104 Hematologic Disorders
RBC Disorders
Hemolytic Anemias
Clinical Presentation and Differential Diagnosis
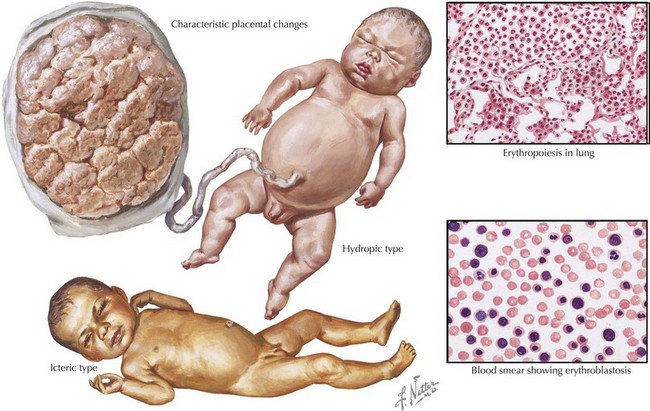
Both ABO incompatibility and Rh disease are associated with jaundice within the first 24 hours of life. In cases of severe hemolytic disease (i.e., erythroblastosis fetalis), infants also present with signs of hydrops fetalis (ascites, pleural or pericardial effusions, edema), pallor (secondary to anemia), petechiae or purpura (caused by thrombocytopenia), and hepatosplenomegaly (result of extramedullary hematopoiesis and splenic sequestration) (Figure 104-1). Each manifestation has a long list of possible causes, but the combination of jaundice and anemia with any of the above findings should focus clinical attention on diseases associated with hemolysis.
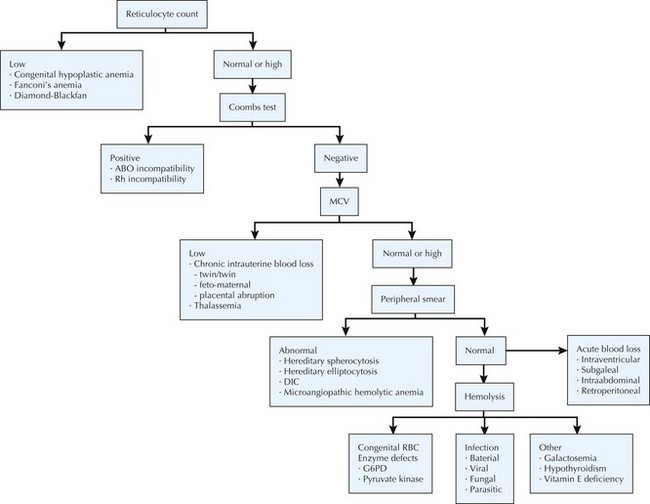
The differential diagnosis of neonatal anemia includes chronic or acute blood loss, congenital disorders of erythrocyte production (e.g., Fanconi’s anemia, Diamond-Blackfan), erythrocyte membrane defects (e.g., hereditary spherocytosis, hereditary elliptocytosis), congenital enzyme deficiencies (e.g., G6PD [glucose-6-phosphate dehydrogenase], pyruvate kinase), infection, and hemoglobin disorders (Figure 104-2). Of the hemoglobinopathies, α-thalassemias are the most common and severe. The switch from fetal (α2γ2) to adult (α2β2) hemoglobin occurs during the first year of life. As a result, defects in α-globin synthesis manifest in utero, whereas defects in β-globin synthesis become apparent in late infancy. Deletion of three (hemoglobin H disease) or four (hemoglobin Barts) α-globin genes can cause significant hemolytic anemia and present as hydrops fetalis. Newborn screening enables early detection and treatment of infants with major hemoglobinopathies and therefore reduces the mortality and morbidity associated with these conditions.
Management and Therapy
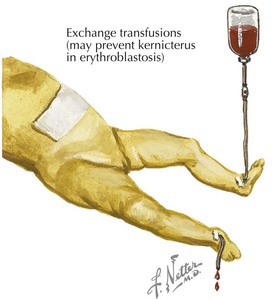
Treatment of neonatal hyperbilirubinemia with ET was introduced in the early 1950s. Although ET is proven to reduce mortality and the risk of kernicterus, it is associated with serious complications, including hemodynamic instability, apnea, coagulopathies, electrolyte imbalance, vascular thromboses, sepsis, arrhythmias, and necrotizing enterocolitis (NEC) (see Chapter 100). Efforts to reduce perinatal mortality and the need for ET have led to the development of several prenatal care strategies, including RhoGAM, use of Doppler ultrasound to detect fetal anemia, and intrauterine blood transfusions.
