Chapter 74
Hemodialysis Access
Dialysis Catheters
Karen Woo, Vincent L. Rowe
Central venous catheters for hemodialysis are categorized by their intended duration as either acute or chronic. Chronic hemodialysis catheters are placed in a tunneled fashion and have a subcutaneous cuff at the skin exit site for tissue ingrowth. Acute catheters do not have a cuff and are placed percutaneously without a tunnel. As a result, acute catheters are at higher risk for infectious complications and can easily become dislodged. Thus, acute catheters should be placed only in hospitalized patients and used for a short time.1
This chapter focuses on tunneled hemodialysis catheters and their management, but the potential complications related to placement of acute hemodialysis catheters mirror those of tunneled hemodialysis catheters. Because of the short duration of use of acute catheters, long-term complications are less frequent but infectious complications are more common.
Indications
The tunneled hemodialysis catheter serves a valuable role in the treatment of patients with end-stage renal disease and has distinct advantages over autogenous (arteriovenous fistula [AVF]) and prosthetic (arteriovenous graft [AVG]) arteriovenous hemodialysis access. The benefits of tunneled hemodialysis catheters include immediate use for hemodialysis, uncomplicated and needle-free connection to the dialysis circuit, elimination of cannulation site complications, and simple insertion technique that can be performed by many different interventional specialists.2
The most common indication for placement of a tunneled hemodialysis catheter is for urgent hemodialysis while the patient is waiting for an AVF to be created or to mature. Hemodialysis through a mature AVF represents the most optimal form of hemodialysis access, and ideally patients should be referred for placement of permanent access long before the need for hemodialysis. In this way, patients have an adequate amount of time for AVF maturation, and the requirement for a tunneled hemodialysis catheter is eliminated altogether. Unfortunately, demise of renal function is not a linear estimation. At the same time, care before end-stage renal disease is often inadequate, resulting in late referral to a nephrologist and to an access surgeon.
Other indications include patients in whom an AVF or AVG is not anatomically feasible or who are not operative candidates because of advanced comorbidities. Temporary hemoaccess is also indicated after revision of a permanent hemodialysis access for management of a complication (e.g., access revision for pseudoaneurysm formation or infection), after placement of a peritoneal dialysis catheter, and for a chronic ambulatory peritoneal dialysis patient requiring abdominal or inguinal surgery.
Types of Catheters
The feasibility of using a felt cuffed catheter for prolonged hemodialysis was first described in 1988.3 The authors documented a median catheter survival of 8 weeks for a tunneled hemodialysis catheter placed in the subclavian vein. Since this initial report, catheter design and preferred anatomic position for placement have evolved. Early cuffed catheters were straight in configuration and stiff. These have been largely supplanted by precurved flexible catheters with various tip designs.
Design Characteristics
Catheter designs aim to achieve one main goal: adequate dialysis clearance at a relatively high flow rate. Currently used hemodialysis machines maintain flow rates of 300 to 350 mL/min through indwelling catheters to provide reasonable clearance times for the patients.4 To support the high-flow demand, some manufacturers have increased the catheter lumen size up to 16F. In addition, the phenomenon of recirculation must be minimized to ensure adequate clearance. The “arterial lumen” of the catheter is the outflow to the dialysis machine from the patient; the “venous lumen” is defined as the inflow from the machine back to the patient. Access recirculation is the reentry of dialyzed blood from the venous lumen directly into the arterial lumen, thus bypassing the systemic circulation and leading to inefficient dialysis.5
Design Categories
Numerous manufacturer modifications exist in an effort to satisfy the requirements of high flow rates and minimal recirculation. The modifications fall into four general categories.
Split Tip.
Split-tip catheters have a double-lumen, single-body configuration in the midbody but separate into two distinct distal tips, each with side holes in all directions (Fig. 74-1A).
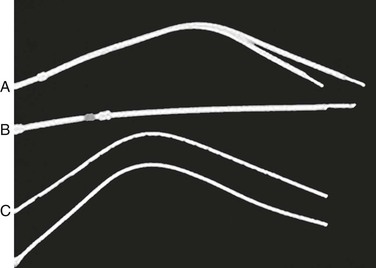
Figure 74-1 Three types of tunneled hemodialysis catheters: split-tip catheter (A), staggered-tip or step-tip catheter (B), and dual catheter (C). (From Richard HM, 3rd, et al: A randomized, prospective evaluation of the Tesio, Ash split, and Opti-flow hemodialysis catheters. J Vasc Interv Radiol 12:431-435, 2001.)
Step Tip.
The staggered-tip or step-tip catheter is a double-lumen, single-body catheter with the venous limb extending at least 2.5 cm beyond the inflow tip (Fig. 74-1B).
Dual Catheter.
The dual catheter design consists of two completely separate catheters that can be inserted in two different locations (Fig. 74-1C).
Symmetric Tip.
The Tal Palindrome (Covidien, Mansfield, Mass) is the only catheter that has a symmetric tip design with equal length of arterial and venous limbs and biased spiral ports. The design of the ports allows inflow to occur through the most proximal portion of the port and outflow to occur as a jet directed away from the catheter tip (Fig. 74-2).6

Figure 74-2 Tal Palindrome catheter. (Courtesy Covidien.)
