Hematolymphoid Tumors of the Gastrointestinal Tract, Hepatobiliary Tract, and Pancreas
Judith A. Ferry
Introduction
The gastrointestinal (GI) tract is the most common primary site of extranodal lymphomas. Between 4% and 20% of all non-Hodgkin lymphomas arise in this organ system. The stomach is most often involved, followed by the ileocecal region, the small intestine, and the colon.1–4 In a small proportion of cases, multiple portions of the GI tract are involved.2,5 Lymphoma also occasionally arises in the hepatobiliary tract, and, rarely, in the pancreas. The types of lymphomas encountered differ to some extent from those encountered in lymph nodes (Tables 31.1 and 31.2). Although lymphomas arising in lymph nodes and in some extranodal sites are largely idiopathic, in the GI tract there are many clues to the pathogenesis of distinct types of lymphoma. Predisposing factors include infection (especially by Helicobacter pylori), celiac disease, inflammatory bowel disease (IBD), and a variety of immunodeficiency syndromes. Thus, the development of different types of lymphoma appears to be related to chronic antigenic stimulation, inadequate immune regulation, or a combination of these factors.
Table 31.1
Lymphomas of the Gastrointestinal Tract and Hepatobiliary Tree, Including Rare Types
| Stomach* | Small Intestine | Colon | Anus | Appendix | Liver | Gallbladder | Pancreas |
| MZL | DLBCL | DLBCL | DLBCL | DLBCL | DLBCL | DLBCL | DLBCL |
| DLBCL | MZL (subtype: IPSID) | MZL | Burkitt | Burkitt | MZL | Burkitt ALCL |
|
| Burkitt | Burkitt | Mantle cell | MZL | MZL | Follicular | MZL | |
| Mantle cell | EATL | Follicular | HSγδTCL HSαβTCL |
||||
| Follicular | Mantle cell | Burkitt | |||||
| PTCL | Follicular | PTCL | |||||
| Hodgkin | PTCL NOS NK/T-cell, nasal type Hodgkin |
Hodgkin |
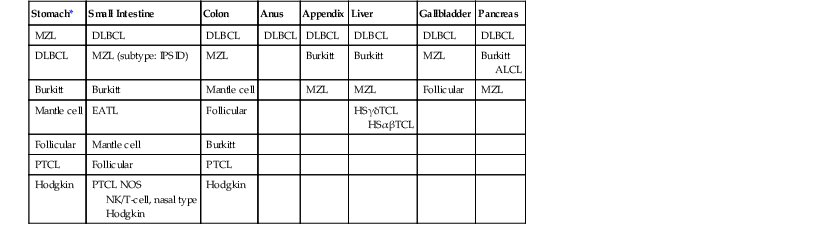
* The types of lymphoma are listed according to frequency, with the most common at the top.
ALCL, Anaplastic large-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; EATL, enteropathy-associated T-cell lymphoma; HSαβTCL, hepatosplenic α/β T-cell lymphoma; HSγδTCL, hepatosplenic γ/δ T-cell lymphoma; IPSID, immunoproliferative small-intestinal disease; MZL, extranodal marginal-zone lymphoma; NK, natural killer; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma.
Table 31.2
Gastrointestinal Lymphomas: Pathologic Features
| Type of Lymphoma | Composition | Usual Immunophenotype | Genetic Features |
| B-Cell Lymphomas | |||
| Extranodal marginal-zone lymphoma, MALT type | Small lymphocytes, marginal zone B cells, plasma cells, reactive follicles, lymphoepithelial lesions | Monotypic sIg+, cIg+/− (IgM > IgG or IgA), CD20+, CD5−, CD10−, Bcl6−, Bcl2+, CD43−/+, cyclin D1− | Immunoglobulin heavy chain clonally rearranged; t(11;18) (API2-MALT1) in some gastric and intestinal cases; trisomy 18 or trisomy 3 in some cases; t(14;18) (IGH@-MALT1) in some hepatic cases |
| DLBCL | Large centrocytes, and centroblasts; immunoblastic and anaplastic large B cells | Monotypic sIg+, CD20+, Bcl6+/−, CD10−/+, CD43+/− | IGH@ clonally rearranged; BCL6 abnormalities are common; t(8;14) (MYC-IGH@) sometimes found; t(14;18) (IGH@-BCL2) uncommon |
| Burkitt lymphoma | Medium-sized atypical lymphoid cells with round nuclei, basophilic cytoplasm, tingible body macrophages | Monotypic sIgM+, CD20+, CD10+, Bcl6+, Bcl2−, Ki67 ≈ 100% | IGH@ clonally rearranged; t(8;14), t(2;8) or (8;22) (MYC); endemic cases and minority of sporadic cases EBV+ |
| Mantle cell lymphoma | Small to medium-sized, slightly irregular cells with scant cytoplasm | Monotypic sIgMD+, CD20+, CD5+, CD10−, CD43+, cyclin D1+ | IGH@ clonally rearranged; t(11;14) (BCL1-IGH@) |
| Follicular lymphoma | Mixture of centrocytes and centroblasts, follicular dendritic cells | Monotypic sIg+, CD20+, CD10+, Bcl6+, Bcl2+, CD5−, CD43−, cyclin D1− | IGH@ clonally rearranged, t(14;18) (IGH@-BCL2) usually found |
| Plasmablastic lymphoma (DLBCL, plasmablastic type) | Plasmablasts, sometimes with more mature plasmacytoid cells, high mitotic rate | CD20−, MUM1+, CD79a+, CD138+, cIg−/+, Ki67 high | EBV+, most cases; MYC rearrangement common |
| T/NK Cell Lymphomas | |||
| EATL type I | Medium-sized and/or large, sometimes bizarre cells, many admixed reactive cells | CD3+, CD4−/CD8− > CD8+, granzyme+, perforin+ | TCR genes clonally rearranged |
| EATL type II | Small to medium-sized cells, few admixed reactive cells | CD3+, CD8+, CD56+, granzyme+, perforin+, TCRγδ± | TCR genes clonally rearranged |
| Extranodal NK/T-cell lymphoma, nasal type | Small, medium-sized, and/or large atypical lymphoid cells, necrosis, vascular damage | cCD3+, CD2+, CD5−, CD56+, granzyme+, perforin+ | TCR genes germline; EBV+ |
| Hepatosplenic T-cell lymphoma | Medium-sized cells with clear cytoplasm in hepatic sinusoids and splenic red pulp | CD2+, CD3+, CD5−/+, CD4−, CD8−/+, TIA-1+ | TCR genes clonally rearranged, isochromosome 7q and trisomy 8 common; EBV− |
| Hodgkin Lymphoma | |||
| Classic Hodgkin lymphoma | Reed-Sternberg cells and variants in a reactive background | CD15+/−, CD30+, CD20−/+, Pax5+, CD3− | EBV usually +, especially in abnormal immune states |
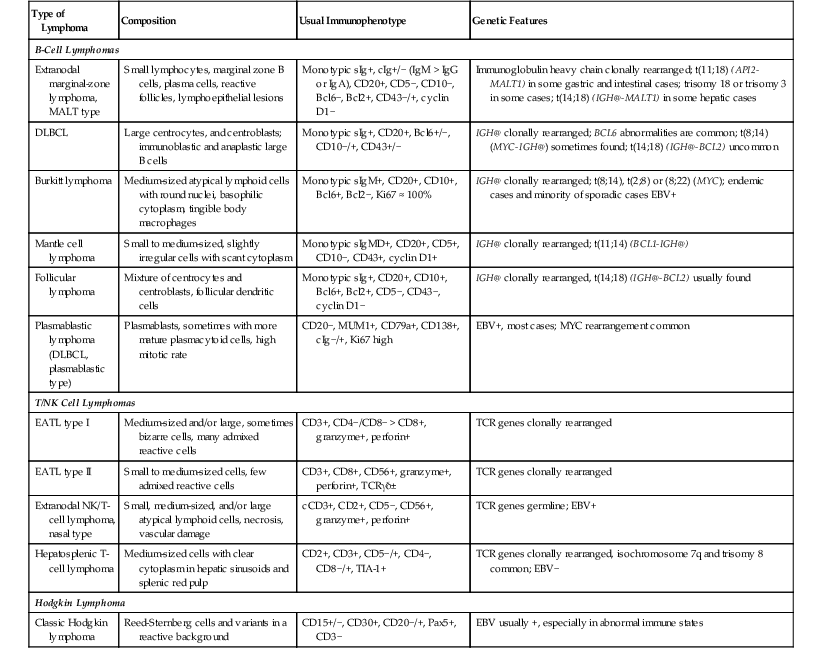
cCD3, Cytoplasmic CD3; cIg, cytoplasmic immunoglobulin; DLBCL, diffuse large B-cell lymphoma; EATL, enteropathy-associated T-cell lymphoma; EBV, Epstein-Barr virus; IGH@, immunoglobulin heavy-chain gene; MALT1, mucosa-associated lymphoid tissue lymphoma translocation gene 1; NK, natural killer; sIg, surface immunoglobulin; TCR, T-cell receptor.
In 55% to 75% of GI lymphomas, the stomach is the primary site. Lymphoma accounts for 1% to 7% of all gastric malignancies.1,2 The most common types in the stomach are diffuse large B-cell lymphoma (DLBCL) and extranodal marginal-zone lymphoma (MZL) of mucosa-associated lymphoid tissue (MALT), which is also known as MALT lymphoma. Some series have more of the former and others more of the latter. Other types of lymphoma are uncommon, but follicular lymphoma, Burkitt lymphoma, mantle cell lymphoma, and peripheral T-cell lymphoma (PTCL) may occasionally arise in the stomach (see Table 31.1). The proportion of gastric T-cell lymphomas appears to be higher in Asian countries than in Western countries, although DLBCL is still more common than T-cell lymphomas.6
In 15% to 35% of GI lymphomas, the small intestine or the ileocecal region is the presenting site.1,2,7,8 Within the intestines, the ileocecal region is the most common site for lymphoma, accounting for almost 40% of cases.3,4 Lymphoma accounts for approximately 25% of small-intestinal neoplasms.1 The proportions of different types of lymphomas vary from one series to another, depending on the age and ethnic background of patients studied and the part of the world in which the study was performed. The most common type in the small intestine is DLBCL, followed by MZL (including immunoproliferative small-intestinal disease), Burkitt lymphoma, various T-cell lymphomas, mantle cell lymphoma, and follicular lymphoma.1,5,8–10 The ileum is more commonly affected than the duodenum or jejunum. Lymphomas that arise in the ileocecal region are almost all diffuse, high-grade B-cell types (DLBCL or Burkitt lymphoma) (see Table 31.1).3,5,11
The large intestine is the primary site in 3% to 20% of GI lymphomas2,7,12,13 and in 21%3 to 35%4 of all intestinal lymphomas. Lymphoma accounts for only approximately 0.5% of malignant neoplasms of this site.1,14 The most common type of lymphoma in the large intestine is DLBCL, followed by MZL, mantle cell lymphoma, and rare cases of follicular lymphoma, Burkitt lymphoma, and PTCL. The cecum is the most common site of involvement in the large intestine, followed by the rectum; other portions of the colon are only rarely affected.1,11,15 Anal lymphomas are very rare; they are usually DLBCLs (see Table 31.1).16
A variety of lymphomas can manifest with multifocal GI involvement, including mantle cell lymphoma, follicular lymphoma, enteropathy-associated T-cell lymphoma (EATL), MZL, and DLBCL.5,11
The most common presenting findings associated with GI lymphomas are abdominal pain, anorexia or weight loss, obstruction, palpable mass, diarrhea, nausea or vomiting, fever, perforation, and bleeding. Intussusception may be seen with bulky lymphomas in the ileocecal region. In a few cases, the lymphoma is discovered as an incidental finding.2–5,7–9,14,17–19
Symptoms distinctive for various types of lymphoma are described in this chapter. The more common types of lymphoma, based on pathologic subclassification, are discussed individually. Lymphoma of the appendix is also discussed separately.
