Chapter 701 Heavy Metal Intoxication
The main threats to humans from heavy metals are associated with exposure to lead (Chapter 702), cadmium, mercury, and arsenic. The most prevalent of these exposures is lead. This chapter discusses mercury and arsenic.
Arsenic
Epidemiology
Arsenic is a metalloid that exists in four forms: elemental arsenic, arsine gas, inorganic arsenic salts (pentavalent arsenate form or trivalent arsenite form), and organic arsenic compounds. Toxic manifestations are higher in the more soluble and higher-valence compounds. Arsine gas is the most toxic form of arsenic. Mass poisonings due to exposure to arsenic have occurred throughout history, including one in 1998 in Wakayama, Japan, in which 70 people were poisoned. Children may be poisoned after exposure to inorganic arsenic found in pesticides, herbicides, dyes, homeopathic medicines, and certain intentionally or accidentally contaminated folk remedies from China, India, and Southeast Asia (Chapter 59). Soil deposits contaminate artesian well water. Groundwater contamination is a common problem in developing countries. Food products (e.g., rice) cooked in contaminated water may actually absorb arsenic, thus concentrating it in the food. The World Health Organization (WHO) has set 10 µg/L as the upper limit of safety. In many parts of Asia and South America, this limit is frequently exceeded. Arsenic concentrations in one quarter of the wells in Bangladesh exceed 50 µg/L and 35-77 million of the 125 million inhabitants of Bangladesh regularly consume arsenic-contaminated water. Occupational exposure may occur in industries such as glass manufacturing, pottery, electronic component, semiconductor and laser, manufacturing, mining, smelting, and refining. Although arsenic is no longer produced in the USA, it is produced in many countries and is imported into the USA for industrial use. Organic arsenic compounds may be found in seafood, pesticides, and some veterinary pharmaceuticals. In contrast to mercury, the organic forms of arsenic found in seafood are nontoxic.
Clinical Manifestations
Arsine gas is colorless, odorless, nonirritating, and highly toxic. Inhalation causes no immediate symptoms. After a latent period of 2-24 hr, massive hemolysis occurs, along with malaise, headache, weakness, dyspnea, nausea, vomiting, abdominal pain, hepatomegaly, pallor, jaundice, hemoglobinuria, and renal failure (Table 701-1). Acute ingestion of arsenic produces gastrointestinal toxicity within minutes to hours and manifested as nausea, vomiting, abdominal pain, and diarrhea. Hemorrhagic gastroenteritis with extensive fluid loss and third spacing may result in hypovolemic shock. Cardiovascular toxicity includes QT interval prolongation, polymorphous ventricular tachycardia, congestive cardiomyopathy, pulmonary edema, and cardiogenic shock. Acute neurologic toxicity includes delirium, seizures, cerebral edema, encephalopathy, and coma. Lethal doses of arsenates are 5-50 mg/kg; lethal doses of arsenites are less than 5 mg/kg.
| ORGAN SYSTEM | EFFECTS OF ARSENIC |
|---|---|
| Gastrointestinal system | Submucosal vesicles, watery or bloody diarrhea, severe hematemesis |
| Cardiovascular system | Reduced myocardial contractility, prolonged QT intervals, tachyarrhythmias |
| Vasodilation, hypotension | |
| Kidneys | Hematuria, proteinuria, acute tubular necrosis |
| Nervous system | Toxic encephalopathy with seizures, cerebral edema, and coma |
| Chronic exposure: peripheral painful sensorimotor neuropathy | |
| Hematologic and lymphatic system | Anemia and thrombocytopenia; acute hemolysis with arsine gas |
| Liver | Fatty degeneration with central necrosis |
| Skin | Desquamation, alopecia, hyperkeratosis, nail changes |
| Chronic exposure: hyperkeratosis, hyperpigmentation | |
| Teratogenic | Neural tube defects in the fetus |
Late sequelae include hematuria, proteinuria, and acute tubular necrosis. A delayed sensorimotor peripheral neuropathy may appear days to weeks after acute exposure, secondary to axonal degeneration. Neuropathy manifests as painful dysesthesias followed by diminished vibratory, pain, touch, and temperature sensation; decreased deep tendon reflexes; and, in the most severe cases, an ascending paralysis with respiratory failure mimicking Guillain-Barré syndrome (Chapter 608). Adult survivors of infant arsenic poisoning experience higher mortality from disorders of the nervous system compared to adults without such exposure.