Shape and Material
Because of the complication of catheter-associated central vein stenosis, the anatomic preference for catheter placement migrated cephalad from the subclavian veins to the internal jugular veins.1 During the same time, straight catheter designs evolved to adopt a premade curve to avoid kinking of the catheter at the vein entry site in the neck. The material composition of the tunneled hemodialysis catheter also affects flow rates. Silicone rubber and Silastic catheters are softer and more pliable, increasing patient comfort when the catheter is inserted into the internal jugular vein and tunneled over the clavicle. Newer polyurethane catheters have a stiffer construct and higher tensile strength and thereby allow thinner walled catheters with smaller outer diameters. Numerous reviews directly compare performance of individual tunneled hemodialysis catheters with specific outcome variables of flow rate, recirculation time, and patency. Unfortunately, despite isolated beneficial characteristics of a particular catheter, no universal significant benefit has been demonstrated by one catheter over the competitors.2,4,5,7
Preoperative Evaluation
History and Physical Examination
As with any clinical assessment, the proper preoperative evaluation of a patient begins with a detailed history and physical examination. Specific inquiries should include details such as prior long-term central line placement, prior AVF or AVG placement, prior tunneled hemodialysis catheter infections, history of a coagulation disorder, and history of a pacemaker. Physical examination of the neck and chest is critical in the evaluation of the hemodialysis patient. Evidence of a previously placed tunneled hemodialysis catheter, previous permanent accesses, upper extremity or facial edema, and ipsilateral venous distention with visible venous collaterals should alert the clinician to the possibility of central veno-occlusive disease.
Numerous specialists have acquired the skill set to properly place a tunneled hemodialysis catheter. However, there is a significant advantage in terms of continuity of care when the same practitioner places the tunneled hemodialysis catheter and the permanent access or when a team approach coordinates these procedures. There are a finite number of access sites available for both tunneled catheter and permanent access placement. Therefore, a comprehensive plan that embraces all forms of hemodialysis access may provide maximum benefit of each crucial access site.
Central Venous Imaging
Color-Flow Venous Duplex Imaging
Similar to the screening of hemodialysis patients for permanent dialysis access, noninvasive color-flow duplex imaging is the first-line method of preoperative imaging for the tunneled hemodialysis catheter. Patency of internal jugular veins and axillary veins is easily identified with compression of the vein. However, as imaging moves toward the central chest, the air interface with the lung tissue as well as obstructing bone structures makes central vein imaging virtually impossible, limiting color-flow venous duplex imaging mainly to the vein entry site.
Magnetic Resonance Venography
Contrast-enhanced magnetic resonance venography (MRV) is a noninvasive modality for imaging of the central veins. Three-dimensional gadolinium-enhanced MRV has been evaluated in the assessment of central venous steno-occlusive disease specifically in hemodialysis patients. In a series of 14 patients, three-dimensional gadolinium-enhanced MRV had a 93% sensitivity in identifying central vein occlusions and stenoses greater than 50% when it was directly compared with digital subtraction angiography.8 Despite the accuracy of MRV, gadolinium must be administered with caution in patients with a glomerular filtration rate of less than 30 mL/min because of the risk of gadolinium-induced nephrogenic systemic fibrosis. Although nephrogenic systemic fibrosis is rare even in this patient population, the condition has not been described in patients with normal renal function.
Computed Tomographic Venography
Computed tomographic venography (CTV) is similar to MRV in being able to image multiple vessels in the chest in one setting. However, CTV does have the advantages of being readily available in most medical centers, fast acquisition times, and fewer deleterious contrast agent concerns. In medical centers dedicated to use of this imaging modality for the evaluation of hemodialysis patients, anecdotal evidence points to an acceptable level of accuracy for the evaluation of central venous disease. A small study of 18 patients was performed to compare CTV and digital subtraction venography for the diagnosis of benign thoracic central venous obstruction. The results of the study demonstrated that CTV findings correlated closely with those of digital subtraction venography.9
Catheter-Based Contrast Venography
Catheter-based contrast venography remains the “gold standard” for diagnosis of central vein stenosis or occlusion. Contrast venography has the distinct advantage of allowing the clinician to initiate endovascular treatment if a significant stenosis is detected at the time of venography. In addition, it is often possible to perform catheter-based venography with a much smaller volume of contrast material than what is required for CTV, reducing the risk of nephrotoxicity.
Catheter Insertion
Site Selection
Numerous venous access sites exist for insertion of a tunneled hemodialysis catheter. However, the right internal jugular vein is the preferred access site because it has the best patency, presumably owing to less kinking. In a prospective evaluation, factors affecting long-term survival of tunneled hemodialysis catheters were analyzed in a cohort of 812 catheters in 492 patients.2 Factors that were associated with immediate failure of the tunneled hemodialysis catheter were malposition and kinking. A tunneled hemodialysis catheter placed in the right internal jugular vein demonstrated significantly longer survival compared with one placed in the left internal jugular vein. Tunneled hemodialysis catheters placed in the femoral vein had the worst long-term survival. The subclavian vein should be avoided if possible to prevent catheter-induced subclavian stenosis, which would negatively affect the future placement of ipsilateral permanent access.2,4
A tunneled hemodialysis catheter from the right internal jugular vein should be 17 to 19 cm in length; catheters from the left are slightly longer. When femoral access is used, long catheters (24 to 31 cm) should be placed so that the tip can reach into the inferior vena cava to produce the best flow rates.
Technique
Tunneled hemodialysis catheter insertion should be performed in a procedure room under fluoroscopic guidance. Bedside placement is discouraged because of the need for proper sterility, the possibility of additional endovascular procedures (such as venography and venoplasty), and the requirement for fluoroscopy. The procedure is usually performed under local anesthesia with conscious sedation. Patients who have had numerous prior catheters have an increased risk of central venous stenoses or occlusions that may increase the complexity of the procedure. In such cases, general anesthesia is preferred. Preoperative antibiotic prophylaxis directed at gram-positive bacterial strains is administered before skin incision in each case. The skin is then prepared with isopropyl alcohol and chlorhexidine solutions.
