32 Hemispheric asymmetries
The two cerebral hemispheres are asymmetrical in certain respects. Some of the asymmetries have to do with handedness, language, and complex motor activities; other, more subtle differences come under the general rubric of cognitive style. (Limbic asymmetries are described in Ch. 34.)
Handedness and Language
Language areas
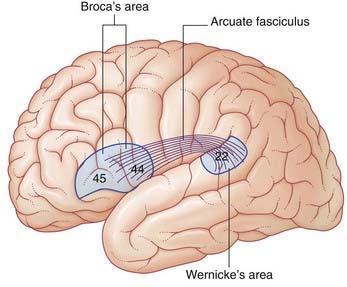
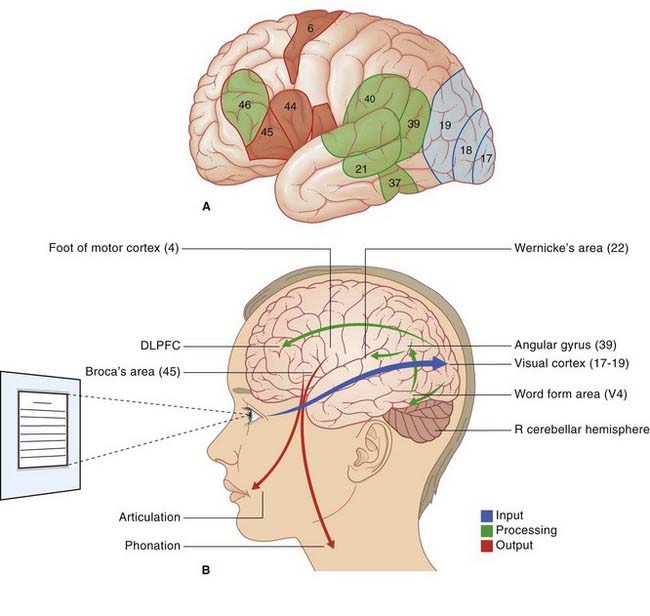
Broca’s area (Figure 32.1)
Lesions involving Broca’s area are associated with expressive aphasia (see Clinical Panel 32.1). Some workers believe that expressive aphasia requires that the lesion also includes the lower end of the precentral gyrus.
Clinical Panel 32.1 The aphasias
Motor (anterior) aphasia
If the lesion involves a substantial amount of the cortical territory of the middle cerebral artery, there will be a motor weakness of the right lower face and right arm. Because the lips and tongue are affected on the right side, the patient will also have dysarthria (difficulty in speech articulation) in the form of slurring of certain syllables. In the example shown in Figure CP 32.1.1A, Broca’s aphasia would be associated with right-sided weakness of the lower face but not of the arm, together with some dysarthria.
Sensory (posterior) aphasia
In the example shown in Figure CP 32.1.1B, Wernicke’s aphasia would be associated with alexia (angular gyrus), ideomotor apraxia (supramarginal gyrus) and probably with a right upper quadrant visual deficit (lower fibers of left optic radiation in the temporal white matter).
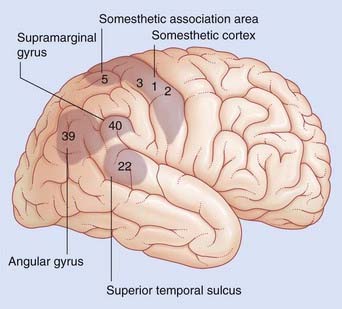
Wernicke’s area (Figure 32.1)
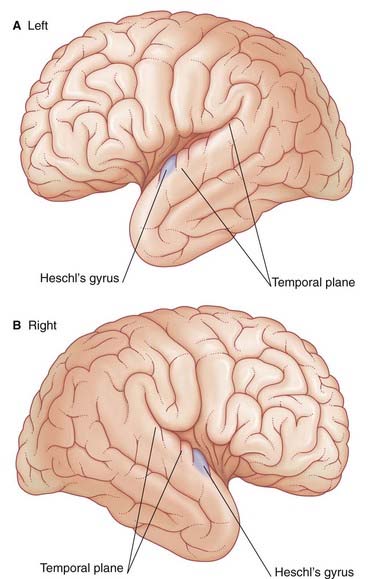
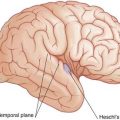
The German neurologist Karl Wernicke made extensive contributions to language processing in the late 19th century. He designated the posterior part of area 22 in the superior temporal gyrus of the left hemisphere as a sensory area concerned with understanding the spoken word. The upper surface of Wernicke’s area is called the temporal plane (Figure 32.2). The volume of cerebral cortex in the temporal plane is larger on the left side in 60% of subjects. The horizontal part of the lateral fissure is longer in consequence – a feature readily identified on MRI scans. Lesions involving Wernicke’s area in adults are associated with receptive aphasia (see Clinical Panel 32.1).
