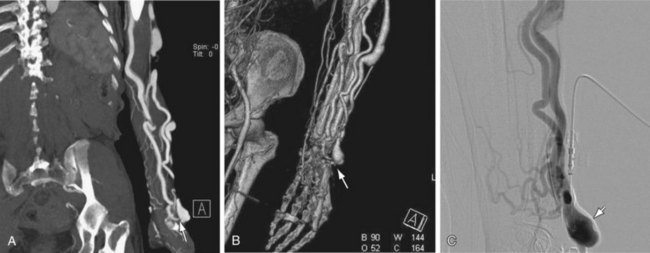
 FIGURE 119-4
FIGURE 119-4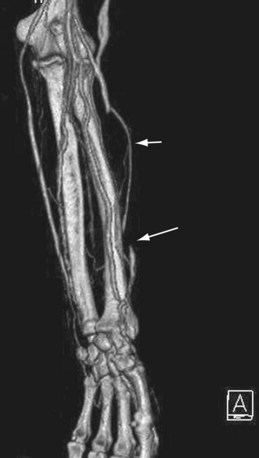
 FIGURE 119-5
FIGURE 119-5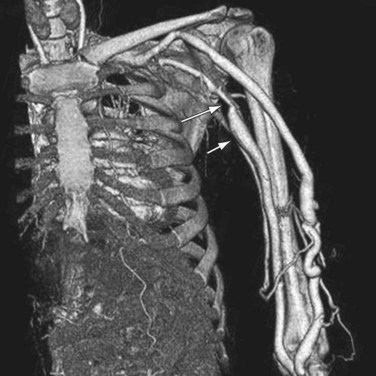
 FIGURE 119-6
FIGURE 119-6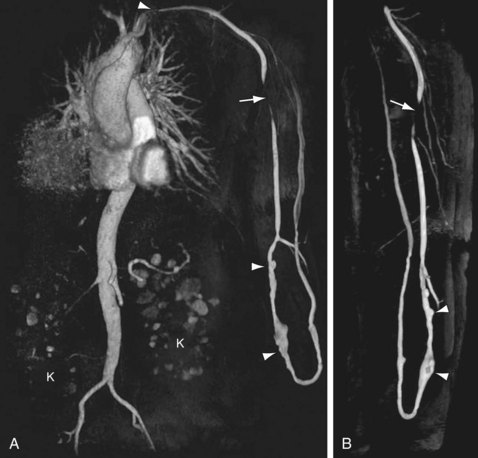
 FIGURE 119-7
FIGURE 119-7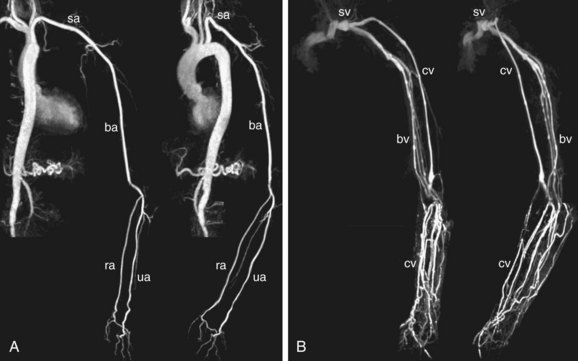
 FIGURE 119-8
FIGURE 119-8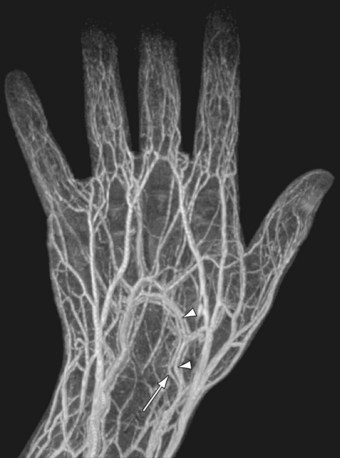
 FIGURE 119-9
FIGURE 119-9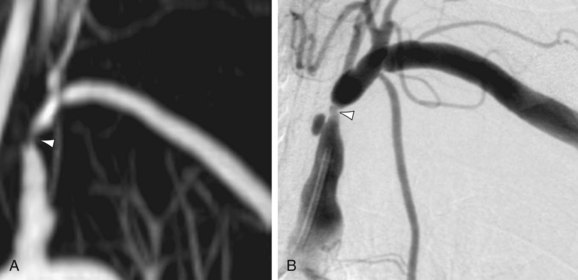
 FIGURE 119-10
FIGURE 119-10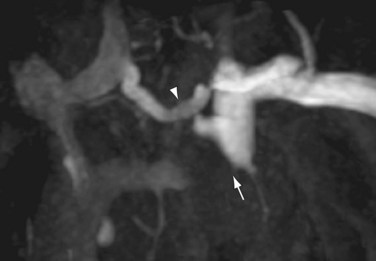
 FIGURE 119-12
FIGURE 119-12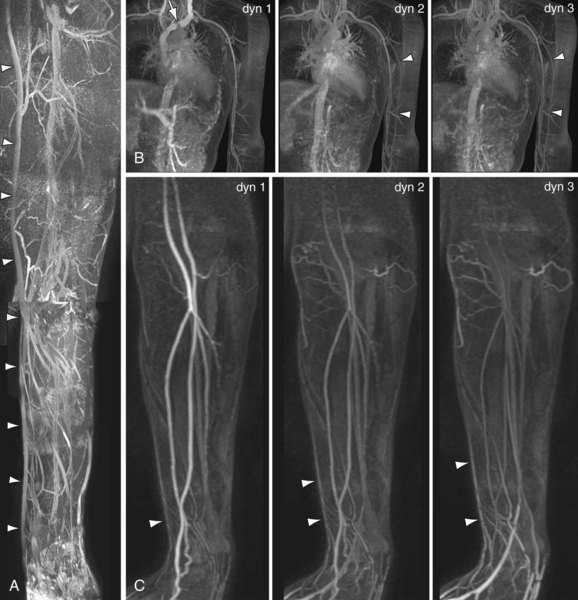
 FIGURE 119-13
FIGURE 119-13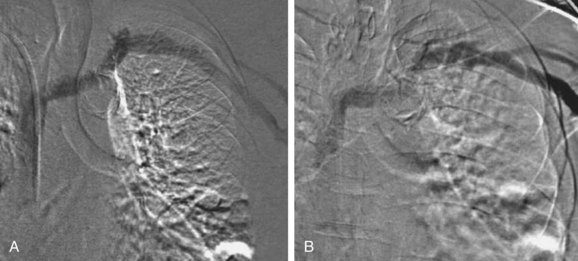
 FIGURE 119-14
FIGURE 119-14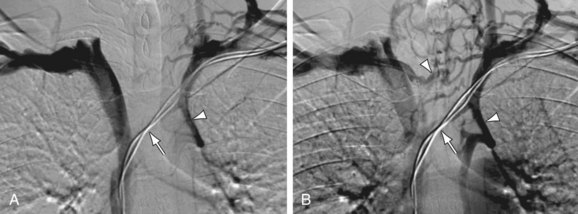
 FIGURE 119-15
FIGURE 119-15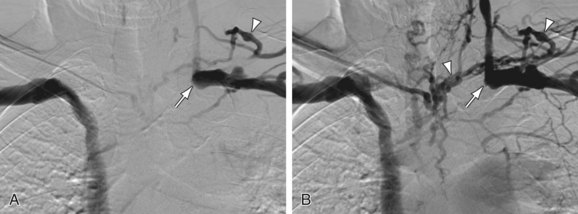
 FIGURE 119-16
FIGURE 119-16