Ultrasound evaluation with a sterile covered transducer confirms access vein patency. The site of vein cannulation should be 3 to 4 cm cephalad to the clavicle. Real-time ultrasound guidance is used to access the vein. The puncture is performed with a micropuncture 21-gauge needle, 0.018-inch guide wire, and 5F coaxial catheter (Fig. 74-3). After successful vein cannulation with the micropuncture needle, the introducer and 0.018-inch wire are removed and exchanged for a 0.035-inch wire. In preparation for the large-bore hemodialysis catheter, a 1-cm skin incision is made surrounding the wire entry site (Fig. 74-4, short arrow). Fluoroscopic imaging should be used during all wire maneuvers to confirm wire position.
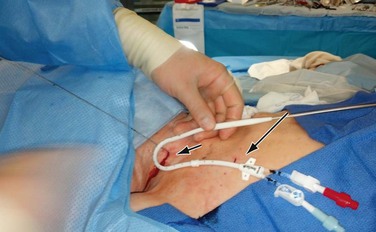
Figure 74-4 Placement of the tunneled hemodialysis catheter alongside the anticipated catheter course to determine where to position the exit site. Internal jugular vein puncture site (short arrow); upper chest catheter exit site (long arrow).
Antegrade Placement
A small incision is made on the anterior chest where the tunneled hemodialysis catheter is to exit the subcutaneous tunnel (Fig. 74-4, long arrow). The exit site of the tunneled hemodialysis catheter should be inferior and lateral to the vein entry site in location. The exit site should also be positioned such that the felt cuff on the catheter shaft is 1 cm proximal to the exit site. Placement of the tunneled hemodialysis catheter alongside the anticipated catheter course is useful in determining where the exit site should be positioned. The tunneled hemodialysis catheter is then attached to the tunneling device and passed subcutaneously from the chest incision up through the vein entry site incision in the neck, traversing anterior to the clavicle en route (Fig. 74-5). The track of the wire is sequentially dilated, and the final introducer and peel-away sheath unit is inserted until the sheath is “hubbed” at the skin (Fig. 74-6). The introducer and wire are removed, and the tunneled hemodialysis catheter is inserted through the peel-away sheath (Fig. 74-7). Once the tunneled hemodialysis catheter is fully inserted, the peel-away sheath is withdrawn.
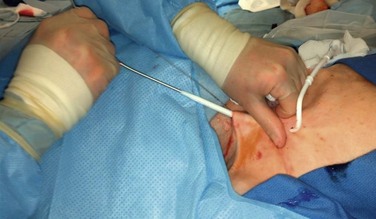
Figure 74-5 Tunneling of the catheter subcutaneously from the chest incision to the neck incision anterior to the clavicle.
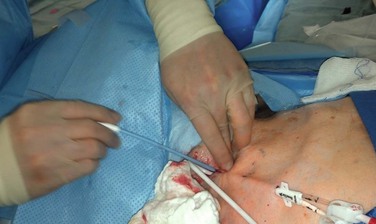
Figure 74-6 Dilatation of the track of the wire.
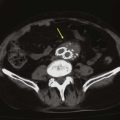
Each lumen of the catheter is aspirated and flushed with dilute heparin (100 units of heparin sulfate per milliliter of normal saline). Both of these actions should be easily accomplished without resistance. If resistance is encountered, the tunneled hemodialysis catheter should be examined under fluoroscopy to rule out a kink in the path of the catheter or malpositioning of the tip. Ideally, the most distal tip of the catheter should be in the distal superior vena cava/proximal right atrium border. This preferred position corresponds to the shadow of the right mainstem bronchus (Fig. 74-8).
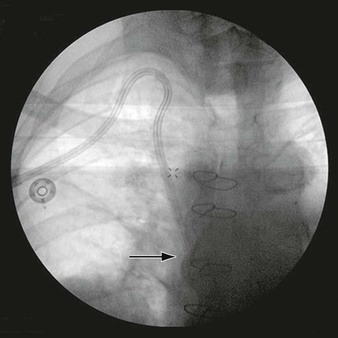
Figure 74-8 Chest radiograph demonstrating proper position of the tip of the tunneled hemodialysis catheter (arrow) at the level of the right mainstem bronchus.
The chest wall and vein entry site incisions are closed with absorbable sutures, and the tunneled hemodialysis catheter is secured to the chest wall with nonabsorbable sutures. Each lumen of the tunneled hemodialysis catheter is filled with the manufacturer-indicated volume of concentrated heparinized saline (1000 units/mL) to protect against intracatheter thrombosis.
Retrograde Placement
More recently, some manufacturers have developed tunneled hemodialysis catheters that can be placed in a “reverse-tunneled” or “retrograde” fashion. These tunneled hemodialysis catheters do not have the ports attached initially. The catheter is inserted into the vein before tunneling in the same fashion as the standard tunneled hemodialysis catheter. The catheter is then tunneled from the neck incision to the chest incision, and the ports are attached after tunneling. The advantage of this type of insertion is that it allows precise positioning of the catheter tip.
Unconventional Catheter Sites
Patients who have been hemodialysis dependent for an extended time often have exhausted conventional sites for vascular access placement. In a 1996 report, 5% of dialysis patients withdrew from dialysis secondary to lack of sites for dialysis access.10 This statistic is likely to have increased with the expanding dialysis population, the increased life expectancy on dialysis, and the relatively stable number of kidney transplants performed annually. Patients who have exhausted conventional sites for vascular access may require placement of tunneled hemodialysis catheters in unconventional sites, such as the transhepatic and translumbar routes (see Chapter 75).
Translumbar catheters are generally placed with the patient in the prone position by percutaneous puncture of the inferior vena cava above the right iliac crest. The catheter is tunneled through a right lateral abdominal exit site. For transhepatic catheters, percutaneous access to the right or middle hepatic vein is obtained through the eighth intercostal space in the midaxillary line under fluoroscopic guidance. The catheter is then tunneled to a lateral anterior chest wall exit site. The tip of the catheter is positioned in the right atrium in both approaches.