Wernicke’s area is linked to Broca’s area by association fibers of the arcuate fasciculus curving around the posterior end of the lateral fissure within the underlying white matter (Figure 32.1). The two areas are also linked through the insula.
Maldevelopment of the left temporal plane is a significant feature in cases of schizophrenia (Clinical Panel 32.4).
Right hemisphere contribution
During normal conversation there is some increase in blood flow in areas of the right hemisphere matching those of the left. These areas are believed to be concerned with melodic aspects of speech – the cadences, emphases, and nuances, collectively called prosody. Disturbances of the melodic function are called aprosodias (Clinical Panel 32.1).
Angular gyrus
The angular gyrus (area 39) belongs descriptively to the inferior parietal lobule. The left angular gyrus receives a projection from the inferior part of area 19 (the lingual gyrus, shown in Figure 2.6), and itself projects to the temporal plane. It is commonly included as a part of Wernicke’s area.
Listening to spoken words
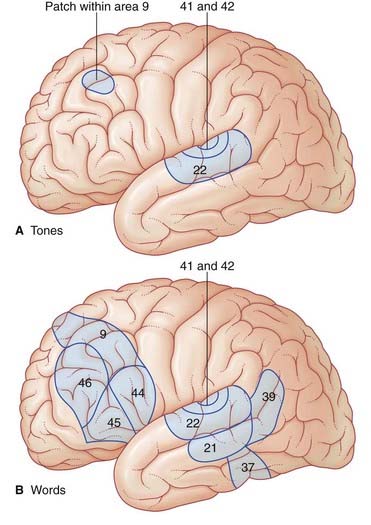
Figure 32.3 contrasts regional increases in blood flow during PET scanning when a volunteer listens to words (‘active listening’) vs random tone sequences (‘passive listening’). As expected, tone sequences activate the primary auditory cortex (bilaterally). Wernicke’s area (left side) also becomes active, probably in screening out this non-verbal material from further processing. Area 9 in the frontal lobe is thought to be part of a supervisory, vigilance system.
When listening to one’s own voice, the areas of the temporal lobe identified above become active. An important function being served here is meta-analysis (post hoc analysis) of speech, whereby ‘slips of the tongue’ can be identified. Speech meta-analysis is singularly lacking in cases of receptive aphasia (Clinical Panel 32.1).
Cognitive Style
Neuroanatomy of reading (Figure 32.4)
Glossary
Reading sequence
Developmental dyslexia is considered in Clinical Panel 32.2.
Clinical Panel 32.2 Developmental dyslexia
Commonly used classroom tests/indications on 5–7 year olds include:
Speech:
Habib M. The neurological basis of developmental dyslexia. Brain. 2000;123:2373-2399.
Leonard CM, Eckert MA, et al. Anatomical risk factors for phonological dyslexia. Cereb Cortex. 2001;11:148-157.
Rumsey JM, Donohue BC, Brady DR, et al. An MRI study of planum temporale asymmetry in males with developmental dyslexia. Arch Neurol. 1997;54:1481-1489.
Temple E. Brain mechanisms in normal and dyslexic readers. Curr Opin Neurobiol. 2002;12:178-183.
Schizophrenia, a psychiatric disorder involving the left hemisphere more than the right, is described in Chapter 34.
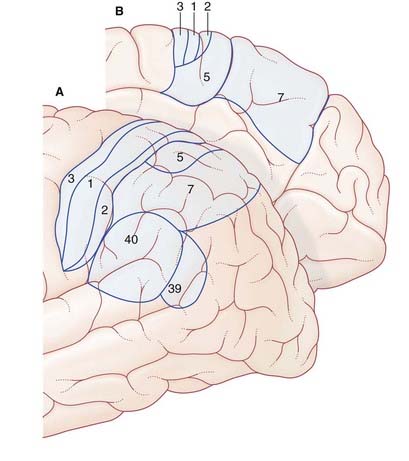
Parietal Lobe (Figure 32.5)
Superior parietal lobule and the body schema
Hemineglect is much more common following a right parietal lobe lesion than a left one. Under normal conditions, however, each parietal lobe exchanges information freely with its partner through the corpus callosum and the left and right hand are equally adept at distinguishing a key from a coin in a coat pocket without the aid of vision (stereognosis, Ch. 29).