Polycythemia
Management and Therapy
Partial ET (PET) is used as a method to lower Hct and treat hyperviscosity. Studies have shown that PET reduces pulmonary vascular resistance and increases cerebral blood flow velocity. PET should be performed in symptomatic infants with Hct greater than 65% or asymptomatic infants with Hct greater than 70%. Either crystalloid (normal saline) or colloid (albumin) solution may be used. The volume to be exchanged is based on the observed and desired Hct (generally 50%–55%). Exchange volume (mL) = Blood volume × (Observed Hct – Desired Hct)/Observed Hct. Blood volume is estimated to be 80 to 90 mL/kg. During this procedure, blood is removed using a central venous or arterial line and replaced with fluid infused via a peripheral intravenous line. Complications of PET are thought to be similar to a single- or double-volume ET (Figure 104-3).
Platelet Disorders and Coagulopathies
Thrombocytopenia
Hemorrhagic Disease of the Newborn
Diagnostic Approach
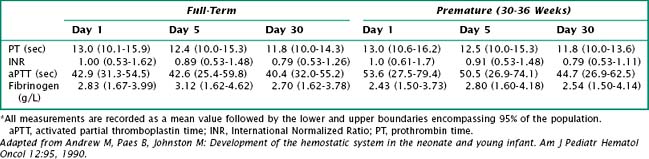
Vitamin K deficiency results in a prolonged prothrombin time (PT) and an International Normalized Ratio (INR) above 1. PT depends on various clotting factors, several of which are vitamin K dependent. INR compares the blood coagulation status of an individual to that of the normal population. Thus, an INR greater than 1 indicates that coagulation is slower than in the control group (Table 104-1).
Leukocyte Disorders
Neutropenia
Etiology and Pathogenesis
When evaluating neonates with neutropenia, it is important to first determine whether the cytopenia results from a defect in cellular production or peripheral destruction or is of mixed etiology (Box 104-1). The most common causes of neonatal neutropenia are maternal PIH, sepsis, and congenital viral infections (e.g., CMV, parvovirus, HIV, hepatitis B, rubella). Infants with severe and prolonged neutropenia should be evaluated for immune-mediated conditions and inherited genetic mutations.
Aher S, Malwatkar K, Kadam S. Neonatal anemia. Semin Fetal Neonatal Med. 2008;13(4):239-247.
Alcock GS, Liley H: Immunoglobulin infusion for isoimmune haemolytic jaundice in neonates. Cochrane Database Syst Rev (3):CD003313, 2002.
Sarkar S, Rosenkrantz TS. Neonatal polycythemia and hyperviscosity. Semin Fetal Neonatal Med. 2008;13(4):248-255.
Soll R, Schimmel MS, Ozek E: Partial exchange transfusion to prevent neurodevelopmental disability in infants with polycythemia (protocol). Cochrane Database Syst Rev (1):CD005089, 2009.
Steiner LA, Gallagher PG. Erythrocyte disorders in the perinatal period. Semin Perinatol. 2007;31(4):254-261.
Platelet Disorders and Coagulopathies
Puckett RM, Offringa M: Prophylactic vitamin K for vitamin K deficiency bleeding in neonates. Cochrane Database Syst Rev (4):CD002776, 2000.
Roberts I, Stanworth S, Murray NA. Thrombocytopenia in the neonate. Blood Rev. 2008;22(4):173-186.
van den Akker ES, Oepkes D, Lopriore E, et al. Noninvasive antenatal management of fetal and neonatal alloimmune thrombocytopenia: safe and effective. BJOG. 2007;114(4):469-473.
Carr R, Modi N, Doré C: G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev (3):CD003066, 2003.
Rivers A, Slayton WB. Congenital cytopenias and bone marrow failure syndromes. Semin Perinatol. 2009;33(1):20-28.
Segel GB, Halterman JS. Neutropenia in pediatric practice. Pediatr Rev. 2008;29(1):12-23.