The reported mean total catheter service lifespan of transhepatic and translumbar catheters in the literature ranges from 70 days to 450 days.11,12 Also, unique to this type of access is the high rate of subsequent interventions for maintenance of adequate hemodialysis access. Two of the largest published series reported that more than 60% of their patients required at least one catheter exchange. Catheter migration and catheter thrombosis are the two most commonly reported reasons for catheter exchange.11–13 Translumbar catheter exchanges may be more difficult than exchanges through the transhepatic approach because of retroperitoneal fibrosis that develops along the track.11
Perioperative Care and Complications
The perioperative management of patients after placement of a tunneled hemodialysis catheter centers on the evaluation for and treatment of potential acute complications. These complications involve needle or catheter injuries to the pleura, lung, vascular or nerve structures in the neck, thoracic inlet, mediastinum, and heart. The keys to successful management of these complications are awareness, prompt recognition, and expeditious treatment. In a compilation of data from 1794 central venous cannulations in critically ill patients, there were a total of 127 complications for an overall rate of 7.1%.14 Failure to place the central line and arterial puncture were the most common mechanical complications, followed by pneumothorax.
Pneumothorax
An end-expiratory upright chest radiograph should be performed after placement of a tunneled hemodialysis catheter to confirm tip placement and to exclude a pneumothorax.15 Bilateral attempts at venous catheterization should not be made until pneumothorax has been ruled out on the initial side by chest radiography to avoid the potential disaster of bilateral pneumothorax. If acute, severe symptoms and signs of pneumothorax develop, a tube thoracostomy should be performed immediately and the patient should be given oxygen by mask. In the setting of tension pneumothorax, decompression of the pleural space by use of an intravenous catheter can be lifesaving. The needle should be placed in the second intercostal space in the midclavicular line. More frequently, the air leak is relatively small, and the pneumothorax develops during a period of several hours. In such cases, there is time to confirm the diagnosis and to determine its size with a chest radiograph.
When a small pneumothorax is encountered (<20%) and there are no symptoms or signs of respiratory compromise, decrease in peripheral oxygen saturation, or hemodynamic impairment, a “watchful waiting” approach with repeated chest radiographs is appropriate.16 If symptoms develop or there is an increase in the size of the pneumothorax on subsequent chest radiographs, tube thoracostomy should be performed. If the pneumothorax is initially noted to be large (>20%), tube thoracostomy should be performed regardless of whether symptoms are present. A mobile thoracic vent device (e.g., Heimlich valve) is an alternative to thoracostomy that is significantly more comfortable for the patient. If the central venous catheter is well positioned and functioning appropriately, it can be left in place.
Hemothorax
Placement of tunneled hemodialysis catheters may be complicated by the development of a hemothorax when the back wall of either a vein or artery and the parietal pleura are perforated by an advancing needle tip, dilator, or sheath.17 The subclavian vein or artery, the innominate vein, or even the superior vena cava may be involved. The lack of effective tamponade combined with negative respiratory pressure may result in a large blood loss through a small puncture. Clinically, patients may develop respiratory compromise accompanied by dullness to percussion and decreased breath sounds on the affected side. A decreased hematocrit and evidence of fluid in the pleural cavity on the chest radiograph strongly support the diagnosis. Drainage of the pleural space with a tube thoracostomy is generally adequate therapy and should be performed for significant hemothorax to prevent entrapment of the lung. Rarely, bleeding must be controlled surgically or, if possible, percutaneously with the use of covered stents.
Subcutaneous Hematoma
Subcutaneous hematoma can occur when vessels in the cervical and clavicular area are lacerated. Prolonged compression (15 minutes or more), with or without catheter removal, usually controls the bleeding unless there is a major vascular injury.18 Elevation of the patient’s upper body to decrease venous pressure is also helpful. If direct pressure with catheter removal is inadequate to control the bleeding, surgical intervention or covered stent placement may be necessary.
Wire Embolism
Wire embolism occurs when control of the wire is lost during the procedure or the guide wire is sheared off by the access needle as it is withdrawn. If resistance to wire removal is encountered, fluoroscopic imaging should be used, and removal of the wire and needle together as a unit may be required. Fortunately, most foreign bodies, including segments of guide wires, can usually be removed in the angiography suite with use of a wire snare retrieval system.19
Cardiac Arrhythmia
Cardiac complications related to central venous catheterization are rare. Arrhythmia during placement of a tunneled hemodialysis catheter is associated with the guide wire’s irritating the myocardium. Patients who have a history of a cardiac arrhythmia or those with altered plasma electrolytes are at a higher risk. The problem can be minimized by use of guide wires that have distance markings and fluoroscopy to visualize the location of the tip of guide wires and catheters. If cardiac arrhythmias develop after the placement of a tunneled hemodialysis catheter, the catheter position should be checked fluoroscopically or with a chest radiograph and the catheter tip should be withdrawn if the tip is near or traversing the tricuspid valve. Rarely, patients will have arrhythmias that require chemical or electrical cardioversion. In a series of 300 patients who underwent central venous catheterization, the incidence of cardioversion for a procedure-associated arrhythmia was 0.9%.20
Cardiac Perforation
The soft, flexible hemodialysis catheters that are currently used are unlikely to perforate the heart. More common causes of cardiac perforation are guide wires, dilators, and rigid introducers. Cardiac perforation may result in acute pericardial tamponade from bleeding or fluid infusion into the pericardial space. Signs and symptoms of pericardial tamponade can develop rapidly and include shock and cyanosis with marked cervical venous distention. Tachycardia and muffled heart sounds are generally present, and a large globular cardiac silhouette may be present on a chest radiograph. Any intraluminal catheter devices should be removed and pericardiocentesis or a pericardial window created. If the tamponade recurs after pericardiocentesis or creation of a window, median sternotomy with formal cardiac repair may be necessary.