Gastric MALT Lymphoma
Clinical Features
The stomach is the most common site for the development of MZL. Gastric MZL (MALT lymphoma) affects similar numbers of men and women, with a slight male preponderance in some series. Most patients are older adults, with a median age in the sixth or seventh decade. Infrequently, young adults and even adolescents are affected.20–28 Patients have epigastric pain or dyspepsia; nausea and vomiting, bleeding, and weight loss may occur but are unusual.29 The symptoms often suggest gastritis or peptic ulcer disease rather than lymphoma.30
Pathologic Features
On endoscopy or on gross examination of a gastrectomy specimen, this type of lymphoma often consists of erosions, shallow ulcers, mucosal granularity, or thickened mucosal folds; alternatively, it may appear as a diffusely infiltrative, ill-defined lesion. Superficial lesions are more common than large masses. The appearance can mimic gastritis.20,23,29–31 Lymphomas usually involve one portion of the stomach, but the tumor may be multifocal and widespread. 23,30–32 Multifocal, widespread, or ill-defined lymphomas may be associated with positive resection margins in gastrectomy specimens.10,27 A discrete, localized, polypoid tumor10 or an exophytic lesion mimicking carcinoma33 may be found, but this is much less common. Most lymphomas are confined to the mucosa or submucosa. Lymph node involvement is unusual with superficially invasive lymphomas, but it is common when there is invasion into the muscularis propria.27
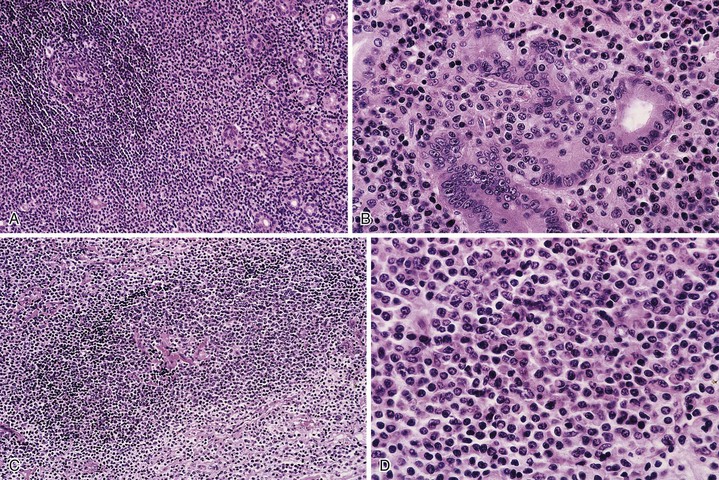
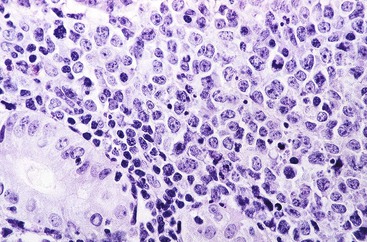
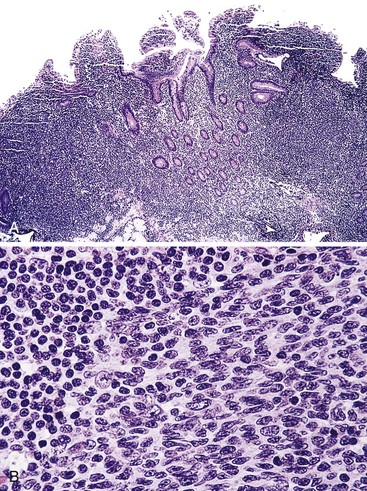
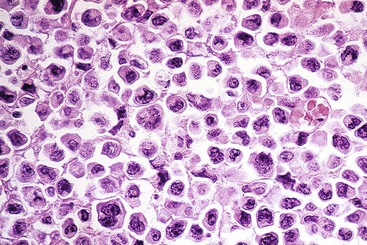
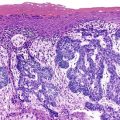
Microscopic examination reveals a diffuse or vaguely nodular infiltrate of marginal-zone cells, small or medium-sized cells with slightly irregular nuclei, and a distinct rim of clear cytoplasm. In approximately one third of cases, there is prominent plasmacytic differentiation in the form of a bandlike infiltrate of plasma cells in the most superficial portion of the lymphoma. The plasma cells may have the appearance of normal, mature plasma cells, or they may have cytoplasm with crystalline inclusions or nuclei with Dutcher bodies. Small clusters of neoplastic cells often infiltrate and disrupt gastric glands to form lymphoepithelial lesions. It is usually possible to identify reactive lymphoid follicles in the lymphoma, often with infiltration and replacement by neoplastic cells (follicular colonization). The cells that colonize the follicles are most commonly marginal-zone–type cells, but plasma cells or large lymphoid cells may also be found. Even when follicles cannot be seen on routinely stained sections, evidence of preexisting follicles can usually be found using antibodies to follicular dendritic cells (FDCs), such as CD21 or CD23. A few large cells can often be found scattered among the marginal-zone cells (Fig. 31.1).10,30
Biopsies of the gastric mucosa also frequently show evidence of infection with H. pylori, detectable on routinely stained sections in some cases, or with special stains such as the thiazine or Steiner stain, or by immunohistochemistry. Alternatively, evidence of H. pylori infection can be detected by use of the rapid urease test, the urea breath test, tissue culture, or serology.20 The likelihood of finding H. pylori is greater with lymphomas that are superficial (i.e., confined to the mucosa or submucosa) and those that are entirely low grade (rather than having areas of progression to a diffuse large B-cell type).34 In addition, the frequency of H. pylori positivity varies among studies. Although some series report that 80% to 100% of patients harbor H. pylori,6,28 in other studies it is only a minority.32
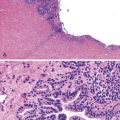
After therapy to eradicate H. pylori, complete histologic regression of lymphoma is characterized by a lamina propria with a distinctive empty appearance, basal aggregates of small lymphocytes, and scattered plasma cells (Fig. 31.2). Partial regression shows areas of empty lamina propria with foci of atypical lymphoid cells or lymphoepithelial lesions, or both.35
Early involvement of lymph nodes often takes the form of bands of marginal-zone cells along sinuses, sometimes with parafollicular aggregates with a marginal-zone pattern, progressing to confluent sheets of marginal-zone cells with or without monocytoid B cells and follicular colonization.30,36 The appearance is indistinguishable from that of nodal MZL (Fig. 31.3).
Immunohistochemistry
Immunophenotyping shows that these lymphomas are positive for CD20 and negative for CD5, CD10, Bcl6, and cyclin D1; they have monotypic surface immunoglobulin (sIg)-positive B cells and plasma cells expressing polytypic or monotypic cytoplasmic immunoglobulin (cIg). The immunoglobulin is most often IgM, but in some cases it is IgA or IgG. The neoplastic cells are Bcl2+, although Bcl2 may be lost in colonized follicles. CD43 is coexpressed in up to one third of cases. An immunostain for cytokeratin can be helpful for highlighting lymphoepithelial lesions. The proliferation fraction, as measured with Ki67, is low, although it is characteristically high in residual reactive germinal centers.27,30,31,37
Molecular Features
Molecular genetic studies show clonally rearranged immunoglobulin heavy- and light-chain genes. Analysis of heavy-chain genes shows somatic hypermutation, consistent with neoplastic cells at a postgerminal center stage of development. The BCL1 (cyclin D1, official symbol CCND1) and BCL2 genes are germline.10,30 Translocation t(11;18)(q21;q21) is the most common cytogenetic abnormality in gastric MZL,38 although its frequency varies widely,38–40 from 5% to 50% of cases in different series. This translocation involves the API2 gene (official symbol BIRC3) on chromosome 11 and the MALT1 gene on chromosome 18; it results in a chimeric transcript that can be detected by reverse transcriptase polymerase chain reaction (RT-PCR). The fusion is believed to confer a survival advantage to the neoplastic cells. This translocation is found almost exclusively in extranodal MZL.41 The t(11;18) is associated with a number of clinical and pathologic correlates. MZLs that fail to regress with anti–H. pylori therapy may harbor the t(11;18), whereas t(11;18) is consistently absent in cases that do regress with such therapy. This translocation tends to be associated with higher-stage disease.42,43 In patients with H. pylori infection, the translocation shows a tendency to be more frequent when the H. pylori strain is positive for the bacterial cytotoxin-associated gene A (cagA). cagA-positive strains are associated with a more prominent neutrophilic infiltrate and therefore with more reactive oxygen species capable of causing DNA damage, predisposing to the acquisition of translocations.44 A higher proportion of H. pylori–negative gastric MZLs is also associated with t(11;18)(q21;q21); this could be related to a different pathogenesis43 or to the tendency of lymphomas with this translocation to manifest at an advanced stage, when the lymphoma is able to grow independently of the presence of the bacteria. The t(11;18) is only very rarely found in cases of large B-cell lymphoma, suggesting that MZLs with this translocation are very unlikely to undergo large-cell transformation. In general, the proportion of European gastric MZLs that are positive for t(11;18) has been higher than in Asia or North America. The reason for this variation is uncertain, but possibilities include host factors related to genetic makeup; the prevalence of different strains of H. pylori; study of resection specimens as opposed to biopsies—because larger, more deeply invasive tumors more often harbor the t(11;18); inclusion of MZLs in some series secondarily involving stomach; and possibly the techniques used (RT-PCR versus fluorescent in situ hybridization [FISH]) (see Table 31.2).
Another cytogenetic abnormality associated with gastric MZL, although less commonly, is t(1;14) (p22;q32), which involves the genes BCL10 and IGH@ (immunoglobulin heavy chain). This change results in deregulation of the BCL10 gene, with resultant loss of its normal pro-apoptotic activity and acquisition of oncogenic potential.42 The t(1;14) is also associated with failure of lymphoma to regress with antibiotics. The t(1;14) is associated with strong nuclear expression of Bcl10 protein; the t(11;18) is associated with moderate nuclear expression of Bcl10. Some cases lacking both translocations demonstrate moderate Bcl10 expression, so the protein expression is not specific for a certain cytogenetic change.44 Rare cases show a t(14;18) involving the genes IGH@ and MALT1.38,40 The three translocations—t(11;18), t(1;14), and t(14;18)—are mutually exclusive. All three are believed to contribute to lymphomagenesis via activation of nuclear factorκB (NFκB).44–48 Trisomy 3 and trisomy 18 are found in a subset of cases.39,40,49
Staging, Treatment, and Outcome
Staging reveals disease confined to the stomach in an estimated 63% to 88% of cases20,26,31,32,50; regional lymph nodes are involved in the remaining cases, and in a few, there is more widespread disease.26,31,50 Patients with widespread disease may have involvement of another MALT site, most commonly the small intestine or colon, but also the lung, kidney, salivary gland, thyroid, ocular adnexa, and others; a few have bone marrow involvement.2,10,31,32,38,51 The lymphoma is indolent and may remain localized for years, even without specific therapy.10
In 1993, Wotherspoon and colleagues described their remarkable observation of regression of MZL after eradication of H. pylori using antibiotics.52 Since then, many other medical centers have reported the same phenomenon, with response rates typically ranging from 60% to 90%.20–22,29,35 The interval to histologic regression can be prolonged, with a range of 1 to 35 months and a median of 3 to 10 months.24,28,31,35 Features associated with failure of regression include invasion beyond the submucosa, spread beyond the stomach, absence of H. pylori, and presence of certain chromosomal abnormalities [t(11;18) and t(1;14)].10,28,31,33,42 A component of DLBCL also decreases the chance of regression. In some cases of DLBCL arising in association with MZL confined to the stomach, complete regression with antibiotic therapy alone has been described 53–57; regression appears more likely to occur when the lymphoma is superficial (i.e., confined to the mucosa or submucosa) on endoscopic ultrasound.53,55–57
A minority of patients experience relapse of lymphoma; in some cases, this is precipitated by H. pylori reinfection.22 In patients who are histologically negative for lymphoma after H. pylori eradication, clonal B cells can be detected by PCR even months to years later.10,21,31,35 Microdissection studies suggest that the clonal cells reside in basal lymphoid aggregates.35 Clonal B cells can be detected by PCR at presentation in endoscopically normal mucosa in a subset of cases; this finding may predict a longer time needed to achieve histologic remission.24
If the lymphoma does not respond, or relapses, after H. pylori eradication, other modalities (e.g., surgery, radiation, chemotherapy) may be used,20,28,31–33,35 and cure can usually be obtained. The 5-year survival rate of patients with gastric B-cell MZL is at least 90%,10,25,26,28,50 and the 10-year survival rate is between 80% and 90%.20,33 Of note, patients with H. pylori infection with or without MALT lymphoma appear to be at increased risk for gastric carcinoma. Follow-up of MALT lymphoma patients reveals development of gastric carcinoma in some cases. The carcinomas may occur in a background of atrophy with intestinal metaplasia, in the same site previously involved by lymphoma.28
Pathogenesis
Most gastric MZLs are believed to arise from a background of gastritis with a component of acquired MALT induced by H. pylori infection. Persistent infection with chronic antigenic stimulation leads to the appearance of a clonal population of B cells. Surprisingly, the B cells do not have specificity for H. pylori; instead, they produce antibodies that may be reactive with a variety of autoantigens. It is the T cells in the infiltrate that have strain-specific reactivity for H. pylori. In the early phase of the disease, the B cells require H. pylori and the T cells to proliferate. Accordingly, in this phase, the lymphoma remains localized and may respond to antibiotic therapy directed against H. pylori. With time, the clonal B cells acquire genetic abnormalities associated with autonomous growth, leading to a histologically low-grade lymphoma that does not regress with H. pylori eradication and that may spread beyond the stomach. Additional genetic abnormalities, such as TP53 inactivation and allelic loss,10 CDKN2A (gene for p16Ink4A) deletion,58 and MYC translocation,30,58,59 may occur and may lead to large-cell transformation.
