Chapter 12 Heart Murmurs
Introduction and Basic Issues
Table 12-2 Classification of Murmurs Described in This Chapter*
| A. Functional (27–65) | B. Systolic (66–194) | C. Diastolic (197–258) |
|---|---|---|
| Systolic | Semilunar Ejection | Atrioventricular Stenosis |
| • Still’s murmur (46–50) | • Aortic stenosis | • Mitral stenosis (197–209) |
| • Valvular (82–115) | ||
| • Subvalvular hyper-trophic (116–128) | ||
| • Subvalvular “fixed” (129) | ||
| • Supravalvular (130) | ||
| • Pulmonary systolic ejection murmur (51) | • Pulmonic stenosis (132–133) | • Mitral diastolic flow murmur (210) |
| • Supraclavicular arterial bruit (52) | • Ventricular septal defect (134–138) | • Tricuspid stenosis (211–212) |
| • Aortic sclerosis (56–65) | • Tricuspid diastolic flow murmur (213–215) | |
| Continuous | AV Regurgitation | Semilunar Regurgitation |
| • Venous hum (53) | • Mitral regurgitation (142–170) | • Aortic regurgitation (216–251) |
| • Mammary soufflé (54) | • Mitral valve prolapse(171–184) | • Pulmonic regurgitation (252–258) |
| • Tricuspid regurgitation (185–194) | ||
| Diastolic | ||
| • Very rare, and always associated with either S3 or a diastolic rumble (33–34) |
* Not including continuous extracardiac murmurs like that of patent ductus arteriosus. The numbers in parentheses refer to the pertinent questions.
Cardiac auscultation is the centerpiece of physical diagnosis, and recognizing murmurs is its most challenging aspect. It requires the identification of sounds jam-packed in less than 0.8 second, often overlapping, and not infrequently at the threshold of audibility. Stethoscopy is like learning a musical instrument and similarly rewarding. Hence, despite being as old as the battle of Waterloo, this little tool and its skillful use still occupy an important role in 21st-century medicine.
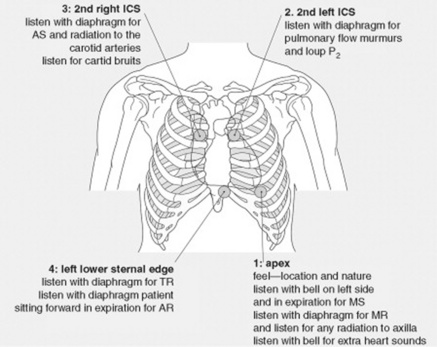
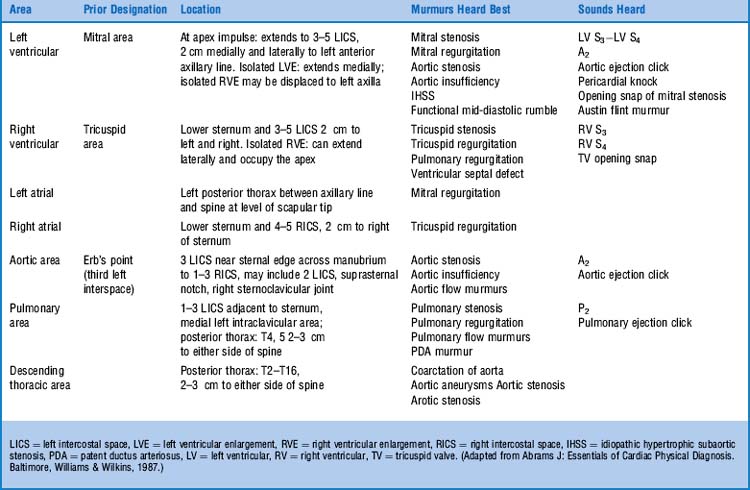
1 What are the auscultatory areas of murmurs?
The classic ones are shown in Fig. 12-1 and Table 12-1. Auscultation typically starts in the aortic area, continuing in clockwise fashion: first over the pulmonic, then the mitral (or apical), and finally the tricuspid areas. Since murmurs may radiate widely, they often become audible in areas outside those historically assigned to them. Hence, “inching” the stethoscope (i.e., slowly dragging it from site to site) can be the best way not to miss important findings.