Thoracic Duct Laceration
Percutaneous catheterization of the superior vena cava through the left internal jugular or the subclavian approach carries a small risk of thoracic duct laceration. Cirrhotic patients are more prone to the development of this complication. If a lymphatic leak becomes apparent, the catheter should be removed and a pressure dressing should be applied. Nearly all such leaks resolve spontaneously.
Nerve Injuries
The brachial plexus is the nerve structure that is most vulnerable during percutaneous catheterization by virtue of its large size and proximity to the subclavian vein and artery.21,22 Acute upper extremity pain referred along a neural anatomic pathway suggests impingement on the brachial plexus and necessitates immediate withdrawal of needles or catheters. Permanent injury is rare. The vagus, recurrent laryngeal, and phrenic nerves are also in proximity to the internal jugular vein. However, these are small nerves and are infrequently injured. The development of hoarseness after catheter placement suggests injury to the vagus or recurrent laryngeal nerve. Phrenic nerve injuries are generally asymptomatic and are incidentally identified on radiographic examination when an elevated hemidiaphragm is seen.23 The development of Horner’s syndrome has also been reported by inadvertent trauma to the stellate ganglion during percutaneous cannulation of the internal jugular vein.
Catheter Misplacement
In a prospective study of 1619 patients, the incidence of catheter tip malposition, defined as extrathoracic or ventricular positioning, was 3.3%.24 The use of fluoroscopy during catheter placement should eliminate the occurrence of catheter tip malposition. If there is a question as to the location of the tip of the catheter during placement, contrast material can be injected through the catheter under fluoroscopic guidance to help define the anatomy.
Venous
If the catheter tip is left in the subclavian, axillary, jugular, or hepatic vein, the catheter tip or pressure generated during hemodialysis frequently causes intimal injury, which leads to thrombosis of the vein.25 Stiff catheters or introducers left abutting the wall of the superior vena cava or more peripheral veins can also erode through the vessel wall and produce a hemomediastinum or hydromediastinum.
Arterial
Catheters may be inadvertently placed into the subclavian or carotid artery without the practitioner’s recognizing the problem. This is more common in a hypotensive or poorly oxygenated patient who may not have return of bright red, pressurized blood when an artery is punctured. As tunneled hemodialysis catheters are generally placed on an elective basis, arterial placement is rare. If there is a question of arterial placement, the pressure can be transduced through the catheter to see if the waveform is arterial or venous. A sample from the catheter can also be sent for a blood gas analysis to determine if the values are consistent with an arterial or venous blood gas. If the injury is recognized in a timely fashion (<4 hours), most of the catheters placed in the carotid artery can be simply removed and pressure applied for a prolonged time (15-20 minutes).26 If the injury is recognized later, the catheter should be removed in the operating room with open repair of the artery because the incidence of track formation and accumulation of thrombus on the catheter is increased.26 Injuries to the subclavian artery are more difficult to compress because of the lack of bone structures to compress against and should be monitored closely for evidence of hemothorax. Open or endovascular repair of the artery may be required.27
Long-Term Care and Complications
The long-term management of tunneled hemodialysis catheters is primarily performed by the staff at the dialysis center where the patient receives dialysis. The National Kidney Foundation Kidney Dialysis Outcomes and Quality Initiative (NKF KDOQI) guidelines provide recommendations for the routine care of tunneled hemodialysis catheters, which include cleaning with 2% chlorhexidine and redressing the exit site at each dialysis treatment, masks for the provider and the patient when the catheter lumina or exit sites are exposed, and use of aseptic technique when the catheter is manipulated.1 Patients with tunneled hemodialysis catheters usually do not come to the attention of the vascular surgeon unless a late complication of the catheter develops.
Air Embolism
Air embolism is a rare but potentially lethal complication of central venous catheterization that can be either an acute or a late complication.17 In its late form, air embolism generally occurs when air enters the catheter either before attachment of the tubing or when the tubing becomes disconnected.28,29 Air embolization can also occur through cracks in the catheter or its hub as well as through the catheter track after the removal of a central venous catheter.30 Patients should be instructed that in the event the catheter becomes disconnected or uncapped, a critical complication could result. Patients should be instructed to cap the open catheter with a thumb or finger and to call for help immediately. An occlusive dressing should be placed over the skin puncture site when the catheter is removed to allow adequate sealing of the track.
If sudden cardiorespiratory collapse develops in a patient with a tunneled hemodialysis catheter, air embolism must be strongly considered. Further embolization must be prevented by capping or clamping of the catheter while the patient is simultaneously placed in the Trendelenburg and left lateral decubitus position (Durant maneuver).31 This position displaces air away from the pulmonic valve, which relieves the right ventricular outflow obstruction. The catheter can be advanced into the heart to aspirate the air.32
Catheter Embolism
Fractures can develop in the material of chronically indwelling catheters. This usually occurs at sites of stress, such as the thoracic inlet.33,34 Fractures can result in breaking off and embolization of the distal aspect of the catheter. Catheter embolism is diagnosed when an incomplete catheter is removed from a patient. The retained catheter segment can typically be removed in the angiography suite with use of a wire snare retrieval system.35
Catheter Occlusion
Catheter occlusion occurs in 30% to 40% of patients with tunneled hemodialysis catheters.36 Occlusion of the catheter generally is a consequence of the development of a fibrin sleeve or plug at the catheter tip. The ability to infuse fluid into the catheter but the inability to withdraw blood often indicates impending occlusion.