Important pathogenetic questions remain. MZL develops in a small proportion of individuals with H. pylori gastritis. It is possible that host factors and environmental factors play a role in the pathogenesis of this type of lymphoma. One study suggested that certain polymorphisms in the T-cell regulatory gene CTLA4 (cytotoxic T-lymphocyte antigen 4) may be associated with an increased or decreased risk for MZL.60 Others have described an increased risk of gastric MZL with certain polymorphisms of genes involved in inflammatory response and antioxidative capacity, with those individuals with a stronger inflammatory response to H. pylori and diminished antioxidative capacity more likely to develop gastric MZL.61 Also, in some cases, there is no evidence of H. pylori infection,32 even in patients studied by histology, immunohistochemistry, urease test, serology, or a combination of these methods.43 In such cases, it is not clear whether infection was present previously and resolved at a stage after the lymphoma attained autonomous growth or whether there are other, as yet unrecognized, etiologic agents for gastric MZL. A few H. pylori–negative MZLs (as well as a small proportion of cases of chronic gastritis) may be caused by non–H. pylori species, such as Helicobacter heilmannii. H. heilmannii is detectable on histologic examination as long, thin, spiral bacilli adjacent to the surface epithelium.62,63
Differential Diagnosis
Marginal-Zone Lymphoma versus Gastritis
On routinely stained sections, the presence of an expansile, destructive infiltrate with loss of glands; cytologic atypia of the infiltrating cells (having the appearance of marginal-zone rather than normal small lymphocytes); frequent lymphoepithelial lesions; and Dutcher bodies favors a diagnosis of lymphoma. Immunohistochemical studies are often of assistance. Demonstration of monotypic immunoglobulin light-chain expression confirms a diagnosis of lymphoma. A diffuse infiltrate of B cells and coexpression of CD43 by B cells favor a diagnosis of lymphoma. In selected cases, PCR can be useful to distinguish marked chronic gastritis from early involvement by MZL.64
Marginal-Zone Lymphoma versus Other Low-Grade Lymphomas
In the stomach, MZL is much more common than either follicular lymphoma or mantle cell lymphoma. However, either of these can involve the stomach and mimic MZL. MZL is composed of CD5−, CD10− B cells with relatively abundant clear cytoplasm and may show plasmacytic differentiation and an admixture of a few large cells. Mantle cell lymphoma typically is composed of a monotonous population of small to medium-sized CD5+, cyclin D1+ B cells with scant cytoplasm, without a large-cell or plasmacytic component. However, in a small proportion of mantle cell lymphomas, the neoplastic cells have pale cytoplasm and resemble marginal-zone cells,65 so relying on morphology alone may occasionally be misleading. Follicular lymphoma has a more distinct follicular architecture and is typically composed of CD10+, Bcl6+ B cells with nuclei that are usually more irregular, and cytoplasm that is usually more scant, compared with marginal-zone cells (see Table 31.2).
Transformation to Large B-Cell Lymphoma versus Marginal-Zone Lymphoma with Increased Large Cells
Sheets or confluent clusters of large, transformed cells found outside of follicles in a background of MZL represent large-cell transformation. Most authorities require clusters of at least 20 large cells for large-cell transformation25,59 and report that diffusely scattered large cells representing 5% to 10%,25 or even 20%,50 of the total population are not associated with a worse prognosis as long as clusters of large cells are not seen. Before diagnosing focal large-cell transformation, it is important to exclude the possibility that the large cells are residual reactive germinal center cells or neoplastic large cells colonizing follicles. Immunohistochemical stains to demonstrate a follicular dendritic network can be helpful in resolving these uncertainties. The DLBCLs that arise in association with gastric MZLs are often positive for Bcl6, and immunostaining for Bcl6 may be helpful in highlighting possible areas of large cell transformation.66 However, reactive germinal centers are also positive for Bcl6; stains for FDCs can be performed to investigate this as a possibility.
Poorly Preserved High-Grade Lymphoma versus Marginal-Zone Lymphoma
In a small biopsy with artifactual degenerative change in large B-cell lymphoma or Burkitt lymphoma, there may be cellular shrinkage and distortion leading to a false impression of low-grade lymphoma. The presence of apoptotic debris or mitotic figures suggests a higher-grade tumor. Staining with the proliferation marker Ki67 can be quite helpful in distinguishing low- from high-grade lymphomas. Clinical information, such as the endoscopic appearance of the tumor, can also provide a clue to the correct diagnosis.10
Plasmacytoma versus Marginal-Zone Lymphoma
Convincing cases of GI plasmacytoma are rare. Most reported cases are more likely MZLs with prominent plasmacytic differentiation. In favor of a diagnosis of lymphoma are the presence of a component of B lymphocytes, lymphoepithelial lesions, and IgM+ neoplastic cells (MZLs may express other heavy chains, but IgM expression by a plasma cell neoplasm would be very rare).
Intestinal MALT Lymphoma
Clinical Features
Intestinal MZL is the second most common type of lymphoma to arise in the intestines (after DLBCL); it accounts for approximately 10%3 to 18%4 of all primary intestinal lymphomas. Almost all patients are middle-aged adults or older. Both men and women may be affected.9,40,67 This type of lymphoma may be located in any portion of the small or large intestine, but a disproportionately large number arise in the rectum.3,11,40,67 In most cases, disease is localized to the bowel, with or without regional lymph node involvement.17,30,67 A few patients have serum M components.9
The prognosis of intestinal MZL is favorable but generally less favorable than that of gastric MZL. However, MZL is reported to have a better prognosis than any other type of intestinal lymphoma.3,8,17 In one study, 80% of patients with MZL were alive and well at last follow-up.9 In another study of intestinal lymphoma, patients with low-grade B-cell lymphoma had a 5-year survival rate of 94% and a 10-year survival rate of 82%; most of the lymphomas were MZLs, and this type of lymphoma showed a better outcome than the other low-grade lymphomas.17 In one additional study in which follow-up was available for 45 patients, only 1 patient died of lymphoma.67 The 5-year overall survival rate for intestinal MZL in one large series was 88%.3
Rare patients with intestinal MZL respond to antibiotic therapy,17,68–70 suggesting that H. pylori70 or some other organism71 plays a role in the pathogenesis of at least a subset of these lymphomas.
Pathologic Features
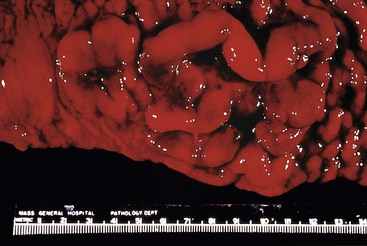
On gross examination, these lymphomas form raised or polypoid masses, sometimes with ulceration,9,17,18,68 or, infrequently, they have the appearance of multiple, small, slightly raised lesions with erosion and erythema,70,71 akin to early gastric MZL. Most are single lesions, but occasionally they are multiple.18,67 In some series, most of the cases are transmurally invasive,9,18 but in others, the lymphomas are only superficially invasive.17,67 In a few cases, the lymphoma has an appearance of multiple lymphomatous polyposis (see Mantle Cell Lymphoma).72
The histologic features of MZL of the intestine are similar to those seen in the stomach.8,9,30 Rarely, there is associated amyloid deposition, without evidence of systemic amyloidosis.72 The immunophenotypic and genetic features are also similar to those of gastric MZL.30 The t(11;18) resulting in the API2-MALT1 fusion is found in 12% to 42% of cases in different series.39,40,67,73 In one series, the presence of t(11;18) was associated with MZLs that were larger and higher stage and more often affected men.67 As for gastric MZL, trisomy 3 and trisomy 18 are relatively common, and t(1;14) (involving the BCL10 and IGH@ genes) and t(14;18) (involving the IGH@ and MALT1 genes) are rare or absent (see Table 31.2).39,40
A substantial number of MZLs involving the intestine are secondary; in these cases, the stomach is the most common primary site. Secondary intestinal MZLs have an even higher proportion of cases with t(11;18), correlating with the tendency of this translocation to be associated with higher-stage disease.40
Immunoproliferative Small-Intestinal Disease
The frequency of small-intestinal lymphoma is higher in the Middle East than in Western countries. The small intestine is the most common primary site for extranodal lymphoma, accounting for 50% of such cases, and for 75% of GI lymphomas in adults in the Middle East. Approximately half of these small-intestinal lymphomas are of the distinctive immunoproliferative small-intestinal disease (IPSID) type. During the past several decades, however, the incidence of IPSID appears to have declined. Although IPSID is found mainly in the Middle East and in countries around the Mediterranean Sea, occasional cases have been described in South Africa, the Far East, Europe, and the United States. Because of its geographic distribution, IPSID has also been called Mediterranean lymphoma.74–76 IPSID is now considered to be a distinct subtype of extranodal MZL. In an association similar to that between H. pylori and gastric MZL, Campylobacter jejuni is hypothesized to be an important factor in the development of IPSID.77,78
Clinical Features