5 What, then, should be the approach to a newly detected murmur?
The first step should be to use the cardiovascular exam to separate pathologic from functional murmurs (see below, questions 28–33). This is essential to avoid expensive and possibly dangerous laboratory tests. Then, if the murmur is identified as organic, the physical examination should provide clues to its site of origin, its hemodynamic cause, and, possibly, its severity.
Mechanisms of Production
9 What are the structural abnormalities that produce local narrowing and turbulent flow?
Any of the following abnormalities in the orifice through which blood flows:
 Abnormal size (the smaller the orifice, the greater the turbulence; the greater the turbulence, the louder the murmur). This phenomenon also may occur when blood moves from a small into a large space, such as a dilated aortic root.
Abnormal size (the smaller the orifice, the greater the turbulence; the greater the turbulence, the louder the murmur). This phenomenon also may occur when blood moves from a small into a large space, such as a dilated aortic root.
 Irregular shape (for example, an irregular valve opening)
Irregular shape (for example, an irregular valve opening)
 Irregular edge (the sharper the edge of the orifice, the higher the turbulence)
Irregular edge (the sharper the edge of the orifice, the higher the turbulence)
Classification
10 How are murmurs classified?
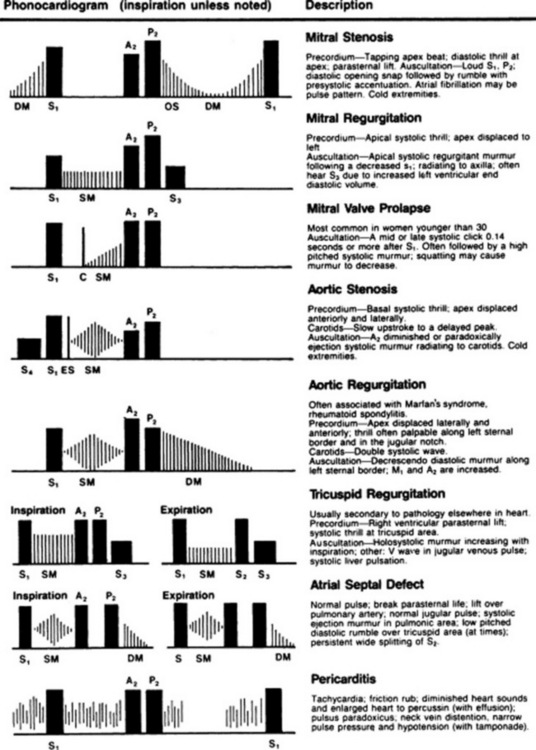
The first (and most important) separation is purely clinical: pathologic versus functional. The real classification, however, is based on the phase of the cardiac cycle where the murmur is located. Accordingly, murmurs are divided into systolic, diastolic, and continuous. This is clinically relevant, since diastolic and continuous murmurs are (almost) always pathologic, whereas systolic murmurs are often functional (Fig. 12-2).
14 Once the phase of the cardiac cycle has been identified, which other characteristics of a murmur should be analyzed and described?
1. The timing: Murmurs can span throughout systole (holosystolic) or diastole (holodiastolic), or they may occur only in the early, mid, or late phases of each interval:
 Systolic murmurs: Holosystolic and late systolic murmurs are clinically more important than early or mid-systolic murmurs because they are usually pathologic. Since benign systolic murmurs are flow generated, and since flow is maximal during the early part of systole, all benign systolic murmurs tend to be short and early peaking. They are never beyond S2. In fact, a murmur that touches S2 (whether holosystolic or late systolic) is pathologic and reflective of atrioventricular (A-V) regurgitation. Conversely, a murmur that occurs during the first half of systole (whether early or mid-systolic) is often benign and reflective of semilunar ejection. Hence, the longer the murmur, the worse the disease.
