Chapter 71 Hematologic Manifestations of HIV/AIDS
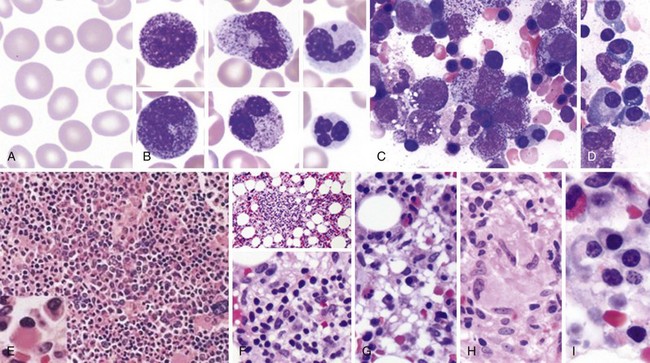
Peripheral Blood Smear and Bone Marrow Morphology in HIV/AIDS
AIDS, Acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
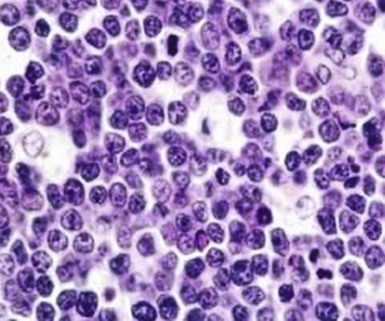
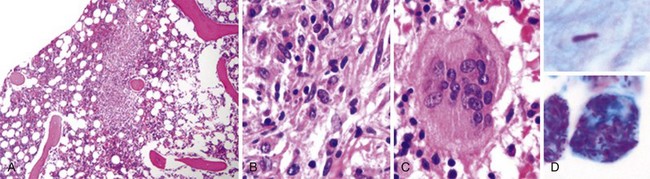
Figure 71-3 BURKITT LYMPHOMA INVOLVING THE BONE MARROW OF A PATIENT WITH ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS).
(Zhao X, Sun NC, Witt MD, et al: Changing pattern of AIDS: A bone marrow study. Am J Clin Pathol 121:393, 2004.)
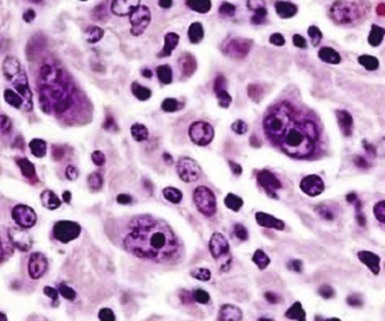
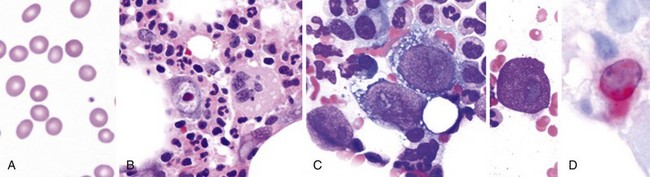
Figure 71-4 CLASSIC HODGKIN LYMPHOMA INVOLVING THE BONE MARROW OF A PATIENT WITH ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS).
(Zhao X, Sun NC, Witt MD, et al: Changing pattern of AIDS: A bone marrow study. Am J Clin Pathol 121:393, 2004.)
Table 71-1 Surveillance Case Definition for HIV Infection in Adults and Adolescents (Age Over 13 Years)
| Stage | Laboratory Evidence | Clinical Evidence |
|---|---|---|
| Stage1 | Laboratory confirmation of HIV infection and CD4+ T-lymphocyte count of ≥500 cells/µL or CD4+ T-lymphocyte percentage of ≥29* | No AIDS-defining condition (see Table 71-2) |
| Stage 2 | Laboratory confirmation of HIV infection and CD4+ T-lymphocyte count of 200-499 cells/µL or CD4+ T-lymphocyte percentage of 14-28* | No AIDS-defining condition (see Table 71-2) |
| Stage 3 | Laboratory confirmation of HIV infection and CD4+ T-lymphocyte count of <200 cells/µL or CD4+ T-lymphocyte percentage of <14* | Documentation of an AIDS-defining condition with laboratory confirmation of HIV infection (see Table 71-2) |
| Stage unknown | Laboratory confirmation of HIV infection and no information on CD4+ T-lymphocyte count or percentage | No information on presence of an AIDS-defining condition |
AIDS, Acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
*The CD4+ T-lymphocyte percentage is the percentage of the total lymphocyte count.
Table 71-2 Surveillance Definitions of AIDS-Defining Conditions
AIDS, Acquired immunodeficiency syndrome.