Most patients are young adults (median age, 25 years), ranging from adolescence to middle age. Males and females are equally affected. IPSID tends to be associated with lower socioeconomic status. Presenting symptoms include abdominal pain, malabsorption, diarrhea, and weight loss of months’ to years’ duration. Many patients also have digital clubbing.74,75,79 Obstruction, bleeding, and perforation are uncommon, in contrast to other types of small-intestinal lymphoma.75 At laparotomy, patients may have one or more recognizable intestinal masses, diffuse mural thickening and/or luminal dilation, or normal-appearing bowel. The abnormalities may involve the proximal small intestine, the entire small intestine, or, rarely, just the ileum; the stomach or colon may also be involved.75 Mesenteric lymph nodes are often enlarged.30,75,76,79 In the early phase of the disease, as with gastric MZL, the lymphoma may respond to broad-spectrum antibiotics such as tetracycline. Nonresponders may be treated with chemotherapy. Later in the course of disease, when muscle invasion or transformation to a high-grade lymphoma has occurred, the lymphoma behaves in an aggressive manner.10 For resectable stage Ie or IIe1 disease, the 5-year survival rate is 40% to 47%. For higher-stage, unresectable disease, the 5-year survival rate has been reported to be from 0% to 25%.1 However, patients treated with anthracycline-containing combination chemotherapy have a better outlook, with a complete remission rate of approximately 60%.74,79
In approximately half of cases, there is a highly characteristic laboratory abnormality: the serum contains free α heavy chains without associated light chains. Free α heavy chain may also be found in body fluids, such as intestinal fluid, urine, and saliva. Secreted α heavy chain is more likely to be found in early stages of IPSID. Cases with this abnormality have been called α heavy-chain disease. Closer analysis of this paraprotein reveals that it is a truncated α1 heavy chain lacking the variable region and the first constant region, without associated light chain. The corresponding messenger RNA (mRNA) shows an internal deletion of domains VH and CH1. It has been suggested that IPSID develops in patients with recurrent or persistent intestinal infection, which leads to chronic antigenic stimulation of IgA-secreting lymphoid tissue in this site; the resultant clonal population acquires mutations, leading to the production of α heavy chain with the internal deletion described earlier.79
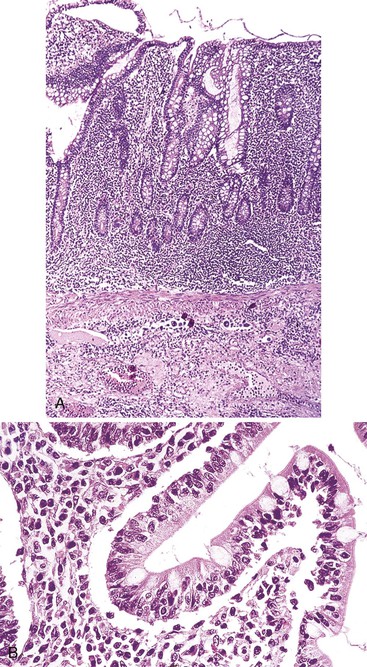
Pathologic Features
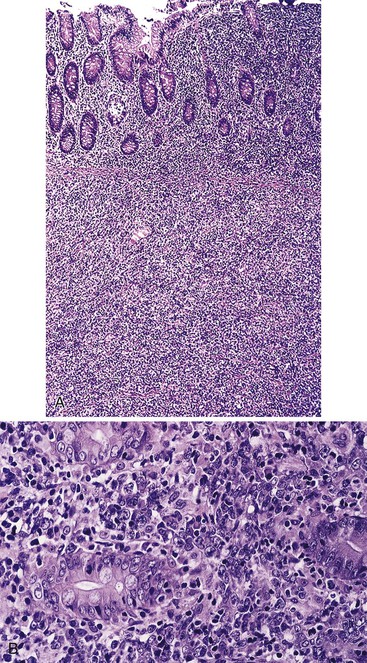
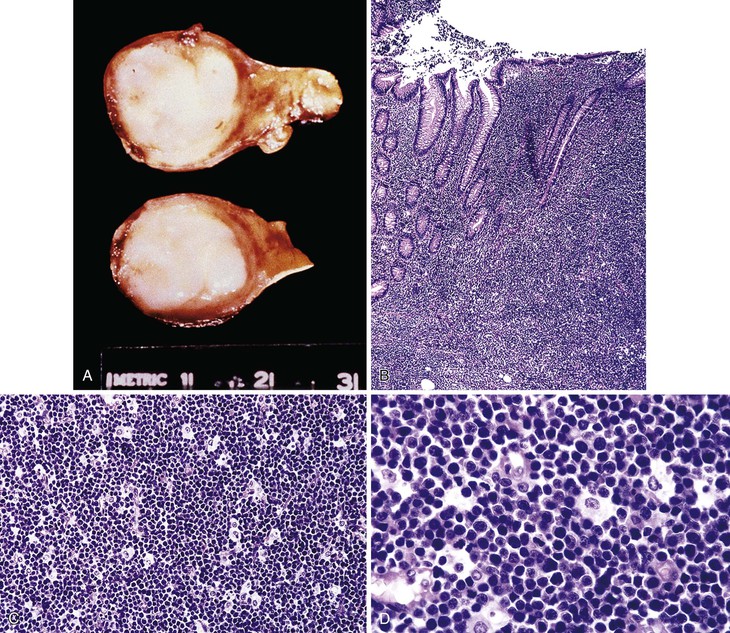
IPSID is typically characterized by a dense, continuous, bandlike mucosal lymphoid or lymphoplasmacytic infiltrate that is uninterrupted along the length of the small intestine.75 The mucosa also shows broad villi, although the intestinal epithelial cells usually remain intact.76,79 The extent of the infiltrate explains the pathogenesis of the malabsorption that affects patients. The histologic features are similar to those of B-cell MZLs in other extranodal sites, except that in IPSID there consistently is marked plasmacytic differentiation. The following staging system has been proposed to subclassify the infiltrate in IPSID.79a
Stage A
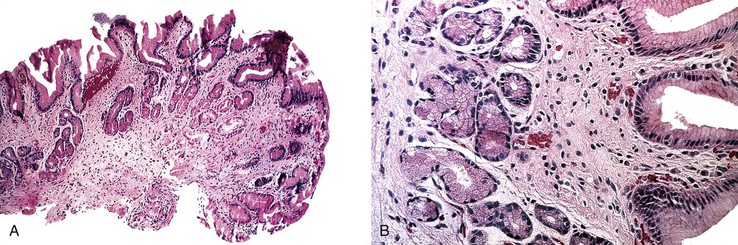
In stage A, lymphoma is confined to the small intestinal mucosa and mesenteric lymph nodes. The infiltrate consists predominantly of plasma cells, with smaller numbers of marginal-zone cells. The B cells may be inconspicuous. An immunostain for CD20 can help with recognition of B cells and also of lymphoepithelial lesions.30 There is variable villous atrophy (Fig. 31.4).
Stage B
In addition to the findings characteristic of stage A, the infiltrate in stage B has areas of nodularity that correspond to reactive lymphoid follicles colonized by neoplastic cells. There are also occasional atypical, immunoblast-like cells. The infiltrate invades beyond the muscularis mucosae. There is total or subtotal villous atrophy.
Stage C
Stage C is characterized by high-grade lymphoma, with formation of one or more large masses. The lymphoma is composed of large cells, sometimes with the appearance of immunoblasts or plasmacytoid immunoblasts. In other cases, the neoplastic cells are pleomorphic and bizarre.
Immunohistochemical analysis usually shows expression of α heavy chain without light chain, correlating with the serum paraprotein. In a minority of cases, monotypic light chain is expressed. Molecular genetic analysis has shown clonal rearrangement of immunoglobulin heavy and light chains, even in early cases responsive to antibiotic therapy. Previously, early phases of IPSID were thought to be inflammatory, but this information indicates that the infiltrate is neoplastic despite response to antibiotics.30,74
Differential Diagnosis
IPSID and celiac sprue are both characterized by lymphoplasmacytic infiltrates and villous atrophy. Patient demographic data should provide a strong clue to the diagnosis, because patients with celiac disease are predominantly of northwestern European descent and improve on a gluten-free diet, in contrast to IPSID patients. Histologic features in favor of a diagnosis of celiac disease include total villous atrophy (in contrast to the villous broadening seen in early-stage IPSID); hyperplastic, elongated crypts; intraepithelial lymphocytosis; and surface epithelial damage.79 High-grade lymphomas may be found in association with either disease, but the lymphoma that complicates celiac disease is a T-cell lymphoma prone to cause multifocal perforation.
It can be difficult to distinguish nonspecific chronic inflammation from early-stage IPSID, particularly on a small biopsy sample. In favor of a diagnosis of IPSID is a dense, predominantly plasmacytic infiltrate that distorts the mucosal architecture in a patient with clinical features compatible with IPSID. Immunohistochemical stains that show plasma cells expressing only α heavy chain help establish the diagnosis.
Gastric Diffuse Large B-Cell Lymphoma
Clinical Features
Gastric DLBCL is mainly a disease of older adults, with a median age in the seventh decade; younger adults are affected only occasionally. There is a slight male preponderance.23,25–27,80 Patients may have a palpable mass on physical examination.10 Some cases arise in association with MZL, consistent with large-cell transformation of the low-grade lymphoma, whereas other large B-cell lymphomas appear to arise de novo; the proportion of cases in the two categories varies among series.2,23,25,26,50,81
Pathologic Features
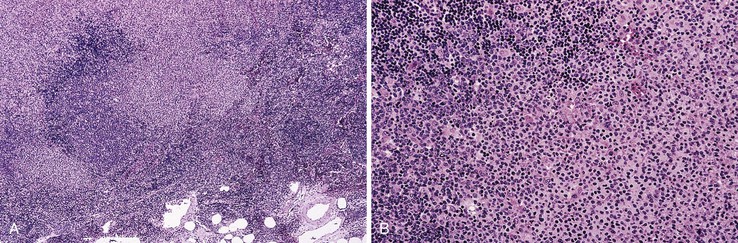
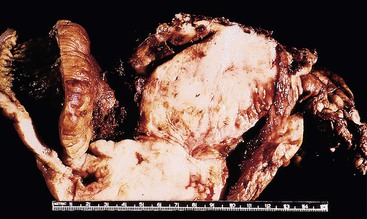
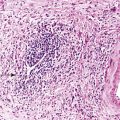
On gross examination, the lymphomas are usually single, but occasionally one may encounter multiple large, ulcerated or exophytic lesions that are usually transmurally invasive and may invade adjacent viscera.10,23 Any portion of the stomach (upper, middle, or lower third) can be involved, and occasionally more than one portion is involved.23 Microscopic examination reveals a diffuse proliferation of large cells with round, oval, irregular, or lobated nuclei, distinct nucleoli, and a narrow but distinct rim of cytoplasm (Fig. 31.5).
Immunophenotyping reveals CD20+ B cells expressing monotypic sIg and occasionally cIg and coexpressing CD43 in approximately half of cases.50 In contrast to MZL, the large-cell lymphomas that arise from them may be Bcl2− and often express Bcl6, although they remain CD10−.26,30 A minority of gastric DLBCLs are CD10+, approximately half are MUM1+, and a variable proportion are Bcl6+.26,81 On immunophenotyping,82 one third or fewer are shown to be of the germinal center B-cell (GCB) type (CD10+ or CD10−, Bcl6+, MUM1−), and the majority are non–germinal center B-cell/activated B-cell (non-GCB) type (either CD10−, Bcl6+, MUM1+ or CD10−, Bcl6−) (see Table 31.2).6,81,83
Most DLBCLs arising in the GI tract would be subclassified as DLBCL, not otherwise specified (NOS) according to the World Health Organization (WHO) tumor classification system. The WHO has defined a number of “diffuse large B-cell lymphoma subtypes” and “other lymphomas of large B cells,” and some of these may involve the GI tract.84 One of the subtypes is Epstein-Barr virus (EBV)-positive DLBCL of the elderly, which is defined as an EBV+ clonal B-cell lymphoproliferation occurring in patients older than 50 years of age with no known immunodeficiency or prior lymphoma.85 The pathogenesis is thought to be related to the deterioration of immunity that occurs over time. This lymphoma can involve a variety of extranodal sites (as well as lymph nodes), but the GI tract is among the more common sites. The composition may be monomorphous, with a predominance of large, atypical lymphoid cells that may have the appearance of immunoblasts or Reed-Sternberg–like cells and variants. In some cases, the composition is more polymorphous, with B cells in a range of stages of maturation, including large atypical cells and also reactive cells such as histiocytes, small lymphocytes, and plasma cells. Necrosis is common. Tumor cells are usually CD20+ and/or CD79a+ and often CD30+ and MUM1/IRF4+, but negative for CD15. By definition, EBV is present in tumor cells.
Among the “other lymphomas of large B cells” is anaplastic lymphoma kinase (ALK)-positive DLBCL; a case of this type of lymphoma arising in the stomach of a 21-year-old man has been reported.86 Also in this category are plasmablastic lymphoma and human herpesvirus 8 (HHV8)-positive large B-cell lymphoma. Both of these rare types of lymphoma may be encountered in the GI tract. Because they occur most often in human immunodeficiency virus (HIV)-infected individuals, they are discussed later (see GI Lymphoproliferative Disorders in Abnormal Immune States).
Molecular Features
Immunoglobulin heavy and light chains are clonally rearranged. Complex cytogenetic abnormalities are common.87 In contrast to lymph nodal DLBCL, BCL2 gene rearrangement is rare and MYC rearrangement is common in gastric DLBCL.88 Abnormalities of the BCL6 gene are more common in gastric than in nodal DLBCL. BCL6 abnormalities include both translocation and somatic hypermutation.81 In one study, gastric lymphomas with germline BCL6 had advanced-stage disease more often and showed a trend toward decreased chance of complete remission and worse survival.89 Another study documented frequent (42% of cases) loss of heterozygosity (LOH) on chromosome 6q in sites of putative tumor suppressor genes, as well as a smaller number of cases with LOH of tumor suppressor genes, including TP53 and APC. Amplification of genomic material in the BCL6 locus, the MLL (mixed-lineage leukemia) gene, and others were also found. There was a significant association between MLL amplification and LOH of TP53.87 Homozygous deletion of CDKN2A, encoding p16Ink4A, has also been described in high-grade gastric lymphoma.