Systolic murmurs: Holosystolic and late systolic murmurs are clinically more important than early or mid-systolic murmurs because they are usually pathologic. Since benign systolic murmurs are flow generated, and since flow is maximal during the early part of systole, all benign systolic murmurs tend to be short and early peaking. They are never beyond S2. In fact, a murmur that touches S2 (whether holosystolic or late systolic) is pathologic and reflective of atrioventricular (A-V) regurgitation. Conversely, a murmur that occurs during the first half of systole (whether early or mid-systolic) is often benign and reflective of semilunar ejection. Hence, the longer the murmur, the worse the disease. Diastolic murmurs: Early diastolic murmurs (starting immediately after S2) reflect semilunar regurgitation, whereas mid- to late-diastolic murmurs (starting slightly after S2) reflect A-V stenosis. Mid- to late-diastolic murmurs may extend into S1 as a result of presystolic accentuation (due to strong atrial contraction), but are never truly holodiastolic. Conversely, murmurs of severe semilunar regurgitation may cover the entire length of diastole. This may provide useful clues to differential diagnosis. Note, however, that holodiastolic murmurs can be so faint in late diastole to become almost inaudible.
Diastolic murmurs: Early diastolic murmurs (starting immediately after S2) reflect semilunar regurgitation, whereas mid- to late-diastolic murmurs (starting slightly after S2) reflect A-V stenosis. Mid- to late-diastolic murmurs may extend into S1 as a result of presystolic accentuation (due to strong atrial contraction), but are never truly holodiastolic. Conversely, murmurs of severe semilunar regurgitation may cover the entire length of diastole. This may provide useful clues to differential diagnosis. Note, however, that holodiastolic murmurs can be so faint in late diastole to become almost inaudible.2. The intensity (or loudness): Traditionally graded by the Levine system from 1/6 to 6/6:
 1/6: A murmur so soft as to be heard only intermittently and always with concentration and effort. Never immediately.
1/6: A murmur so soft as to be heard only intermittently and always with concentration and effort. Never immediately.18 Which interventions and maneuvers can be used at the bedside to modify the intensity and characteristics of murmurs and make them more easily recognizable?
22 What is the effect of Valsalva on sounds and murmurs?
Valsalva not only has important hemodynamic effects (that can be used for the recognition of congestive heart failure—see Chapter 2, questions 121–127), but may also elicit a diagnostic auscultatory response in patients with HOCM or mitral valve prolapse (MVP). This is mediated by a reduction in left ventricular diameter (caused by the strain), which in turn increases the left ventricular gradient of HOCM, thus intensifying its subvalvular systolic ejection murmur. This is the opposite of what happens to other murmurs of left ventricular outflow obstruction (such as, for example, valvular AS or PS), which instead soften with Valsalva (because of decreased venous return, with a resulting decrease in transvalvular gradients). The strain phase of Valsalva also anticipates the prolapse of a floppy mitral valve (by making the ventricle smaller, and thus “loosening up” the chordae tendineae). As a result, the click will occur earlier, and the murmur will lengthen. Hence, only two murmurs get enhanced by the straining phase of Valsalva: HOCM and MVP. In HOCM, the murmur gets louder, whereas in MVP it gets longer. Note that the release period of Valsalva may have opposite effects, based on the site of origin of the acoustic event being examined: right-sided murmurs will generally revert to their baseline intensity within 2–3 cardiac cycles, whereas left-sided murmurs will instead take a little longer (up to 5–10 cardiac cycles).
A. Functional Murmurs
28 How can physical examination help differentiate functional from pathologic murmurs?
There are two golden and three silver rules.
1. The first golden rule is to always judge (systolic) murmurs like people: by the company they keep. Hence, murmurs that keep bad company (like symptoms; extra sounds; thrill; abnormal arterial or venous pulse, ECG, or chest x-ray) should be considered pathologic until proven otherwise. These murmurs should receive lots of evaluation, technology-based included.
2. The second golden rule is that a diminished or absent S2 usually indicates a poorly moving and abnormal semilunar valve. This is the hallmark of pathology. As a flip side, functional systolic murmurs are always accompanied by a well-preserved S2, with normal split.
34 What are the causes of benign diastolic murmurs?
Rapid ventricular filling—very much like the physiologic S3. In fact, these benign diastolic murmurs are always associated with either a physiologic S3 or some other type of benign rumbling murmur of filling (like Still’s—see below, questions 46–50). They never occur in isolation.