Anemia in HIV/AIDS
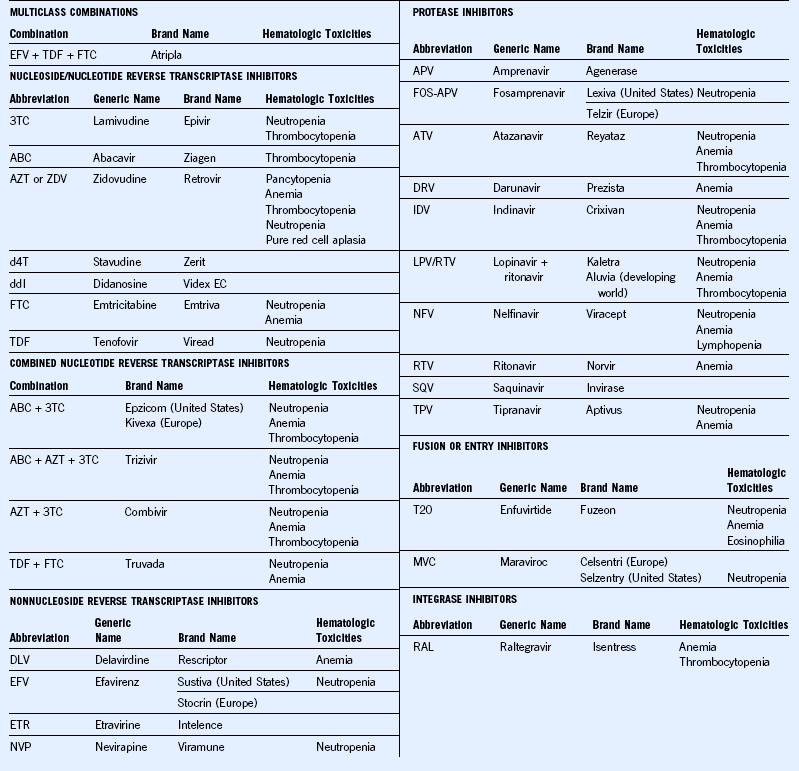
The most common cause of anemia in HIV disease is decreased RBC production. Frequently encountered mechanisms responsible for decreased RBC production in patients with HIV include anemia of acute and chronic inflammation (chronic disease anemia), infection or infiltration of bone marrow by infectious agents such as atypical mycobacterium, tuberculosis, cytomegalovirus, and/or fungal organisms or malignancies such as lymphoma. Parvovirus B19 can cause isolated red cell aplasia in the more severely immunocompromised individuals. Medications used for treatment of HIV or other illnesses and for prophylaxis of opportunistic infections can cause anemia mostly due to decreased RBC production. The list of medications as in Tables 71-3 and 71-4 is extensive. Hence it is very important that a thorough review of all the medications is done. Nutritional deficiencies, including vitamin B12 and folic acid deficiency, are causes of anemia due to ineffective production.
AIDS, Acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Table 71-4 Agents for Treatment and Prevention of Opportunistic Infections With Hematologic Toxicities
| Drug Class | Drug Toxicities | Hematologic |
|---|---|---|
| Antifungal agents | Amphotericin B deoxycholate and lipid formulations | Anemia |
| Anidulafungin | Deep venous thrombosis (rare) | |
| Flucytosine | Bone marrow suppression | |
| Micafungin | Hemolysis, leukopenia | |
| Anti-Pneumocystis pneumonia (PCP) agents | Dapsone | Methemoglobinemia, hemolytic anemia (especially in patients with G6PD deficiency), neutropenia |
| Primaquine | Methemoglobinemia, hemolytic anemia (especially in patients with G6PD deficiency) | |
| Trimethoprim-sulfamethoxazole (TMP-SMX) | Bone marrow suppression | |
| Antitoxoplasmosis agents | Pyrimethamine | Neutropenia, thrombocytopenia, megaloblastic anemia |
| Sulfadiazine | Bone marrow suppression | |
| Antimycobacterial agents | Rifampin | Thrombocytopenia, hemolytic anemia. |
| Rifabutin | Neutropenia anemia, thrombocytopenia | |
| Antiviral agents | Ganciclovir | Neutropenia, thrombocytopenia, anemia |
| Interferon-α and peginterferon-α | Neutropenia, thrombocytopenia | |
| Ribavirin | Hemolytic anemia | |
| Valacyclovir | At a high dose of 8 g/day: thrombotic thrombocytopenic purpura/hemolytic uremic syndrome reported in advanced human immunodeficiency virus patients and in transplant recipients | |
| Valganciclovir | Neutropenia, thrombocytopenia, anemia | |
| Antiparasitic agents | Albendazole | Neutropenia |
| Benznidazole | Bone marrow suppression | |
| Fumagillin (investigational) | Oral therapy: neutropenia, thrombocytopenia Ocular therapy: minimal systemic effect or local effect |
|
| Miltefosine | Leukocytosis, thrombocytosis | |
| Treatment for syphilis | Pentavalent antimony (sodium stibogluconate) | Leukopenia, anemia, thrombocytopenia |
| Penicillin G | Bone marrow suppression (rare), drug fever |
PCP, Pneumocystis carinii pneumonia.
Table 71-5 Etiology of Anemia in HIV
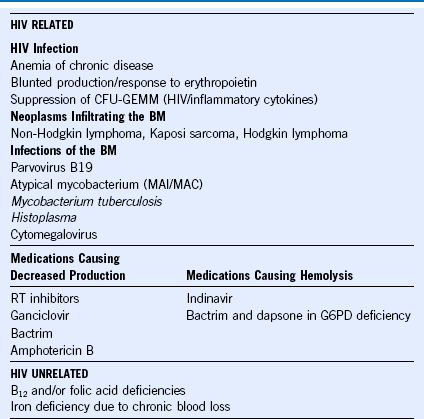
BM, Basement membrane; CFU-GEMM, colony-forming unit–granulocyte, erythrocyte, macrophage, megakaryocyte; HIV, human immunodeficiency virus, MAI/MAC, Mycobacterium avium-intracellulare/Mycobacterium avium complex; RT, reverse transcriptase.
Neutropenia in HIV/AIDS
AIDS, Acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Thrombocytopenia in HIV Infection
AIDS, Acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.