Laboratory Findings
The diagnosis of arsenic intoxication is based on characteristic clinical findings, a history of exposure, and elevated urinary arsenic values, the last of which confirm the exposure. A spot urine arsenic level should be determined for symptomatic patients before chelation, although initially the result may be negative. Because urinary excretion of arsenic is intermittent, definitive diagnosis depends on a 24-h urine collection. Concentrations greater than 50 µg/L in a 24-h urine specimen are consistent with arsenic intoxication (Table 701-2). Urine specimens must be collected in metal-free containers. Ingestion of seafood containing nontoxic arsenobetaine and arsenocholine can cause elevations of urinary arsenic. Blood arsenic levels rarely are helpful because of their high variability and the rapid clearance of arsenic from the blood in acute poisonings. Elevated arsenic values in the hair or nails must be interpreted cautiously because of the possibility of external contamination. Abdominal radiographs may demonstrate ingested radiopaque arsenic.
| ARSENIC | MERCURY | |
|---|---|---|
| Molecular weight | 74.9 d | 200.59 d |
| Normal blood level | <5 µg/L (<0.665 nmol/L) | <10 µg/L (<50 nmol/L) |
| Normal urine level | <50 µg/L (<6.65 nmol/L) 24-h urine sample | <20 µg/L (<100 nmol/L) |
| Intervene at blood level | >35 µg/L (>175 nmol/L) | |
| Intervene at urine level | >100 µg/L (>13.3 nmol/L) 24-h urine sample | >150 µg/L (>750 nmol/L) |
Mercury
Epidemiology
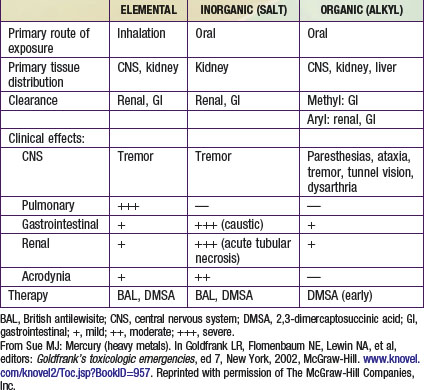
Mercury exists in three forms: elemental mercury, inorganic mercury salts, and organic mercury (Table 701-3). Elemental mercury is present in thermometers, sphygmomanometers, barometers, batteries, and some latex paints produced before 1991. Workers in industries producing these products may expose their children to the toxin when mercury is brought home on contaminated clothing. Vacuuming of carpets contaminated with mercury and breaking of mercury fluorescent light bulbs may result in elemental mercury vapor exposure. Severe inhalation poisonings have resulted from attempts to separate gold from gold ore by heating mercury and forming a gold-mercury amalgam. Elemental mercury has been used in folk remedies by Asian and Mexican populations for chronic stomach pain and by Latin Americans and Caribbean natives in occult practices. Dental amalgams containing elemental mercury release trace amounts of mercury that do not pose a credible risk to health. An expert panel for the National Institutes of Health concluded that existing scientific evidence does not indicate that dental amalgams pose a health risk; they should not be replaced merely to decrease mercury exposure.
Thimerosal is a mercury-containing preservative used in some vaccines. Thimerosal contains 49.6% mercury by weight and is metabolized to ethyl mercury and thiosalicylate. During an ongoing review of biologic products in response to the U.S. Food and Drug Administration (FDA) Modernization Act of 1997, the FDA determined that infants who received thimerosal-containing vaccines at multiple visits might have been exposed to more mercury than recommended by federal guidelines. As a precautionary measure, the American Academy of Pediatrics, American Academy of Family Physicians, Advisory Committee on Immunization Practices, and U.S. Public Health Service issued a joint recommendation in 1999 that thimerosal be removed from vaccines as quickly as possible. In the USA, thimerosal has been removed from all vaccines in the recommended childhood immunization schedule. Infants and children who have received thimerosal-containing vaccines do not need to undergo blood, urine, or hair testing for mercury because the concentrations of mercury would be quite low and would not require treatment. The benefits and risks of vaccines containing thimerosal should be discussed with parents (as with all vaccines). The larger risks of not vaccinating children far outweigh any known risk of exposure to thimerosal-containing vaccines. Studies do not demonstrate a link between thimerosal-containing vaccines and autistic spectrum disorders (Chapter 28.1), and no evidence supports a change in the standard of practice with regard to administration of thimerosal-containing vaccines in areas of the world where they are used. A rise in blood mercury levels following a single dose of hepatitis vaccine was seen in preterm infants, but the clinical significance is unknown. The American Academy of Pediatrics recommends that the initiation of hepatitis vaccine series be deferred until 2-6 mo of age in children who are born to hepatitis B surface antigen–negative mothers.
Laboratory Findings
The diagnosis of mercury intoxication is based on characteristic clinical findings, a history of exposure, and elevation of whole blood or urine mercury values, the last of which confirms the exposure. Thin-layer and gas chromatographic techniques can be used to distinguish organic from inorganic mercury. Blood should be collected in special tubes for trace elements from laboratories that are capable of performing those tests. Levels less than 10 µg/L in whole blood and less than 20 µg/L in a 24-h urine specimen are considered normal (see (Table 701-2). Although blood mercury levels may reflect acute exposure, they decrease as mercury redistributes into the tissues. Urine mercury levels are most useful for identifying long-term exposures, except in the case of methyl mercury, which undergoes minimal urinary excretion. Urinary mercury levels are used in monitoring efficacy of chelation therapy, whereas blood levels are used primarily in monitoring organic mercury poisonings. Hair analysis for mercury is not reliable because hair reflects endogenous as well as exogenous mercury exposure (hair avidly binds mercury from the environment). Abdominal radiographs may demonstrate ingested radiopaque mercury.