35 What are the clinical implications of functional murmurs?
36 How common are these functional murmurs?
Extremely common. In young adults, systolic murmurs have a 5–52% prevalence, with echocardiography being normal in 86–100%. They also are extremely commonly in pregnant women, with as many as 80% having a benign ejection murmur during gestation. Finally, systolic murmurs are present in 29–60% of elderly medical outpatients or nursing home residents (see aortic sclerosis, questions 56–65), with echocardiography being normal in 44–100% of cases. Hence, functional murmurs are the most common heart murmurs encountered by the generalist. Indeed, each of us probably had a functional murmur at some point in life.
39 Is high flow velocity present in nonpediatric patients?
Yes. For example, in tachycardia, anemia, fever, or, quite simply, during and after exercise.
40 How significant is a murmur that appears only after exercise, anemia, or fever?
It might still be quite significant because:
 Exercise increases flow velocity and thus may elicit not only a functional but also a pathologic murmur that would be otherwise inaudible. Hence, mild exercise (such as going from supine to sitting or left lateral decubitus) can be quite helpful in unmasking subtle findings.
Exercise increases flow velocity and thus may elicit not only a functional but also a pathologic murmur that would be otherwise inaudible. Hence, mild exercise (such as going from supine to sitting or left lateral decubitus) can be quite helpful in unmasking subtle findings.
 Anemia accelerates circulatory time, which in turn elicits innocent findings (such as the hemic murmur of patients with normal valves and no pressure gradient) or possibly pathologic ones.
Anemia accelerates circulatory time, which in turn elicits innocent findings (such as the hemic murmur of patients with normal valves and no pressure gradient) or possibly pathologic ones.
43 What are the functional murmurs caused by reduced flow velocity?
The most common is aortic sclerosis, wherein the aorta is dilated and tortuous.
44 How many types of functional murmurs are known?
In order of frequency, the four systolic murmurs are:
 Precordial vibratory murmur (Still’s)
Precordial vibratory murmur (Still’s)
 Pulmonary ejection systolic murmur
Pulmonary ejection systolic murmur
The first three occur in children and adolescents, whereas the fourth one is typical of the elderly.
45 What is the mechanism responsible for the generation of these functional murmurs?
It depends of the phase of the cardiac cycle:
 Functional systolic murmurs are due to rapid and vigorous ejection, across normal semilunar valves and into the large vessels. Because their site of origin is probably the large vessels themselves, these findings are loudest at the cardiac base (i.e., over the pulmonic [second to third left interspace] or aortic area [second to third right interspace]). Since the left ventricle generates higher pressures than the right, most functional systolic murmurs tend to occur over the aortic area.
Functional systolic murmurs are due to rapid and vigorous ejection, across normal semilunar valves and into the large vessels. Because their site of origin is probably the large vessels themselves, these findings are loudest at the cardiac base (i.e., over the pulmonic [second to third left interspace] or aortic area [second to third right interspace]). Since the left ventricle generates higher pressures than the right, most functional systolic murmurs tend to occur over the aortic area.
 Functional continuous murmurs (like the venous hum and the mammary soufflé) are caused instead by turbulent flow in either the great veins or the great arteries.
Functional continuous murmurs (like the venous hum and the mammary soufflé) are caused instead by turbulent flow in either the great veins or the great arteries.
 Finally, functional diastolic murmurs reflect a rapid and vigorous ventricular filling, with no intracardiac pressure gradient and no structural abnormalities. Hence, they have the same hemodynamic basis of a physiologic S3, which they often accompany as a sort of extension. In fact, they are never an isolated finding. And yet, they are so rare as to validate the rule that every diastolic murmur should be considered pathologic until proven otherwise.
Finally, functional diastolic murmurs reflect a rapid and vigorous ventricular filling, with no intracardiac pressure gradient and no structural abnormalities. Hence, they have the same hemodynamic basis of a physiologic S3, which they often accompany as a sort of extension. In fact, they are never an isolated finding. And yet, they are so rare as to validate the rule that every diastolic murmur should be considered pathologic until proven otherwise.
53 What is a venous hum?
It is a continuous and physiologic venous sound—not uncommon in children, but rare in adults. For a more extensive description, refer to Chapter 10, questions 120–123.