Activation of the NFκB pathway is important in the pathogenesis of lymphomas of a variety of types. Recently, mutations in A20 (official symbol TNFAIP3), in CARD11, and in ABIN1 (TNIP1) and ABIN2 (TNIP2)—A20-binding inhibitors of NFκB that are likely to contribute to activation of the NFκB pathway—have been documented in DLBCLs of the GI tract.90 Mutations of A20 were associated more often with DLBCLs having the non-GCB phenotype (according to the Hans algorithm,82 described earlier) and with significantly worse overall survival and event-free survival.90
Unless a component of low-grade lymphoma is identified, it may not be possible to distinguish between de novo DLBCL and DLBCL arising from histologic progression of MZL (or other low-grade lymphoma). Genetic and cytogenetic features may be helpful. Despite the high frequency of t(11;18) and trisomy 3 in gastric B-cell MZLs, they are uncommon in gastric DLBCL, suggesting that they are not important in transformation of MZL to large B-cell lymphoma.49,58 One report suggests that trisomies (most often involving chromosomes 12 and 18) are more common in large-cell lymphomas that have arisen through transformation of MZL than in de novo large B-cell lymphomas.49
A variety of mechanisms may lead to transformation of MALT lymphoma to DLBCL, including TP53 mutation, alterations in BCL6, and aberrant DNA hypermethylation.66,91 Translocations involving BCL681 and MYC88 may play a role in progression of gastric MZL to DLBCL, and may be involved in the pathogenesis of de novo DLBCL as well. Recently, overexpression of Myc was shown to alter the microRNA signature in gastric lymphomas. In the setting of Myc-repressed microRNAs normally showing tumor suppressive activity, FoxP1 was overexpressed, theoretically promoting lymphomagenesis and large cell transformation.91 Accordingly, MALT lymphomas are typically negative for Myc and FoxP1 by immunohistochemistry; whereas, DLBCLs are often positive.91
Staging and Outcome
In most of the cases,26,50,80 patients have stage I or II disease at presentation; patients with stage II disease usually outnumber those with stage I, and stage IIe1 is more frequent than IIe2.25,26,50 Patients may be treated with surgery, radiation, chemotherapy, or a combination of these modalities. The 5-year survival rate is estimated to be 65%.10 Some studies have documented pathologic features that have an impact on prognosis. In one study, the 5-year survival rate was 92% for large B-cell lymphoma associated with MZL, 89% for CD10+ large B-cell lymphoma, and only 30% for CD10− large B-cell lymphoma without a low-grade component.26 In another study, large B-cell lymphoma associated with a component of MZL or with lymphoepithelial lesions formed by small cells had a 5-year cause-specific survival rate of 84%, compared with 64% for de novo large B-cell lymphoma. Lymphoepithelial lesions formed by large cells were not associated with a favorable prognosis.25 In one study with immunophenotyping to subclassify gastric DLBCL as GCB or non-GCB type, patients with GCB type had a significantly better outcome.81
Stage is also prognostically important. Patients with stage Ie or IIe1 have a better outcome than those with stage IIe2 or higher.26,50 In one study of high-grade gastric lymphoma, four features—age older than 60 years, male sex, high lactate dehydrogenase (LDH) level, and ascites—were each identified as an independent factor associated with poor outcome.80 Survival was worse with an increasing number of these factors.80
Differential Diagnosis
Poorly differentiated carcinoma may be composed of dyshesive-appearing cells that form few or no glands and therefore may be difficult to distinguish from DLBCL. Lymphoid cells may show artifactual vacuolar change and mimic signet ring cells, but mucin stains and immunohistochemical stains are helpful in distinguishing between lymphoma and carcinoma.
Intestinal Diffuse Large B-Cell Lymphoma
Clinical Features
DLBCL is the most common type of intestinal lymphoma, accounting for as much as two thirds of cases.3 Most patients with intestinal DLBCL are middle-aged to older adults. Few cases occur in younger adults or children. There is a slight male preponderance among adults, whereas affected children are almost exclusively male. DLBCL in children is found almost always in the ileocecal area.9,14,15,18,67 In most patients, regional lymph nodes are involved.9,14,67 In most cases, the lymphoma is confined to the bowel with or without regional lymph node involvement; in the remainder, disease is more widespread.67
This type of lymphoma is relatively aggressive but potentially curable.10,15,30,67 In one report, de novo large B-cell lymphoma had a less favorable outcome than large B-cell lymphoma that arose in association with MZL.17
Pathologic Features
On gross examination, the tumors are similar to, or larger than, low-grade lymphomas.17,18 They are elevated or infiltrative, ulcerated lesions that are usually transmurally invasive. A subset show evidence of perforation.8,67 Most are composed of centroblasts, often with an admixture of immunoblasts or multilobated large lymphoid cells; a minority are composed almost exclusively of immunoblasts (Fig. 31.6).8,9
In some cases, a component of MZL is found, which is consistent with large-cell transformation of a low-grade lymphoma. The proportion of cases with an underlying low-grade lymphoma varies greatly in different series, from 10% to more than 50% of large B-cell lymphomas.8,9,17,67 The immunophenotypic features overlap with those found in gastric DLBCL. In contrast to gastric DLBCL, in which most cases have a non-GCB immunophenotype (either CD10−, Bcl6+, MUM1+ or CD10−, Bcl6−) most de novo intestinal DLBCLs have a GCB immunophenotype (CD10+ or CD10−, Bcl6+, MUM1−).83
Intestinal DLBCL is genetically heterogeneous. In one study of 14 cases, 3 had a t(14;18) translocation involving genes IGH@ and BCL2, 1 had a t(8;14) translocation involving IGH@ and MYC, 5 had a BCL6 rearrangement, and 1 had a t(11;18) translocation involving API2 and MALT1.73 Although the t(11;18) characteristic of a subset of MZLs is generally considered to prevent histologic progression to DLBCL, rare cases of intestinal DLBCL harbor t(11;18). This suggests that large-cell transformation of an underlying MZL with this translocation may occur (see Table 31.2).67,73
Differential Diagnosis
The differential diagnosis for intestinal DLBCL is similar to that for gastric DLBCL. In addition, floridly reactive lymphoid tissue in the intestine occasionally raises the question of lymphoma. The bowel normally harbors hyperplastic lymphoid tissue in the terminal ileum (Peyer patches), but hyperplastic lymphoid tissue in other portions of the intestine, if prominent, may suggest lymphoma. This problem may arise with lymphoid polyps, polypoid lesions composed of reactive lymphoid tissue that may be found in the colon. Large collections of hyperplastic lymphoid tissue may develop in the rectum; these have been referred to as rectal tonsils. These are large, discrete nodules of organized lymphoid tissue with reactive follicles, often showing florid hyperplasia, located in the lamina propria or submucosa. Rectal tonsils may be accompanied by overlying cryptitis, mild architectural distortion, intraepithelial lymphocytes (IELs), and lymphoepithelial lesions, typically without crypt obliteration.92 In lymphoid follicular proctitis, a condition characterized by congested, nodular mucosa and often associated with rectal bleeding, reactive lymphoid tissue, including lymphoid follicles, is found in the distal portion of the GI tract.93 Familiarity with these entities and appreciation of the follicular architecture, with large B cells mostly confined to follicles, can help the clinician to avoid overdiagnosis as lymphoma.
Mantle Cell Lymphoma
Clinical Features
Among lymphomas with involvement of the GI tract at presentation, mantle cell lymphoma is uncommon.3,4 Mantle cell lymphoma affects middle-aged or older adults, with a male preponderance. Almost all patients are 50 years of age or older.18,30,72,94,95 Mantle cell lymphoma usually manifests with widespread disease with involvement of lymph nodes and a variety of extranodal sites, and GI involvement is common. Any portion of the GI tract may be affected,17,30 and frequently the lymphoma affects multiple sites (stomach, small intestine, colon, and, on occasion, the esophagus).72,95 Presenting symptoms include abdominal pain, diarrhea, bloody stool, and weight loss. This type of lymphoma often takes the form of innumerable polyps, which is termed multiple lymphomatous polyposis72; this pattern is particularly prevalent in the intestines.95 The ileocecal region tends to contain the largest polyps.17,30 In some cases, particularly when mantle cell lymphoma involves the stomach, it takes the form of a smaller number of protruded, ulcerated, or fold-thickening lesions.95 Mesenteric lymph nodes are usually involved by lymphoma.30 Staging frequently reveals widespread disease away from the GI tract. Although there is usually a positive response to chemotherapy, relapses are common, and survival time is typically only 3 to 5 years. Predisposing factors for the development of mantle cell lymphoma are currently unknown.
Pathologic Features
On gross examination, multiple lymphomatous polyposis has the appearance of multiple, fleshy-white nodules, 0.5 to 2 cm in greatest dimension, involving the mucosa but sometimes with superficial submucosal involvement (Fig. 31.7). Less commonly, mantle cell lymphoma takes the form of a discrete mass or an ulcerated lesion.10
Microscopic examination shows a bandlike infiltrate, or multiple ill-defined nodules, of atypical, monotonous lymphoid cells that are slightly larger and more irregular than normal lymphocytes, with scant cytoplasm and without conspicuous nucleoli. Single epithelioid histiocytes may be scattered among the neoplastic cells. Remnants of reactive follicle centers may be identified in some nodules. The lymphoma tends to displace and obliterate intestinal glands, but formation of true lymphoepithelial lesions is not a feature (Fig. 31.8).30,94
Immunophenotyping typically shows CD20+, CD5+, CD43+, CD10−, CD23−, and nuclear cyclin D1+ B cells expressing monotypic immunoglobulin, IgMD type, with λ being more frequent than κ light chain. With antibodies to FDCs such as CD21, a loose, expanded dendritic network is seen. This lymphoma is associated with t(11;14), a translocation that involves BCL1 and the IGH@ (see Table 31.2). Rare cases of mantle cell lymphoma are negative for cyclin D1. Nuclear expression of Sox11 has been demonstrated in more than 90% of cases of mantle cell lymphoma96; expression of Sox11 can be helpful in identifying the rare cyclin D1–negative cases.
Expression of α4β7, the mucosal homing receptor, by mantle cell lymphoma is highly associated with GI involvement.30,72,97
Differential Diagnosis
On occasion, lymphomas other than mantle cell type have the endoscopic or gross appearance of lymphomatous polyposis. In addition, as stated earlier, not all mantle cell lymphomas take the form of lymphomatous polyposis. Follicular lymphoma,10,17,72 MZL,72 and even T-cell lymphoma98 can have the appearance of lymphomatous polyposis. Mantle cell lymphoma without the classic polyposis appearance can be mistaken for other small B-cell lymphomas. The problem can be resolved readily in most cases with careful study of hematoxylin and eosin (H&E)-stained slides augmented by immunohistochemistry. The distinction is important, because mantle cell lymphomas have a poor outlook compared with other low-grade lymphomas.
A minority of cases of mantle cell lymphoma are characterized by histologic features suggesting more aggressive behavior; these “aggressive variants” include blastoid and pleomorphic variants. In the blastoid variant, neoplastic cells have finely dispersed chromatin, and the mitotic rate is high (usually >20 mitoses/10 high-power fields), so that the appearance is reminiscent of lymphoblastic lymphoma. In the pleomorphic variant, neoplastic cells are larger and more pleomorphic than in the usual mantle cell lymphoma, and, in contrast to typical mantle cell lymphoma, nucleoli are often prominent in at least some of the neoplastic cells. The appearance may mimic DLBCL.65 In the blastoid variant, occurrence in an older adult is a clue that lymphoblastic lymphoma is unlikely, and the immunophenotype (CD20+, CD5+, cyclin D1+, monotypic sIg+) confirms a diagnosis of mantle cell lymphoma and excludes lymphoblastic lymphoma. In the pleomorphic variant, even though there are many large pleomorphic neoplastic cells, there is often an admixture of medium-sized cells more closely resembling those seen in mantle cell lymphoma. Cyclin D1 expression by neoplastic cells confirms a diagnosis of mantle cell lymphoma.