American Academy of Pediatrics, Committee on Environmental Health. Technical report: mercury in the environment. Implications for pediatricians. Pediatrics. 2001;108:197-204.
Argos M, Kalra T, Rathouz PJ, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376:252-258.
Bae M, Wantanabe C, Inaoka T, et al. Arsenic in cooked rice in Bangladesh. Lancet. 2002;360:1839-1840.
Baum CR. Treatment of mercury intoxication. Curr Opin Pediatr. 1999;11:265-268.
Bolger PM, Schwetz BA. Mercury and health. N Engl J Med. 2002;347:1735-1736.
Bose-O’Reilly S, McCarty KM, Steckling N, et al. Mercury exposure and children’s health. Curr Prob Pediatr Adolesc Health Care. 2010;40(8):185-216.
Centers for Disease Control and Prevention. Summary of the joint statement on thimerosal in vaccines. American Academy of Family Physicians, American Academy of Pediatricians, Advisory Committee on Immunization Practices, Public Health Service. MMWR Morb Mortal Wkly Rep. 2000;49:622-631.
Centers for Disease Control and Prevention. Mercury exposure—Kentucky, 2004. MMWR Morb Mortal Wkly Rep. 2005;54:797-798.
Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med. 2003;349:1731-1736.
Cullen NM, Wolf LR, St Clair D. Pediatric arsenic ingestion. Am J Emerg Med. 1995;13:432-435.
Elinder CG. Epidemiology and toxicity of mercury. www.uptodate.com.
Ford M. Arsenic (heavy metals). Goldfrank LR, Flomenbaum NE, Lewin NA, et al, editors. Goldfrank’s toxicologic emergencies, ed 7. McGraw-Hill, New York, 2002. www.knovel.com/knovel2/Toc.jsp?BookID=957.
Goldman RH. Arsenic exposure and poisoning. Available from www.uptodate.com
Golka K, Hengstler JG, Marchan R, et al. Severe arsenic poisoning: one of the largest man-made catastrophies. Arch Toxicol. 2010;84:583-584.
Heron J, Golding J, ALSPAC Study Team. Thimerosal exposure in infants and developmental disorders: A prospective cohort study in the United Kingdom does not support a causal association. Pediatrics. 2004;114:577-583.
Horowitz Y, Greenberg D, Ling G, et al. Acrodynia: a case report of two siblings. Arch Dis Child. 2002;86:453-455.
Huyck KL, Kile ML, Mahiuddin G, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49:1097-1104.
Innis SM, Palaty J, Vaghri Z, et al. Increased levels of mercury associated with high fish intakes among children from Vancouver, Canada. J Pediatr. 2006;148:759-763.
Jacobson JL, Jacobson SW. Risks to child health from methylmercury exposure in immigrant populations. J Pediatr. 2006;148:716-718.
Karagas MR. Arsenic-related mortality in Bangladesh. Lancet. 2010;376:213-214.
Kondo K. Congenital Minamata disease: warnings from Japan’s experience. J Child Neurol. 2000;15:458-464.
Lai MW, Boyer EW, Kleinman ME, et al. Acute arsenic poisoning in two siblings. Pediatrics. 2005;116:249-257.
McDonald C, Hoque R, Huda N, et al. Risk of arsenic-related skin lesions in Bangladeshi villages at relatively low exposure: a report from Gonoshasthaya Kendra. Bull World Health Organ. 2007;85:668-673.
Mitchell RJ, Osborne PB, Haubenreich JE. Dental amalgam restorations: daily mercury dose and biocompatibility. J Long Term Eff Med Implants. 2005;15:709-721.
Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J Nutr. 2007;137:2805-2808.
Parker SK, Schwartz B, Todd J, et al. Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics. 2004;114:793-804.
Pichichero ME, Cernichiari E, Lopreiato J, et al. Mercury concentrations and metabolism in infants receiving vaccines containing thimerosal: a descriptive study. Lancet. 2002;360:1737-1740.
Schober SE, Sinks TH, Jones RL, et al. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA. 2003;289:1667-1674.
Stajich GV, Lopez GP, Harry SW, et al. Iatrogenic exposure to mercury after hepatitis B vaccination in preterm infants. J Pediatr. 2000;136:679-681.
Sue YJ. Mercury (heavy metals). Goldfrank LR, Flomenbaum NE, Lewin NA, et al, editors. Goldfrank’s toxicologic emergencies, ed 7. McGraw-Hill, New York, 2002. www.knovel.com/knovel2/Toc.jsp?BookID=957.
Tanaka H, Tsukuma H, Oshima A: Long-term prospective study of 6104 survivors of arsenic poisoning during infancy due to contaminated milk product in 1955, J Epidemiol (in press).