55 What can one do to sort out functional murmurs from pathologic ones?
1. Start with history, specifically:
 Personal history (age murmur first heard, history of central cyanosis, feeding difficulties, poor weight gain). Also, benign murmurs should never present with cardiac symptoms, such as dyspnea, angina, lightheadedness, fatigue, failure to thrive, and cyanosis.
Personal history (age murmur first heard, history of central cyanosis, feeding difficulties, poor weight gain). Also, benign murmurs should never present with cardiac symptoms, such as dyspnea, angina, lightheadedness, fatigue, failure to thrive, and cyanosis.2. Then continue with the physical examination, looking for clubbing and cyanosis and any abnormalities in the following areas of the cardiovascular exam:
3. Then carefully evaluate the murmur in its main characteristics, especially:
 Variations with Valsalva maneuver (remember that the pulmonary ejection, the Still’s murmur, and the venous hum all disappear with the onset of Valsalva, whereas the murmurs of HOCM and MVP increase—see question 22)
Variations with Valsalva maneuver (remember that the pulmonary ejection, the Still’s murmur, and the venous hum all disappear with the onset of Valsalva, whereas the murmurs of HOCM and MVP increase—see question 22) Postural changes (supine position increases preload and thus exaggerates flow murmurs; squatting/standing enhances HOCM and MVP)
Postural changes (supine position increases preload and thus exaggerates flow murmurs; squatting/standing enhances HOCM and MVP)4. Finally, gather simple laboratory tests (such as an electrocardiogram or a chest x-ray), and look for any associated “bad company.”
B. Systolic Murmurs
66 How common are systolic murmurs?
Extremely common, especially if ejection. Hence, the need to separate functional from pathologic.
67 What are the causes of a systolic murmur?
1. Ejection (i.e., increased “forward” flow over a semilunar valve). This can be:
 Physiologic: Normal valve, but flow high enough to cause turbulence (anemia, exercise, fever, and other hyperkinetic heart syndromes)
Physiologic: Normal valve, but flow high enough to cause turbulence (anemia, exercise, fever, and other hyperkinetic heart syndromes) Pathologic: Abnormal valve, with or without outflow obstruction (i.e., aortic stenosis versus aortic sclerosis)
Pathologic: Abnormal valve, with or without outflow obstruction (i.e., aortic stenosis versus aortic sclerosis)2. Regurgitation: “Backward” flow from a high- into a low-pressure bed. Although this is usually due to incompetent A-V valves (mitral/tricuspid), it also can be due to ventricular septal defect.
68 What characteristics of a systolic murmur help differentiate ejection from regurgitation?
 The relationship of the murmur to S2. Regurgitant systolic murmurs typically extend into S2 (and sometimes even beyond it), whereas ejection murmurs always end before it. This may be difficult to detect, since S2 is often soft (and even absent in severe semilunar stenosis).
The relationship of the murmur to S2. Regurgitant systolic murmurs typically extend into S2 (and sometimes even beyond it), whereas ejection murmurs always end before it. This may be difficult to detect, since S2 is often soft (and even absent in severe semilunar stenosis).
 The change of the murmur after a longer diastole. This is especially evident after a premature beat (see question 26), with ejection murmurs of semilunar stenosis becoming louder, and regurgitant murmurs of atrioventricular insufficiency remaining constant.
The change of the murmur after a longer diastole. This is especially evident after a premature beat (see question 26), with ejection murmurs of semilunar stenosis becoming louder, and regurgitant murmurs of atrioventricular insufficiency remaining constant.
 The response of the murmur to bedside maneuvers (previously discussed)
The response of the murmur to bedside maneuvers (previously discussed)
 The musical quality of the murmur. This is only present in situations of regurgitation.
The musical quality of the murmur. This is only present in situations of regurgitation.
(1) Systolic Ejection Murmurs
74 In addition to pitch, are there other differences between ejection and regurgitant murmurs?
 Clearly location. Ejection murmurs (such as AS) are loudest over the base, whereas regurgitant murmurs (such as MR) are most intense at the apex. The only exception is the Gallavardin phenomenon (see questions 92 and 93).