Follicular Lymphoma
Follicular lymphoma of the GI tract is rare, accounting for fewer than 4% of all primary GI lymphomas.13 However, studies suggest that GI follicular lymphoma is a distinct clinicopathologic entity, not just a rare curiosity. In 1997, Misdraji and coworkers published a report of a 56-year-old woman with follicular lymphoma, grade 1 of 3, arising at the ampulla of Vater, associated with jaundice, clinically mimicking pancreatic carcinoma.99 The lymphoma formed a 4-cm white-tan mass arising in the wall of the duodenum, surrounding the common bile duct, and invading the head of the pancreas. Peripancreatic lymph nodes showed partial nodal involvement by follicular lymphoma. The patient was treated with radiation and was alive and well 5 months postoperatively. Subsequently, a study from Japan analyzed a total of 222 GI lymphomas, among which there were 13 duodenal lymphomas and 8 follicular lymphomas. Five of the eight follicular lymphomas arose in the duodenum, all in the second portion, in the vicinity of the ampulla of Vater. The patients were all women, aged 37 to 66 years (median, 52 years), with follicular lymphoma, grade 1 of 3, producing small polypoid masses and protrusions or mucosal irregularity. Two patients had partial involvement of regional lymph nodes by follicular lymphoma. All patients were alive and well 2 to 50 months after diagnosis.13
More recently, several studies have yielded similar findings: affected patients are mostly middle-aged adults with a mean or median age in the 50s.100–102 There is a female preponderance in some series,100,101,103 whereas in others men and women are equally affected.104,105 Precise evaluation of the distribution of disease is evolving with the availability of techniques allowing more complete evaluation of the small bowel, including double-balloon enteroscopy and capsule endoscopy. However, the small intestine is more often involved than other portions of the GI tract. Within the small bowel, the duodenum, usually the second portion, is the site most often involved in most series.100,102,103,105 Duodenal involvement is frequently accompanied by jejunal or ileal involvement.105 The stomach and colorectum are affected less often. Esophageal involvement is rare.104 Three percent of gastric B-cell lymphomas were follicular lymphomas in one large series.23 Patients are seen with a variety of symptoms, but abdominal pain is the most common. Others have mild or vague GI symptoms. A few have diarrhea, nausea, vomiting, or bleeding. In a minority of patients, mostly with jejunal or ileal involvement, the presenting symptom is small bowel obstruction. Bleeding is more common with colorectal lesions. In centers with active screening for GI cancers, it is common for patients to be asymptomatic at the time of diagnosis.100,104,105 Elevated LDH is distinctly unusual, and the Follicular Lymphoma International Prognostic Index (FLIPI) score is typically low.105
On endoscopy, nodularity of the mucosa is the most common finding, sometimes with the picture of multiple lymphomatous polyposis, which may involve the entire GI tract.72,100,104 The nodules are typically 1 to 2 mm in diameter, white or yellow-white, and scattered or confluent. The lymphoma may also take the form of a large, discrete, polypoid or ulcerated mass, or there may be small numbers of polyps.100,104 A macroscopic appearance with multiple small nodules is more common in follicular lymphomas that involve the duodenal second portion.105 The finding of single lesions, or of small numbers of lesions, may be more common in the stomach104 and colorectum.100 Even in cases with a single dominant mass, multiple smaller nodules of lymphoma are also often present.104 The lymphomas may be confined to the bowel wall, or they may show regional nodal involvement. More distant spread is uncommon, although cases with widespread disease outside the GI tract have been excluded from most series. In one additional case, duodenal follicular lymphoma developed in a 53-year-old man known to have the hereditary nonpolyposis colorectal cancer syndrome, raising intriguing possibilities about the pathogenesis of this disorder.106
The lymphomas consist of follicles, usually lacking mantles, that are composed of a monotonous population of centrocytes with few to scattered centroblasts distorting the normal glandular architecture. Very often, there are increased numbers of lymphoid cells outside the follicles, including in the stroma of small-intestinal villi. Lymphoepithelial lesions are not characteristic. Most follicular lymphomas are low grade (grade 1 or 2 of 3), with far fewer being grade 3.100,104
Follicular lymphomas typically have an immunophenotype similar to that found in nodal follicular lymphomas (CD20+, CD10+, Bcl2+, Bcl6+) (Fig. 31.9).13,100–104,107 Often, some of the lymphoid cells outside follicles are B cells with a similar immunophenotype, although CD10 and Bcl6 may be more dimly expressed. Molecular and cytogenetic studies show clonal immunoglobulin heavy- and light-chain genes and BCL2 rearrangement,106,107 although rare cases negative for Bcl2 protein and for BCL2 rearrangement have been reported.72 The pathologic features of GI follicular lymphoma are therefore similar to those of lymph nodal follicular lymphoma. In contrast to follicular lymphoma arising in lymph nodes, however, expression of α4β7 integrin, a mucosal homing receptor, by GI follicular lymphomas has been described.108 Other investigators have noted ongoing somatic hypermutation in the absence of activation-induced cytidine deaminase expression, hollow rather than intact follicular dendritic meshworks, and restricted use of variable regions of the immunoglobulin heavy chain gene; these features are reminiscent of extranodal MZL and suggest response to antigen is important in the pathogenesis of these lymphomas.109
Treatment has varied widely, and the best therapy has yet to be determined. Patients usually do well, although some have persistent lymphoma, and some experience relapse. Relapses may involve the GI tract, lymph nodes, or other sites. Rare instances of response to antibiotics have been reported,104 although antibiotics are ineffective in general.100 A superior progression-free survival has been associated with female gender, lack of symptoms at diagnosis, and involvement of the second portion of the duodenum.105 Large cell transformation has been described. Death from lymphoma is very uncommon.72,100–103,105
Although GI follicular lymphoma is rare, it has distinctive features: It tends to affect women more often than men and involves the duodenum more often than other parts of the GI tract. Its pathologic features are similar to those of low-grade nodal follicular lymphoma, although with subtle differences that may set it apart from follicular lymphoma arising in lymph nodes.
Burkitt Lymphoma
Clinical Features
Burkitt lymphoma is a highly aggressive, rapidly growing B-cell lymphoma with distinctive pathologic features, including the presence of a translocation involving MYC.110 In the WHO classification of tumors of the hematopoietic and lymphoid tissues,110 three clinical variants of Burkitt lymphoma are described: endemic, sporadic, and immunodeficiency-associated. Endemic Burkitt lymphoma occurs mainly in young children in sub-Saharan Africa. A complex interplay of environmental and host-related factors—climate, malaria, EBV infection, age, and developmental stage of the patient—appear to play a role in the development of this subset of Burkitt lymphoma. Most patients with immunodeficiency-associated Burkitt lymphoma are HIV+, and a few have an underlying iatrogenic111 or congenital immunodeficiency.112 Patients with sporadic Burkitt lymphoma are those who are not immunodeficient but do not fit the epidemiology for endemic Burkitt lymphoma. Sporadic Burkitt lymphoma is rare, although it accounts for 30% to 50% of all cases among children in whom lymphoma develops.110
Burkitt lymphoma accounts for approximately 5% of all intestinal lymphomas.3 Involvement of the ileocecal region is the most common manifestation of sporadic Burkitt lymphoma. Ileocecal disease is occasionally seen with endemic or immunodeficiency-associated Burkitt lymphoma, although presentation with disease outside the GI tract is more common. Infrequently, sites in the GI tract other than the ileocecal area, including the stomach and more distal portions of the colon, are involved.8,15,23 Only 1% of gastric B-cell lymphomas were Burkitt lymphomas in one large series.23 GI Burkitt lymphoma affects children and young adults, with a marked male preponderance.8,15 In some cases, staging reveals disease beyond the GI tract. Immunocompetent patients with Burkitt lymphoma who are treated with aggressive, high-intensity, short-duration chemotherapy have an excellent prognosis.113
Pathologic Features
On gross inspection, the GI tumors are usually bulky exophytic lesions, sometimes with ulceration (Figs. 31.10 and 31.11, A).17 Microscopic examination typically reveals a diffuse infiltrate of densely packed, uniform, medium-sized cells with round nuclei, granular chromatin, three to four small nucleoli, and a distinct rim of deeply basophilic cytoplasm with minimal to absent intervening stromal elements. There are typically numerous tingible body macrophages that produce a “starry sky” pattern. The mitotic rate is usually very high. Occasionally, the nuclei of neoplastic cells show greater pleomorphism and the nucleoli are fewer in number but tend to be more prominent (see Fig. 31.11, B through D). If the immunophenotype and genetic features in such cases are typical of Burkitt lymphoma, they are still acceptable as Burkitt lymphoma. In some instances, neoplastic cells have plasmacytoid differentiation, with eccentric basophilic cytoplasm and a single central nucleolus. Nuclei may be somewhat pleomorphic. This variant is found most often in immunodeficient patients.
Immunophenotyping reveals monotypic IgM+, CD20+, CD10+, Bcl6+, CD5−, Bcl2−, TdT− B cells with a proliferation fraction of almost 100%. Cytogenetic analysis reveals t(8;14), t(2;8), or t(8;22), corresponding to translocations involving the MYC gene and either the IGH@ or the κ or λ light-chain gene. Translocations involving BCL2 or BCL6 are absent. Other than the translocation involving MYC, cytogenetic abnormalities are few or absent. EBV is found in approximately one third of sporadic Burkitt lymphomas and is typically present in endemic cases (see Table 31.2).
Differential Diagnosis
The differential diagnosis of Burkitt lymphoma mainly includes other high-grade B-cell lymphomas, such as DLBCL and the so-called “B-cell lymphomas” unclassifiable, which have features intermediate between those of DLBCL and Burkitt lymphoma.114 Careful attention to histologic and immunophenotypic features, supplemented by cytogenetic analysis, help one to avoid misdiagnosis. Some high-grade lymphomas that resemble Burkitt lymphoma also harbor a translocation involving BCL2; these are designated “double-hit” lymphomas. They are associated with a very poor prognosis. A clue to the diagnosis is that, in contrast to Burkitt lymphoma, double-hit lymphomas usually express Bcl2 protein.115 Double-hit lymphomas account for a subset of the B-cell lymphoma unclassifiable type.114
On a small biopsy, a floridly hyperplastic germinal center could mimic Burkitt lymphoma. As in Burkitt lymphoma, reactive germinal center cells are CD20+, CD10+, Bcl6+, Bcl2−, with Ki67+ in almost 100% of cells. A lymphoid follicle should be associated with a follicular dendritic meshwork, so immunostains for CD21 or CD23 may be helpful; such meshworks are typically absent in Burkitt lymphoma. Clinical information regarding the presence or absence of a large mass is helpful as well.
Enteropathy-Associated T-Cell Lymphoma
An association between malabsorption and intestinal lymphoma has been noted since 1937. Analysis of lymphomas that arise in association with celiac disease suggested that they were a distinctive type of malignant histiocytosis, although later studies showed that the neoplastic cells were T cells consistent with an unusual type of PTCL,116,117 termed enteropathy-associated T-cell lymphoma (EATL).118 These have also been referred to as enteropathy-type T-cell lymphoma119 and intestinal T-cell lymphoma.120 EATL is the most common type of T-cell lymphoma to arise in the intestine, although it is still an uncommon neoplasm. It occurs at a rate of approximately 0.10 to 0.14 per 100,000 persons per year, with some geographic variation.121,122 In a region in which celiac disease is frequent, it accounts for approximately 1.2% of all non-Hodgkin lymphomas.121 It accounts for approximately 5.4% of all PTCL or natural killer (NK)-cell lymphomas, although with substantial geographic variation: EATL represents 9.1% of this group in Europe, 5.8% in North America, and only 1.9% in Asia.123 Although an increased risk for lymphoma among celiac disease patients is greatest for primary GI lymphomas of T lineage, a large population-based study revealed an increased risk for B-cell lymphoma, and for lymphoma arising outside of the GI tract as well.124
According to the WHO classification, EATL is an intestinal T-cell lymphoma derived from the intestinal intraepithelial T cell; it is usually composed of large lymphoid cells, often with an inflammatory background, and often with enteropathic changes in the adjacent mucosa (EATL type I). Less commonly, the lymphoma is composed of monomorphic medium-sized cells (EATL type II).118 EATL type I is typically associated with celiac disease, whereas type II is usually not associated with celiac disease and is of unknown etiology. A case of intestinal T-cell lymphoma with some features of EATL arising in a patient with autoimmune enteropathy has been described125; the full complement of risk factors for the development of GI T-cell lymphoma remains to be defined.