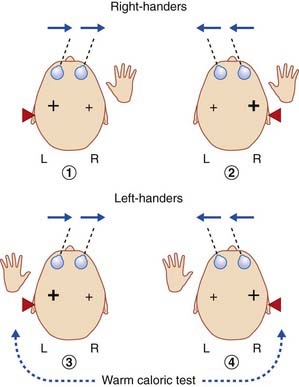
Handedness and balance
In Chapter 18 we noted that the pathway from the vestibular nucleus to the parieto-insular vestibular cortex (PIVC) is mainly ipsilateral. Formal testing of static and dynamic vestibular functions, under fMRI monitoring, has revealed that maximal activation of the PIVC is produced in the minor hemisphere, as illustrated in Figure 32.6. The limited data available indicate a tight linkage between handedness and vestibular cortical activation, to the extent that the ‘preferred’ vestibular hemisphere, in view of its phylogenetic antiquity and early ontogenesis (e.g. presence of postural vestibular reflexes in the newborn), may determine handedness.
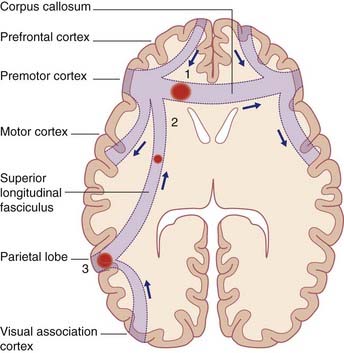
Parietal lobe and movement initiation
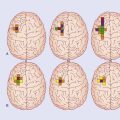
Failure to perform a learned movement on request is called ideomotor apraxia, or limb apraxia. It has been repeatedly observed immediately following vascular lesions at the sites listed and described in Figure 32.7. In that figure, a vascular stroke at site 2 injuring corticospinal fibers descending from the hand area of the motor cortex, would cause clumsiness of movement of both hands, whereas a similar lesion on the right side would only compromise movements of the left hand.
Ideomotor apraxia can be accounted for if the dominant parietal lobe is considered to contain a repertoire of learned movement programs which, on retrieval, elicit appropriate responses by the premotor cortex on one or both sides under directives from the prefrontal cortex. (The basal ganglia would also be involved, as described in Ch. 33.)
Clinical Panel 32.3 provides a brief account of parietal lobe dysfunction.
Clinical Panel 32.3 Parietal lobe dysfunction
Angular gyrus
Lesions of the anteroventral part of the angular gyrus (area 39, Figure 32.3.1) are notably associated with spatial (extrapersonal) hemineglect. The patient tends to ignore the contralateral visual hemispace, even if the visual pathways remain intact, and there is visual extinction (contralaterally) to simultaneous bilateral stimuli, e.g. when the clinician wiggles index fingers in both visual fields simultaneously.
Chokron S, Colliot P, Bartolomeo P, et al. Visual, proprioceptive and tactile performance in left neglect patients. Neuropsychologia. 2002;40:1965-1976.
Golay L, Schneider A, Ptak R. Cortical and subcortical anatomy of chronic spatial neglect following vascular damage. Behav Brain Funct. 2008;4:43-53.
Hanna-Pladdy B, Heilman KM, Foundas AL. Cortical and subcortical contributions to ideomotor apraxia. Brain. 2001;124:2513-2527.
Mort DJ, Malhotra P, Mannan SK, et al. The anatomy of visual neglect. Brain. 2003;126:1986-1987.
Prefrontal Cortex
The medial prefrontal cortex has auditory and verbal associations. The orbitofrontal cortex has been described as the neocortical representative of the limbic system, being richly connected to the amygdala, septal area, and the cortex of the temporal pole – three limbic structures described in Chapter 34.
Aspects of frontal lobe dysfunction are described in Clinical Panel 32.4.
Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212-217.
Buchsbaum BR, Olsen RK, Koch PF, et al. Reading, hearing, and the planum temporale. NeuroImage. 2005;24:444-454.
Dieterich M, Bense S, Lutz S, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13:994-1007.
Di Russo F, Aprile T, Spitoni G, Spinelli D. Impaired visual processing of contralesional stimuli in neglect patients: a visual-evoked potential study. Brain. 2008;131:842-854.
Farne A, Roy AC, Paulignan Y, et al. Visuo-motor control of the ipsilateral hand: evidence from right brain-damaged patients. Neuropsychologia. 2003;41:739-757.
Gross RG, Grossman M. Update on apraxia. Curr Neurol Neurosci Rep. 2008;8:490-496.
Kertesz A, Ferro JM. Lesion size and location in ideomotor apraxia. Brain. 1984;107:921-933.
Liotti M, Gay CT, Fox PT. Functional imaging and language. J Clin Neurophysiol. 1994;11:175-190.
Price CJ. The anatomy of language: contributions from functional imaging. J Anat. 2000;197:335-339.
Reed CL, Caselli RJ, Farah MJ. Tactile agnosia. Brain. 1996;119:875-888.
Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219-224.