Clearly location. Ejection murmurs (such as AS) are loudest over the base, whereas regurgitant murmurs (such as MR) are most intense at the apex. The only exception is the Gallavardin phenomenon (see questions 92 and 93).
 Transmission also is different (to the neck for an ejection murmur and to the axilla for a regurgitant one).
Transmission also is different (to the neck for an ejection murmur and to the axilla for a regurgitant one).
 Changes after a long diastole. AS (but not MR) is louder after a long pause, like that following a premature ventricular contraction (or the long cardiac cycle of atrial fibrillation).
Changes after a long diastole. AS (but not MR) is louder after a long pause, like that following a premature ventricular contraction (or the long cardiac cycle of atrial fibrillation).
 Handgrip intensifies regurgitant murmurs, but softens ejection ones.
Handgrip intensifies regurgitant murmurs, but softens ejection ones.
 Duration. Regurgitant murmurs audibly extend all the way into S2, whereas ejection murmurs end before it.
Duration. Regurgitant murmurs audibly extend all the way into S2, whereas ejection murmurs end before it.
 Shape. Although systolic regurgitant murmurs may at times have a crescendo-decrescendo pattern, this is rather uncommon, and typically associated with higher frequency components than systolic ejection murmurs. Yet, despite all of these differentiating features, bedside separation can at times be very difficult.
Shape. Although systolic regurgitant murmurs may at times have a crescendo-decrescendo pattern, this is rather uncommon, and typically associated with higher frequency components than systolic ejection murmurs. Yet, despite all of these differentiating features, bedside separation can at times be very difficult.
I. Aortic Stenosis
81 What are the three main types of aortic stenosis?
Supravalvular, valvular, and subvalvular. These can be easily separated by evaluating the central arterial pulse (see Chapter 10, questions 18 and 19 and Fig. 10-3). In addition, subvalvular stenosis is further divided into (1) hypertrophic subaortic stenosis (also called HOCM [i.e., hypertrophic obstructive cardiomyopathy]) and (2) fixed, fibrotic subvalvular stenosis.
89 What is the frequency of clinically significant outflow obstruction?
1–2% of adults older than 65, with most patients eventually requiring valve replacement.
92 What is the Gallavardin phenomenon?
95 How can one separate the murmur of AS from that of aortic sclerosis?
By the same rules previously outlined for separating pathologic from functional murmurs:
 “The company it keeps”: A systolic ejection murmur associated with a reduced and delayed arterial pulse, a sustained apical impulse, a palpable precordial thrill, electrocardiographic evidence of left ventricular hypertrophy, and symptoms of outflow obstruction (exertional dizziness/syncope, chest pain, and dyspnea) is much more likely due to aortic stenosis than a similar murmur with none of the associations.
“The company it keeps”: A systolic ejection murmur associated with a reduced and delayed arterial pulse, a sustained apical impulse, a palpable precordial thrill, electrocardiographic evidence of left ventricular hypertrophy, and symptoms of outflow obstruction (exertional dizziness/syncope, chest pain, and dyspnea) is much more likely due to aortic stenosis than a similar murmur with none of the associations.
 Auscultatory characteristics: AS is more likely to be long, late peaking, associated with a soft to absent S2 (at least in its aortic component), and radiated to the right carotid. It may even have the ejection sound of a bicuspid valve. Conversely, aortic sclerosis is short, early peaking, and with a well-preserved (and even loud) S2, since many patients have in fact hypertension. Ejection sounds are uncommon in this condition, and so is radiation to the neck.
Auscultatory characteristics: AS is more likely to be long, late peaking, associated with a soft to absent S2 (at least in its aortic component), and radiated to the right carotid. It may even have the ejection sound of a bicuspid valve. Conversely, aortic sclerosis is short, early peaking, and with a well-preserved (and even loud) S2, since many patients have in fact hypertension. Ejection sounds are uncommon in this condition, and so is radiation to the neck.
 Finally, all things being equal, murmur intensity may help. A murmur of aortic sclerosis is softer. In fact, less than grade 4 in 98% of the cases (and thus never accompanied by a palpable thrill).
Finally, all things being equal, murmur intensity may help. A murmur of aortic sclerosis is softer. In fact, less than grade 4 in 98% of the cases (and thus never accompanied by a palpable thrill).