Type I
Clinical Features
EATL type I (sometimes previously referred to as type A) occurs in adults within a wide age range (20 to 80 years, with a mean or median age in the 50s or 60s); there is a slight male preponderance. 121–123,126–128 The development of this type of lymphoma is closely linked to celiac disease. It is more prevalent among individuals of European descent and, in particular, among those from the United Kingdom, especially Ireland and Wales.127 EATL type I accounts for approximately 80% of EATL in Europeans but is rare to absent among Asians.123,129 Patients often have a history of celiac disease, which may be of long or short duration.127,130 Strict adherence to a gluten-free diet reportedly diminishes, but does not eliminate, the risk for lymphoma, whereas poor compliance with a gluten-free diet is associated with an increased risk.130,131 In one large study, EATL developed in approximately 0.5% of 1757 individuals with celiac disease who were monitored for a mean of 18 years after diagnosis.130 A subset of patients with EATL have no prior history of celiac disease; in some of these cases, there is histologic evidence of enteropathy, suggesting that the patients had subclinical celiac disease. Even if enteropathy is not found, the presence of antibodies to gliadin or endomysium or of the human leukocyte antigen (HLA) genotype characteristic of celiac disease (HLA DQA1*0501, DQB1*0201) may be found.132
Patients with EATL have abdominal pain, weight loss, diarrhea, vomiting, symptoms related to perforation or obstruction, fever, night sweats, or a combination of these findings. Among patients with a history of celiac disease, symptoms often result from an apparent worsening of the disease, with loss of response to a gluten-free diet.8,121,122,127 Only a small minority have a palpable mass on initial physical examination, and peripheral lymphadenopathy is very uncommon.127 Rarely, patients have symptoms related to distant spread of disease.133 Lymphoma is often confined to the small intestine at the time of presentation.122 In almost all cases, the lymphoma affects the jejunum (more frequently), the ileum, or both. The stomach and the colon are uncommonly involved. Mesenteric lymph nodes may be involved by lymphoma. Staging sometimes reveals spread to the liver and, rarely, to the bone marrow.121,127 In most cases, lymphoma is confined to the abdomen at the time of presentation.127 In one series of patients with EATL, the Ann Arbor stage was I in 19% of cases, II in 58%, and IV in 23%.127 Similar results were found in another series: 75% of patients had stage I or II disease.128
Among intestinal lymphomas, EATL has the worst prognosis,8 with the possible exception of extranodal NK/T-cell lymphoma, nasal type (discussed later). Treatment is complicated by the severe malnutrition that characterizes many of these patients. Only approximately half of patients complete their planned chemotherapeutic regimens because of toxicity or disease progression.121,122,127,128 Chemotherapy may be complicated by perforation, GI bleeding, or sepsis.127 Most patients (84% in one series)127 die of lymphoma or of complications of therapy.127,130 Median survival time ranges from 3 to 10 months.121,123,128 However, a few patients treated with chemotherapy achieve remission and become long-term survivors, suggesting that chemotherapy is effective in a small subset of cases.127,128
Overall, patients treated with surgery have a very poor prognosis. The addition of anthracycline-based chemotherapy results in only a slightly improved prognosis. In an attempt to improve outcomes, newer agents or high-dose chemotherapy regimens followed by stem cell transplantation have been tested. In some studies, these more aggressive efforts have not proved to be efficacious,134 but a few studies have shown improved outcomes with 5-year survival rates of 50 to 60%.121,122 The perforation associated with intestinal involvement is the most common cause of death, but in some cases, dissemination of disease to lymph nodes and a wide variety of extranodal sites (liver, spleen, brain, heart, bone marrow, lungs, kidney, and thyroid) and toxicity resulting from therapy contribute to mortality.117,127
EATL in a patient with no history of refractory celiac disease (RCD) is referred to as de novo EATL, and when there is such a history, it is designated secondary EATL. Patients with secondary EATL have had a slightly worse outcome than those with de novo EATL in some series.122
Pathologic Features
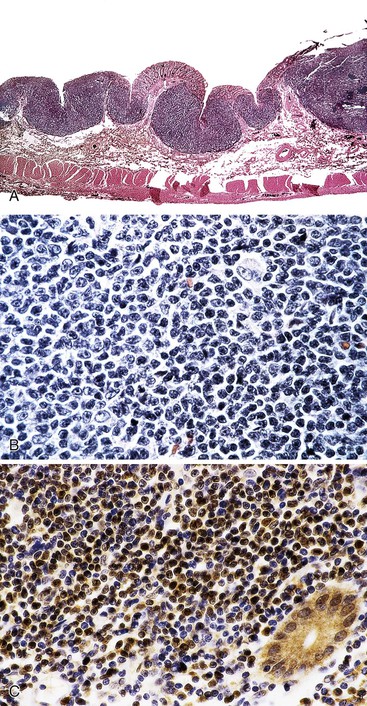
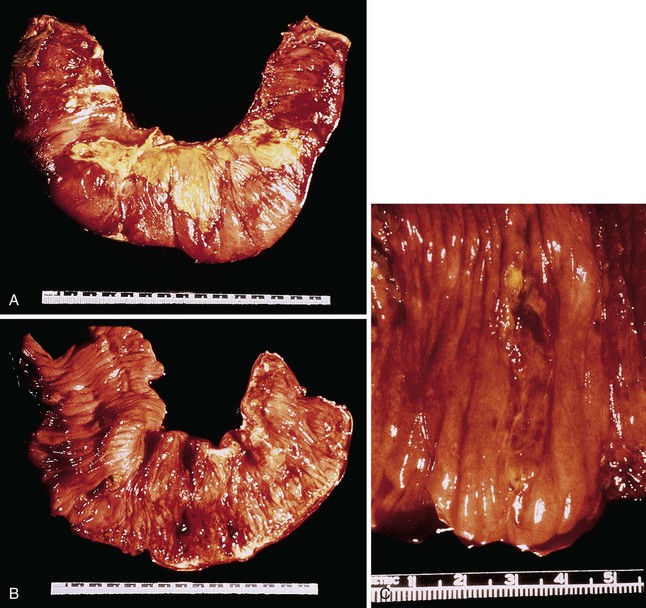
EATL type I most commonly involves the jejunum or the ileum. Presentation with disease outside the small intestine is rare. Lesions may be single or multiple. They take the form of plaques, nodules, or strictures, with circumferential ulceration and often with perforation. Large masses are less common (Fig. 31.12).8,118,127
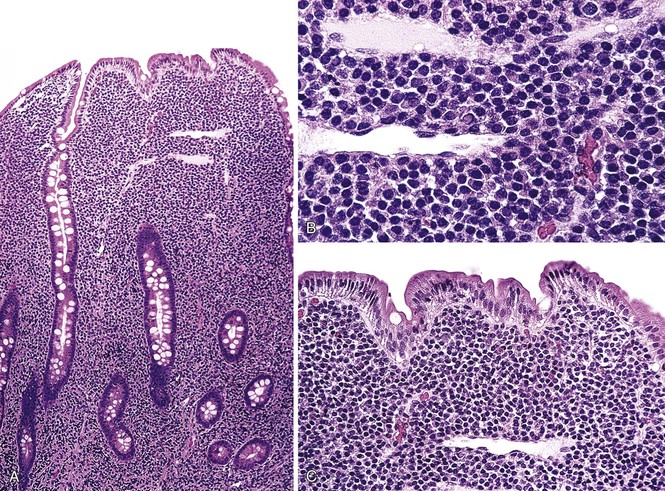
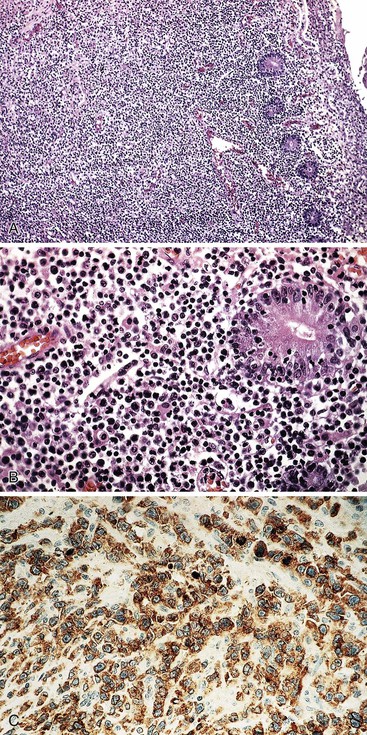
Microscopic examination reveals a dense, diffuse infiltrate of atypical lymphoid cells associated with ulceration and a variable admixture of inflammatory cells. The neoplastic population usually consists of medium-sized or large, atypical lymphoid cells, or a mixture of both, that may be monotonous or pleomorphic, with round or irregular nuclei, prominent nucleoli, and a scant to moderate quantity of pale cytoplasm. Tumor cells may occasionally have the appearance of immunoblasts or may be large and bizarre, with an anaplastic appearance. There is often an admixture of histiocytes, eosinophils, small lymphocytes, and plasma cells.79,117,118,123,127 Occasionally, eosinophils are numerous; in such cases, there may be peripheral eosinophilia.132 Careful examination commonly reveals intravascular clusters of tumor cells. Changes of celiac disease are often seen in the mucosa away from the lymphoma, including increased numbers of IELs, villous atrophy, crypt hyperplasia, and a lymphoplasmacytic infiltrate in the lamina propria (Figs. 31.13 and 31.14).79,117,123,127
Immunohistochemical Features
Immunohistochemistry shows that neoplastic cells usually express leukocyte common antigen (CD45), cytoplasmic CD3, and CD7, but they often lack CD2, usually lack CD5, and consistently lack CD1a, TdT, and CD57. CD8 is expressed in some cases, as noted earlier, and in rare cases CD4 expression is described, but usually CD4 and CD8 are both absent. The α/β T-cell receptor (TCR) is expressed in some cases, indicating origin from α/β T cells, and most cases are considered to be of α/β origin. CD30 is expressed by some lymphomas with anaplastic morphology, but epithelial membrane antigen (EMA) is expressed much less often; Alk1 should not be expressed. The neoplastic cells have a cytotoxic phenotype: They express cytotoxic granule proteins (T cell–restricted intracellular antigen 1 [TIA-1], granzyme B, and perforin).117,118,126 The cytotoxic nature of the neoplastic cells, and of the intraepithelial cells from which they arise, could be responsible for tissue damage, including the villous atrophy, ulceration, and necrosis seen with celiac disease and lymphoma. In most cases, CD103 (human mucosal lymphocyte antigen 1 [HML-1]), is expressed by intestinal IELs and by a subset of lamina propria lymphocytes. Among lymphomas, this antigen is characteristically expressed only in EATL and hairy cell leukemia.135
Type II
Clinical Features
EATL type II (previously called type B) has clinical and pathologic features that distinguish it from EATL type I (also called classic type).128 In contrast to type I, it does not appear to be associated with celiac disease, nor with an increased frequency of HLA types associated with celiac disease.126 EATL type II accounts for approximately 20% of cases of EATL among whites.123,128,129 However, in Asian populations in which celiac disease is uncommon, EATL is reported to be predominantly136,137 or exclusively129,138 type II. Patients are middle-aged and older adults with a median age in the 50s or 60s; there is a male preponderance, with a male-to-female ratio of approximately 3 : 1.129,136,137 Patients with EATL type II have abdominal pain without a prior history of malabsorption, and perforation is common.129,137–139 The lymphoma often involves the small bowel, most commonly the jejunum. There may be concurrent involvement of the colon or stomach. In contrast to EATL type I, some cases of EATL type II are reported to involve the colon without small-intestinal involvement.123,137,140 The lymphoma is usually localized (stage I or II), but occasionally it may involve other intraabdominal structures, omentum, mesentery, and, less often, more distant sites such as bone marrow, inguinal lymph nodes, and brain.129,137–139 The clinical course and response to therapy are similar to those of EATL type I, and the prognosis is equally poor.129,136–138
Pathologic Features
On gross examination, lymphomas may be unifocal or multifocal. They take the form of diffusely infiltrating lesions (or, less often, protruded lesions), usually accompanied by ulceration and often by perforation. Microscopic examination reveals a diffuse, often transmural infiltrate of relatively monotonous small or medium-sized lymphoid cells with dark to stippled chromatin, small to absent nucleoli, and scant to moderate quantity of pale cytoplasm without a conspicuous component of admixed reactive cells such as histiocytes and eosinophils and without conspicuous fibrosis. Most lymphomas show surface ulceration, but away from sites of ulceration and perforation, necrosis is typically absent. At the periphery of the lymphoma, tumor cells often involve the mucosa, with less involvement of deeper portions of the wall of the bowel. Examination of mucosa away from the lymphoma usually shows a marked increase in IELs. Villous blunting and crypt hyperplasia are often absent; if present, they are usually mild. The areas with increased IELs may be adjacent to or at a distance from the lymphoma (Fig. 31.15).129,136,137,140
The most common immunophenotype of the neoplastic cells is CD3+, CD5−, CD4−, CD8+, CD56+, CD30−, and TIA-1+, although there is sometimes modest deviation with respect to one or more of these classic markers.126,129,137,139 In a few cases, aberrant coexpression of CD20 has been reported, although other B-cell markers are not expressed.129,138,141 Most cases express TCRγδ and therefore appear to be of γ/δ T-cell origin, although betaF1+ cases of α/β T-cell origin are also described.129,137 EBV is typically absent.129,138 The immunophenotype of the IELs is similar or identical to that of the neoplastic cells in the lymphoma.129 When the immunophenotype of the IELs deviates from that of the lymphoma, the most common differences involve expression of CD8 or CD56.129,138
Compared with EATL type I, EATL type II is composed of a more monomorphic population of smaller cells. EATL type II more often expresses CD8 and CD56 and less often expresses CD30; has fewer admixed inflammatory cells, less necrosis, and less fibrosis; and less often has typical changes of celiac disease (i.e., prominent villous blunting, crypt hyperplasia) in the surrounding mucosa (Table 31.3, and see Table 31.2).128,129,142 EATL type II is also more often of γ/δ T-cell origin when compared with EATL type I.129