Prevention
A randomized controlled trial of the use of low-dose warfarin to prevent catheter thrombosis demonstrated lack of efficacy.37 Another randomized trial was performed with three arms: aspirin 325 mg/day, warfarin titrated to an international normalized ratio of 2 to 3, and control group. This study found that whereas both aspirin and warfarin were equally effective at increasing catheter patency, there was a significant increase in the risk of gastrointestinal bleeding in both treatment groups.38 As such, use of antiplatelet agents or systemic anticoagulation is not recommended to prevent thrombosis. However, a randomized trial of patients assigned to a catheter-locking regimen of heparin (5000 units/mL) three times a week or recombinant tissue plasminogen activator (1 mg in each lumen) substituted for heparin one session a week did demonstrate a twofold decrease in the risk of catheter malfunction in patients treated with recombinant tissue plasminogen activator once a week with no increase in the risk of bleeding.39
Treatment
Local infusion of fibrinolytic agents has been used in the salvage of occluded central venous catheters.40,41 Currently, alteplase is the only fibrinolytic agent that is approved for treatment of occluded central venous catheters. Most commonly, 2 mg of fibrinolytic agent in 2 mL of solution is injected and allowed to dwell in the catheter for 2 to 3 hours. The catheter is then irrigated and flushed, with removal of any residual clot. This process can be repeated until patency is restored. However, use of this technique may result in only a limited number of additional dialysis sessions before the treatment must be repeated. A study of 570 catheters during a 2.5-year period demonstrated a median of five to seven additional dialysis sessions after each treatment.42 Guide wires should never be passed through catheters in an effort to relieve an obstruction because of the risk of dislodging part or all of the occlusion and causing an embolic event.
Because of the frequency of recurrent occlusion and the difficulty in delivery of a highly concentrated fibrinolytic agent to the fibrin sheath around the tip of the catheter, techniques have been developed to mechanically eliminate the fibrin sheath. These techniques generally involve the insertion of a wire snare device through another venous access site, such as the femoral vein, to strip the fibrin sheath from the catheter and remove it. In a study of 131 sheath stripping procedures on 100 catheters, the technical success of the procedure was 95.6% and the mean primary patency after the first stripping was 89 days.43 An alternative technique is to remove the catheter over a wire, then use an angioplasty balloon to rupture the sheath, and finally replace the catheter over the wire. A retrospective review of these two techniques compared with simple catheter exchange demonstrated no difference in patency.44
Central Venous Thrombosis
Catheter-related thrombus is present in approximately 30% of all patients with central venous catheters.45 However, less than half of these thrombi are clinically significant.45 The incidence of pulmonary embolus from catheter-related thrombus ranges from 0% to 17%, although catheter-related thrombus has rarely been reported to be the cause of death.45 The more clinically significant implication of catheter-related thrombosis is its association with infection. In a study of patients with catheter-related Staphylococcus aureus bacteremia, 71% were found also to have central venous thrombus.46 Nevertheless, the routine use of low-dose warfarin prophylaxis is not recommended.47
Venous thrombosis should be suspected in patients with tunneled hemodialysis catheters who present with arm, neck, or facial swelling; prominent collateral venous patterns; signs or symptoms of embolic complications; or unexplained fever. Duplex scanning generally is diagnostic, although venography is required on occasion for definitive diagnosis and determination of the extent of thrombosis. Conventional therapy consists of anticoagulation and elevation of the symptomatic arm. If possible, the catheter should be removed. As patients with tunneled hemodialysis catheters often do not have other sites available for access placement, anticoagulation therapy with the catheter left in place can be considered.48
Central Venous Stenosis
Catheter-associated central vein stenosis develops as a result of injury to the intima of the vein by the catheter.49 The association between subclavian vein cannulation for hemodialysis access and subsequent subclavian vein stenosis is well described.50,51 The incidence of subclavian vein stenosis after placement of a tunneled hemodialysis catheter is significantly higher than that of internal jugular vein stenosis. In a study comparing venography in 50 patients dialyzed through a subclavian vein catheter and 50 patients dialyzed through an internal jugular vein catheter, 42% of patients in the subclavian group had a stenosis versus 10% in the jugular group.52 As such, cannulation of the subclavian vein for hemodialysis access should be avoided if at all possible. Catheter-associated central vein stenosis can also involve the brachiocephalic vein as well as the superior vena cava.
Presentation
Central vein stenosis can be completely asymptomatic. Often, an ipsilateral upper extremity access is created without knowledge of a central vein stenosis, which results in the rapid development of symptoms, the most common of which is arm edema. Patients with brachiocephalic vein stenosis can also present with facial edema. Other manifestations of central vein stenosis are aneurysmal dilatation of the extremity veins and the ipsilateral AVF, thrombosis of the access, inadequate dialysis, prolonged bleeding after use of the access, and superior vena cava syndrome.49
Treatment
Elevation and compression of the upper extremity can occasionally be enough to relieve the edema associated with central venous stenosis but is unlikely to be effective if there is an access on the ipsilateral extremity. NKF KDOQI guidelines recommend percutaneous transluminal angioplasty with or without stent placement as the preferred treatment for central venous stenosis.1 In a small randomized study of percutaneous transluminal angioplasty and stent placement, 1-year primary patency in both groups was dismal at 12% and 11%, respectively.53 However, the secondary patency at 1 year was 100% for percutaneous transluminal angioplasty and 78% for stenting. This marked difference in primary and secondary patency underscores the fact that multiple procedures are usually needed to maintain patency after endovascular management of central venous stenoses.