96 Of all these characteristics, which are the most useful for ruling in (or ruling out) aortic stenosis?
105 Is there any other acoustic event that may suggest severe AS?
Intuitively, one could think of the S4 and the early systolic click. And yet:
 Presence of an audible S4 does reflect severe left ventricular hypertrophy (with a transvalvular pressure gradient >70 mmHg), but only in younger patients. Older subjects may already have a “normal” S4. Conversely, a palpable S4 always reflects severe disease.
Presence of an audible S4 does reflect severe left ventricular hypertrophy (with a transvalvular pressure gradient >70 mmHg), but only in younger patients. Older subjects may already have a “normal” S4. Conversely, a palpable S4 always reflects severe disease.
 An early systolic (ejection) click usually indicates a valvular AS, typically due to a congenitally bicuspid aortic valve. Still, presence of a click does not correlate with severity of obstruction. Moreover, an early systolic click tends to be more frequent in younger patients, since AS in the elderly is usually due to calcification of a normal trileaflet valve.
An early systolic (ejection) click usually indicates a valvular AS, typically due to a congenitally bicuspid aortic valve. Still, presence of a click does not correlate with severity of obstruction. Moreover, an early systolic click tends to be more frequent in younger patients, since AS in the elderly is usually due to calcification of a normal trileaflet valve.
107 How is the point of maximal impulse (PMI) in AS?
There are two major characteristics:
 Location of PMI: In the typical patient with concentric left ventricular hypertrophy, the PMI is usually well sustained, but only mildly displaced to the left. An increased displacement usually indicates the development of left ventricular failure or concomitant aortic regurgitation.
Location of PMI: In the typical patient with concentric left ventricular hypertrophy, the PMI is usually well sustained, but only mildly displaced to the left. An increased displacement usually indicates the development of left ventricular failure or concomitant aortic regurgitation.
 Apical thrill: A palpable thrill is common in AS and does not reflect disease severity. To detect it, have the patient sit up, lean forward, and hold his or her breath in forced exhalation.
Apical thrill: A palpable thrill is common in AS and does not reflect disease severity. To detect it, have the patient sit up, lean forward, and hold his or her breath in forced exhalation.
108 How are the neck veins in patients with AS?
They may have a prominent “A” wave, as a result of the “Bernheim phenomenon.”
111 What then are the valuable clinical predictors of severe AS?
118 How can one differentiate the systolic ejection murmur of valvular AS from that of HOCM?
 Location: HOCM is subvalvular—and, hence, louder over Erb’s point, or even the apex—whereas typical AS is loudest over the aortic area.
Location: HOCM is subvalvular—and, hence, louder over Erb’s point, or even the apex—whereas typical AS is loudest over the aortic area.
 Timing of onset is also different: The murmur of valvular AS starts immediately after S1, whereas HOCM starts a little later—usually in mid-systole (since the obstruction is dynamic and, hence, more likely to occur when the ventricular lumen is reduced—as during systole).
Timing of onset is also different: The murmur of valvular AS starts immediately after S1, whereas HOCM starts a little later—usually in mid-systole (since the obstruction is dynamic and, hence, more likely to occur when the ventricular lumen is reduced—as during systole).
 Arterial pulse is delayed and reduced in AS, but brisk and bifid in HOCM.
Arterial pulse is delayed and reduced in AS, but brisk and bifid in HOCM.
120 What bedside maneuvers can modify the murmur of HOCM?
Maneuvers/factors that change left ventricular (LV) volume: a smaller volume brings the septum closer to the anterior mitral leaflet, thus causing a greater obstruction and a louder murmur. Conversely, a larger LV volume separates the upper septum from the anterior mitral leaflet, thus causing less obstruction and a softer murmur. Note that these are the same maneuvers that can respectively lengthen or soften the findings of mitral valve prolapse (see below, questions 178 and 179).
121 Which factors increase left ventricular volume?
122 Which factors reduce left ventricular volume?
127 Are there any other associated physical findings in HOCM?
 Brisk arterial pulse: This may be bifid on tracing (spike-and-dome waveform). Yet, in contrast to the pulsus bisferiens of AR, the bifid of HOCM is usually undetectable at the bedside (unless the obstruction is very severe).