Table 31.3
EATL and Associated Conditions
| Histology | Immunophenotype | T-Cell Clonality | Prognosis | |
| Celiac disease (untreated) | Increased IELs, villous atrophy, crypt hyperplasia, lymphoplasmacytic infiltrate in lamina propria | IELs: predominance of CD3+, CD8+, CD103+, TIA-1+ T cells; rare CD4+ cells; rare CD56+ cells; few CD4−/CD8− γ/δ cells | Polyclonal | Good, with proper diet |
| Refractory sprue | As for celiac disease, sometimes with histologic changes of ulcerative jejunitis | IELs: Predominance of CD3+, CD4−/CD8−, TIA-1+ T cells Lamina propria: mixture of CD4+ and CD8+ T cells |
Almost always monoclonal | Moderately poor; patients may die of malnutrition or lymphoma may develop with the same clonal rearrangement as prior refractory sprue |
| Ulcerative jejunitis | One or more mucosal ulcers with many inflammatory cells | IELs: Predominance of CD3+, CD4−/CD8−, TIA-1+ T cells Ulcer base: mixture of CD4+ and CD8+ T cells and other inflammatory cells |
Monoclonal | Moderately poor; patients may die of malnutrition or perforation or lymphoma may develop with the same clonal rearrangement as prior ulcerative jejunitis |
| EATL type I | Lymphoma: medium-sized and large atypical cells or anaplastic large cells | Lymphoma: CD3+, CD4−, CD8−, CD103+/−, TIA-1+, CD30+/− | Monoclonal | Very poor |
| Adjacent mucosa: as for celiac disease | IELs: as for refractory sprue and ulcerative jejunitis | Monoclonal; same clone as the EATL | ||
| EATL type II | Lymphoma: monomorphic medium-sized cells | Lymphoma: CD3+, CD4−, CD8+, CD56+, CD30−, CD103−, TIA-1+ | Monoclonal | Very poor |
| Adjacent mucosa: often shows increased IELs but without other changes of celiac disease | IELs: CD3+, CD4−, CD8+, CD56+ cells | Monoclonal; same clone as the EATL |
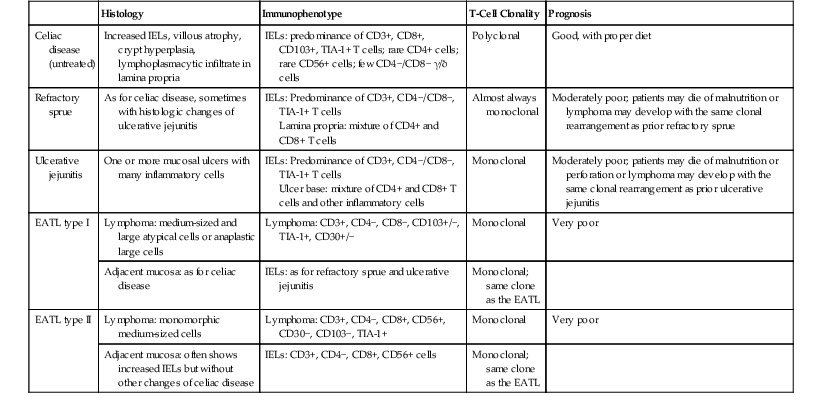
EATL, Enteropathy-associated T-cell lymphoma; IELs, intraepithelial lymphocytes; TIA-1, T cell–restricted intracellular antigen 1.
The relationship between EATL I and EATL type II is uncertain. Because EATL type II appears to be unrelated to celiac disease, some authorities have suggested that it should be removed from the category of EATL. An alternative designation of “monomorphic intestinal T-cell lymphoma” has been suggested.129
Molecular Features
Molecular genetic studies have shown clonal rearrangement of the TCR β- and γ-chain genes.128,136,142,143 EATL type I usually shows monoallelic rearrangement of the TCRγ chain gene, whereas type II cases usually show biallelic TCRγ rearrangement144 In situ hybridization with probes for Epstein-Barr encoded RNA (EBER) to detect EBV is typically negative; the presence of EBV should suggest a diagnosis of extranodal NK/T-cell lymphoma (see Differential Diagnosis).
EATLs of both types show a high frequency of genetic imbalances affecting multiple loci. The most frequent abnormality (70% of cases) is amplification of material in the area of 9q31-9q34. 126,144,145 Most of the cases that lack a 9q abnormality are described to have loss of genetic material from 16q.126 There has been some variation in results, however: One Japanese group did not identify frequent 9q change, suggesting that Asian cases may have underlying genetic abnormalities that differ from those in Western cases. 136 In contrast, a Korean group identified gains of 9q33-34.1 by array comparative genomic hybridization in four of five cases examined.140 Therefore, almost all cases in a number of series have shown gains of 9q or loss of 16q, suggesting that these mutations are important in the pathogenesis of EATL. The affected region on 9q contains the NOTCH1 and ABL1 genes, both of which are important in regulation of hematopoiesis and likely in lymphomagenesis.144,145
Analysis discloses differences in genetic changes between EATL types I and II as well. In type I, gains of 5q (APC gene locus) are common, but they are rare in type II.126,144 In type II, gains in the area of the MYC locus on 8q, leading to MYC amplification, are common in both Western and Asian cases.126,136 Gains of Xp and Xq appear to be common in Asian type II cases.136
LOH at chromosome 9p21, a region containing the tumor suppressor genes p14/p15/p16, is also relatively frequent; this abnormality is more common in type I than in type II but has been described in both.144,146 Cases with loss of genetic material in this area show loss of p16 protein expression, suggesting that this genetic change is functionally significant.146 TP53 protein expression is detected in most EATLs.146 LOH of the TP53 locus is detected in some type 1 and type II cases,144 and loss of genetic material on 17p in the area of TP53 is detected in some cases by comparative genetic hybridization.126
Lymph Node Changes
Mesenteric lymph nodes are almost always enlarged, although they are not always involved by lymphoma. In partially involved lymph nodes, the lymphoma may be found in sinuses or in the paracortex. 30 Enlarged nodes free of tumor may show nonspecific reactive changes, edema, or mesenteric lymph node cavitation. In the latter condition, lymph nodes may be markedly enlarged (as large as 8 cm) and may show cystic change, so that the lymph node consists of a thickened capsule and a thin rim of lymphoid tissue surrounding clear or turbid fluid that most likely represents lymph.147 Mesenteric lymph node cavitation is not specific for EATL; it may be seen in celiac disease without lymphoma and in refractory sprue. The condition may be related to severe malnutrition. When malnutrition is corrected, the lymph node changes may regress.133
Complicated Celiac Disease: Refractory Sprue and Ulcerative Jejunitis
Refractory sprue and ulcerative jejunitis are serious, potentially fatal manifestations of celiac disease. Refractory sprue is characterized by persistent or worsening malabsorption despite strict adherence to a gluten-free diet. Approximately 2% to 5% of celiac disease patients have RCD.122,148 Biopsies typically show changes consistent with untreated celiac disease. Ulcerative jejunitis, or ulcerative jejunoileitis, consists of histologically benign mucosal ulcers occurring in patients with celiac disease. Patients with refractory sprue often have ulcerative jejunitis, and vice versa. EATL may develop in patients with refractory sprue or ulcerative jejunitis, and patients with EATL often have, or have had, refractory sprue or ulcerative jejunitis. These observations suggest a relationship between lymphoma and complicated celiac disease.116,133
Investigators have addressed this issue by comparing the immunophenotypic and genetic features of EATL with those of mucosal lymphocytes in normal intestine, inflamed intestine, and intestine with uncomplicated celiac disease, RCD, or ulcerative jejunitis. Normal jejunal IELs are predominantly positive for TCRαβ, surface CD3 (sCD3), and CD8 and dim positive for CD5. There are also minor populations of CD4−, CD8− γ/δ T cells and CD56+ T cells.128,142 In most patients with refractory sprue, IELs are predominantly sCD3− but cytoplasmic CD3 (cCD3)+, CD4−, and CD8−133,142 This immunophenotype (cCD3+, CD4−, CD8−) is the same as that found in most EATLs. Molecular genetic analysis has shown that clonal T cells are present in almost all cases of refractory sprue and ulcerative jejunitis.133,142,143,149 In addition, when lymphoma follows refractory sprue or ulcerative jejunitis, the same clone is found in the lymphoma as in the histologically benign disorders.143,149 The same clonal rearrangement is also found in lymphoma and in mucosa surrounding the lymphoma.142 In one study, some patients with refractory sprue with abnormal IELs had T cells in the peripheral blood with clonal TCR rearrangements identical to those found in the intestine.133 Patients with normal bowel, other intestinal disorders such as Crohn’s disease, or celiac disease responsive to a gluten-free diet do not have clonal T cells133,142,143,149 or increased numbers of CD4−, CD8− T cells.133,142 It should be noted that EATL type II (CD56+, CD8+) typically does not have an increase in “double-negative” T cells in the surrounding mucosa but does have clonal T cells with an immunophenotype similar to that of the overt lymphoma (see Table 31.3).129,142
RCD has been divided into two types depending on immunophenotype, the distinction typically requiring flow cytometry. RCD type I is characterized by IELs with a normal immunophenotype (sCD3+, CD8+, TCR+). RCD type II is indistinguishable from RCD I on histologic examination, but flow cytometry reveals more than 20% T cells with an aberrant immunophenotype: sCD3− (although cCD3+), CD4−, CD8−, TCR−.148 Patients with RCD I are on average younger than those with RCD II (49 versus 59 years in one series).148 In addition, the HLA-DQ2 genotype is found in 80% of patients with RCD I and 92% of those with RCD II; however, RCD II patients are significantly more likely to have HLA-DQ2 homozygosity.148
In celiac disease, there may be increased numbers of intraepithelial γ/δ T cells, which likely play a role in regulation of the α/β T cells believed to be involved in the pathogenesis of celiac disease.150 γ/δ T cells are negative for CD4 and negative or dim positive for CD5 and CD8. Therefore, cases of celiac disease with increased numbers of reactive γ/δ T cells may appear to have IELs with an atypical immunophenotype. To be certain that an abnormal T-cell population is present, one may send tissue for PCR to investigate the possibility of a clonal T-cell population, as an adjunct to paraffin section immunohistochemistry yielding atypical results.
This information suggests that refractory sprue and ulcerative jejunitis are, in fact, neoplastic disorders. Precise classification is difficult, but investigators have suggested that they be designated as low-grade or cryptic EATL,133 epitheliotropic lymphoma, or intraepithelial lymphoma.146 With disease progression, the abnormal T cells may evolve into an overt, high-grade T-cell lymphoma.116,133
The distinction between RCD I and RCD II appears to be prognostically important. Patients with RCD I appear to have a very low risk of evolution into EATL, whereas RCD II frequently progresses to EATL (52% of cases in one series).148 Features associated with higher risk for EATL among celiac disease patients include older age at presentation, male gender, presence of ulcerative jejunitis, HLA-DQ2 homozygosity, and immunophenotypically aberrant T cells.148
The question that remains is how patients with refractory sprue or ulcerative jejunitis should be treated. Most have been treated with a gluten-free diet combined with steroids, other immunosuppressive agents, or total parenteral nutrition (or a combination of these),133 but the prognosis remains poor. With the information that these diseases represent clonal lymphoproliferative disorders, treatment with chemotherapy should be considered in certain cases and could possibly prevent progression to overt EATL. However, the question of whether chemotherapy should be administered, and if so, what type, is controversial.122 Patients with RCD I appear to respond well to steroids or to steroids in combination with azathioprine. Most RCD II cases do not respond to therapy, although aggressive treatment in a subset has led to remission.148 Many RCD II patients die of malnutrition or progress to EATL and die of lymphoma (see Table 31.3).148