Catheter-Related Infection
There are three categories of catheter-related infection: exit site infection, tunnel infection, and bacteremia.1 The NKF KDOQI guidelines define an exit site infection as “inflammation confined to the area surrounding the catheter exit site, not extending superiorly beyond the cuff if the catheter is tunneled, with exudate culture confirmed to be positive.” A tunnel infection is defined as “the catheter tunnel superior to the cuff is inflamed, painful, and may have drainage through the exit site that is culture positive.” Finally, catheter-related bacteremia is defined as “blood cultures are positive for the presence of bacteria with or without the accompanying symptom of fever.” The incidence of catheter-related bacteremia ranges from 0.6 to 6.5 episodes per 1000 catheter days.54
Bacteriology
The predominant organism isolated from infected lines is gram-positive (52%-84%), with Staphylococcus aureus making up 21% to 43%.54 Methicillin-resistant S. aureus is reported in 12% to 38% of cases.54 Gram-negative bacilli, including Pseudomonas species, Klebsiella pneumoniae, Escherichia coli, and Enterobacter species, have also been isolated from infected lines.55 Fungal infections, predominantly Candida species, are associated with broad-spectrum antibiotic therapy and renal impairment.56 Consequences of bacteremia include infective endocarditis and metastatic abscesses, the incidence of which increases when catheter salvage is attempted.54
Treatment
Ideally, the management of an infected hemodialysis catheter includes removal of the catheter.54 Initial empirical antibiotic therapy should include broad-spectrum coverage of potentially resistant strains of gram-positive organisms as well as gram-negative organisms.57 This coverage should be adjusted to a focused regimen when culture results become available. Amphotericin B or caspofungin, which has a more favorable toxicity profile, should be used if there is evidence of disseminated fungal infection or if patients demonstrate persistent fungemia after catheter removal.47 Uncomplicated S. aureus catheter-related bacteremia is generally treated with 4 to 6 weeks of antibiotic therapy; gram-negative bacilli or enterococcus bacteremia is usually treated with 7 to 14 days of therapy. Bacteremia with Candida is usually treated with a minimum of 14 days of antibiotic therapy. Complicated bacteremia with septic thrombophlebitis or endocarditis is usually treated for 4 to 6 weeks and osteomyelitis for 6 to 8 weeks.57
Catheter exit site infections alone can usually be salvaged with topical and systemic antibiotics without the need for catheter replacement.57 Presence of a tunnel infection or catheter-related bacteremia requires catheter removal with delayed placement of a permanent access. The NKF KDOQI Work Group recommends delay of placement of a new permanent access until culture results have been negative for at least 48 hours after cessation of antibiotic therapy.1 Depending on the length of antibiotic therapy, this may or may not be reasonable. Strategies for catheter salvage include exchange over a wire and catheter salvage with or without antibiotic lock. Whereas catheter salvage has been described, it is associated with failure rates greater than 65%.54 As such, the safest course of action is most often to remove the catheter.
Selected Key References
d’Othee J, Tham JC, Sheiman RG. Restoration of patency in failing tunneled hemodialysis catheters: a comparison of catheter exchange, exchange and balloon disruption of the fibrin sheath, and femoral stripping. J Vasc Interv Radiol. 2006;17:1011–1015.
Hemmelgarn BR, Moist LM, Lok CE, Tonelli M, Manns BJ, Holden RM, LeBlanc M, Faris P, Barre P, Zhang J, Scott-Douglas N. Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. [Prevention of Dialysis Catheter Lumen Occlusion with rt-PA versus Heparin Study Group] N Engl J Med. 2011;364:303–312.
Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S176–S273.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S176–S273.
2. Fry AC, et al. Factors affecting long-term survival of tunneled haemodialysis catheters—a prospective audit of 812 tunneled catheters. Nephrol Dial Transplant. 2008;23:275–281.
3. Schwab SJ, et al. Prospective evaluation of a Dacron cuffed hemodialysis catheter for prolonged use. Am J Kidney Dis. 1988;11:166–169.
4. O’Dwyer H, et al. A prospective comparison of two types of tunneled hemodialysis catheters: the Ash Split versus the PermCath. Cardiovasc Intervent Radiol. 2005;28:23–29.
5. Tal MG. Comparison of recirculation percentage of the palindrome catheter and standard hemodialysis catheters in a swine model. J Vasc Interv Radiol. 2005;16(9):1237–1240.
6. Tal MG, et al. Selecting optimal hemodialysis catheters: material, design, advanced features, and preferences. Tech Vasc Interv Radiol. 2008;11:186–191.
7. Trerotola SO, et al. Randomized comparison of high-flow versus conventional hemodialysis catheters. J Vasc Interv Radiol. 1999;10:1032–1038.
8. Gao K, et al. Three-dimensional gadolinium-enhanced MR venography to evaluate central venous steno-occlusive disease in hemodialysis patients. Clin Radiol. 2012;67:560–563.
9. Kim HC, et al. Role of CT venography in the diagnosis and treatment of benign thoracic central venous obstruction. Korean J Radiol. 2003;4:146–152.
10. Excerpts from the United States Renal Data System 1996 Annual Data Report. Am J Kidney Dis. 1996;28(Suppl 2):S1–S165.
11. Younes HK, et al. Transhepatic hemodialysis catheters: functional outcome and comparison between early and late failure. J Vasc Interv Radiol. 2011;22:183–191.
12. Stavropoulos SW, et al. Percutaneous transhepatic venous access for hemodialysis. J Vasc Interv Radiol. 2003;14:1187–1190.
13. Smith TP, et al. Transhepatic catheter access for hemodialysis. Radiology. 2004;232:246–251.
14. Schummer W, et al. Mechanical complications and malpositions of central venous cannulations by experienced operators. A prospective study of 1794 catheterizations in critically ill patients. Intensive Care Med. 2007;33:1055–1059.
15. Giacomini M, et al. How to avoid and manage a pneumothorax. J Vasc Access. 2006;7(1):7–14.
16. Noppen M, et al. Pneumothorax. Respiration. 2008;76:121–127.
17. Borja AR. Current status of infraclavicular subclavian vein catheterization: review of the English literature. Ann Thorac Surg. 1972;13:615–624.
18. Bishop L, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol. 2007;29:261–278.
19. Bessoud B, et al. Experience at a single institution with endovascular treatment of mechanical complications caused by implanted central venous access devices in pediatric and adult patients. AJR Am J Roentgenol. 2003;180:527–532.
20. Brothers TE, et al. Experience with subcutaneous infusion ports in three hundred patients. Surg Gynecol Obstet. 1988;166:295–301.
21. Bernard RW, et al. Subclavian vein catheterizations: a prospective study. I. Noninfectious complications. Ann Surg. 1971;173:184–190.
22. Ramdial P, et al. Brachial plexopathy after subclavian vein catheterization. J Trauma. 2003;54:786–787.
23. Takasaki Y, et al. Transient right phrenic nerve palsy associated with central venous catheterization. Br J Anaesth. 2001;87:510–511.
24. Pikwer A, et al. The incidence and risk of central venous catheter malpositioning: a prospective cohort study in 1619 patients. Anaesth Intensive Care. 2008;36:30–37.
25. Langston CS. The aberrant central venous catheter and its complication. Radiology. 1971;100:55–59.
26. Mussa FF, et al. Current trends in the management of iatrogenic cervical carotid artery injuries. Vasc and Endovasc Surg. 2006;40(5):354–361.
27. Cayne NS, et al. Experience and technique for the endovascular management of iatrogenic subclavian artery injury. Ann Vasc Surg. 2010;24:44–47.
28. Flanagan JP, et al. Air embolus: a lethal complication of subclavian venipuncture. N Engl J Med. 1969;281:488–489.
29. Hoshal VL Jr, et al. The subclavian catheter. N Engl J Med. 1969;281:1425.
30. Paskin DL, et al. A new complication of subclavian vein catheterization. Ann Surg. 1974;179:266–268.
31. Durant TM, et al. Body position in relation to venous air embolism: a roentgenologic study. Am J Med Sci. 1954;227:509–520.
32. Alvaran SB, et al. Venous air embolism: comparative merits of external cardiac massage, intracardiac aspiration, and left lateral decubitus position. Anesth Analg. 1978;57:166–170.
33. McMenamin EM. Catheter fracture: a complication in venous access devices. Cancer Nurs. 1993;16:464–467.
34. Van der Hem KG, et al. “Spontaneous” catheter fracture and embolization of a totally implanted venous access port. Neth J Med. 1991;38:262–264.
35. Furui S, et al. Intravascular foreign bodies: loop-snare retrieval system with a three-lumen catheter. Radiology. 1992;183:283–284.
36. Besarab A, et al. Catheter management in hemodialysis patients: delivering adequate flow. Clin J Am Soc Nephrol. 2011;6:227–234.
37. Wilkieson TJ, et al. Low-intensity adjusted-dose warfarin for the prevention of hemodialysis catheter failure: a randomized, controlled trial. Clin J Am Soc Nephrol. 2011;6:1018–1024.
38. Obialo CI, et al. Maintaining patency of tunneled hemodialysis catheters—efficacy of aspirin compared to warfarin. Scand J Urol Nephrol. 2003;37:172–176.
39. Hemmelgarn BR, et al. Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. N Engl J Med. 2011;364:303–312.
40. Hurtubise MR, et al. Restoring patency of occluded central venous catheters. Arch Surg. 1980;115:212–213.
41. Shavit L, et al. High dose urokinase for restoration of patency of occluded permanent central venous catheters in hemodialysis patients. Clin Nephrol. 2010;74:297–302.
42. Little MA, et al. A longitudinal study of the repeated use of alteplase as therapy for tunneled hemodialysis catheter dysfunction. Am J Kidney Dis. 2002;39:86–91.
43. Brady PS, et al. Efficacy of percutaneous fibrin sheath stripping in restoring patency of tunneled hemodialysis catheters. AJR Am J Roentgenol. 1999;173:1023–1027.
44. d’Othee J, et al. Restoration of patency in failing tunneled hemodialysis catheters: a comparison of catheter exchange, exchange and balloon disruption of the fibrin sheath, and femoral stripping. J Vasc Interv Radiol. 2006;17:1011–1015.
45. van Rooden CJ, et al. Deep vein thrombosis associated with central venous catheters—a review. J Thromb Haemost. 2005;3:2409–2419.
46. Crowley AL, et al. Venous thrombosis in patients with short- and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med. 2008;36:385–390.
47. Bishop 2011.
48. Lowell JA, et al. Venous access—preoperative, operative and postoperative dilemmas. Surg Clin North Am. 1991;71:1231–1246.
49. Agarwal AK, et al. Central vein stenosis: a nephrologist’s perspective. Semin Dial. 2007;20:53–62.
50. Barrett N, et al. Subclavian stenosis: a major complication of subclavian dialysis catheters. Nephrol Dial Transplant. 1988;3:423–425.
51. Vanherweghem JL, et al. Subclavian vein thrombosis: a frequent complication of subclavian vein cannulation for hemodialysis. Nephrol Dial Transplant. 1986;26:235–238.
52. Schillinger F, et al. Post catheterization vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991;6:722–724.
53. Quinn SF, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenosis in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol. 1995;6:851–855.
55. Bevc S, et al. Non–insertion-related complications of central venous catheterization—temporary vascular access for hemodialysis. Ren Fail. 2007;29:91–95.
56. Ghabril MS, et al. Metabolic and catheter complications of parenteral nutrition. Nutrition. 2004;6:237–334.
57. Mermel LA, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45.