Brisk arterial pulse: This may be bifid on tracing (spike-and-dome waveform). Yet, in contrast to the pulsus bisferiens of AR, the bifid of HOCM is usually undetectable at the bedside (unless the obstruction is very severe).
 Double or triple apical impulse: The “triple-ripple” reflects a strong atrial contraction (which, in turn, can cause an S4) and a double ventricular contraction (early rapid ejection, followed by obstruction, and subsequent late slow ejection. This is also the mechanism for the bifid pulse).
Double or triple apical impulse: The “triple-ripple” reflects a strong atrial contraction (which, in turn, can cause an S4) and a double ventricular contraction (early rapid ejection, followed by obstruction, and subsequent late slow ejection. This is also the mechanism for the bifid pulse).
 S3: This is rare in HOCM, unless the degree of associated MR is severe.
S3: This is rare in HOCM, unless the degree of associated MR is severe.
 Single split S2 (due to delayed closure of the aortic component, from increased impedance to left ventricular emptying): In severe HOCM, S2 can become paradoxically split (10% of cases).
Single split S2 (due to delayed closure of the aortic component, from increased impedance to left ventricular emptying): In severe HOCM, S2 can become paradoxically split (10% of cases).
 Nonejection systolic click: The likely explanation is inequality of the functional length of the mitral chordae tendineae, secondary to asymmetric myocardial hypertrophy.
Nonejection systolic click: The likely explanation is inequality of the functional length of the mitral chordae tendineae, secondary to asymmetric myocardial hypertrophy.
II. Aortic Versus Pulmonic Stenosis
132 How does the murmur of pulmonic stenosis (PS) differ from that of aortic stenosis (AS)?
 Location: The area of maximum intensity for AS is the second right interspace or the apex, whereas that of PS is the left sternal border.
Location: The area of maximum intensity for AS is the second right interspace or the apex, whereas that of PS is the left sternal border.
 Respiration: AS is louder in exhalation, whereas PS is louder in inspiration.
Respiration: AS is louder in exhalation, whereas PS is louder in inspiration.
 Standing: It makes the PS murmur proportionally louder in inspiration.
Standing: It makes the PS murmur proportionally louder in inspiration.
 Valsalva maneuver: Straining softens both murmurs. Still, immediately after release, the PS murmur reaches its highest intensity after only two to three beats, whereas AS takes much longer.
Valsalva maneuver: Straining softens both murmurs. Still, immediately after release, the PS murmur reaches its highest intensity after only two to three beats, whereas AS takes much longer.
133 What other auscultatory features can help differentiate pulmonic from aortic stenosis?
 An ejection click can occur in both PS and AS. Yet, only the one of PS fades or disappears with inspiration (which represents the only exception to the Rivero-Carvallo’s rule that all right-sided findings get louder in inspiration).
An ejection click can occur in both PS and AS. Yet, only the one of PS fades or disappears with inspiration (which represents the only exception to the Rivero-Carvallo’s rule that all right-sided findings get louder in inspiration).
 A widening of the normal and physiologic splitting of S2 argues in favor of PS. A paradoxical splitting of S2, on the other hand, argues in favor of AS.
A widening of the normal and physiologic splitting of S2 argues in favor of PS. A paradoxical splitting of S2, on the other hand, argues in favor of AS.
 Finally, the presence of an S4 gallop during inspiration is more likely to be associated with PS, whereas presence of an S4 gallop during exhalation is more likely to be associated with AS.
Finally, the presence of an S4 gallop during inspiration is more likely to be associated with PS, whereas presence of an S4 gallop during exhalation is more likely to be associated with AS.
III. Miscellaneous Ejection Murmurs
137 Where is the VSD murmur best heard?
Along the left lower sternal border, often radiating left to right across the chest.
(2) Systolic Regurgitant Murmurs
141 What are the auscultatory characteristics of systolic regurgitant murmurs?
They tend to start immediately after S1, often extending into S2. They also may have a musical quality, variously described as “honk” or “whoop.” This is usually caused by vibrating vegetations (endocarditis) or chordae tendineae (mitral valve prolapse, dilated cardiomyopathy) and may help separate the more musical murmurs of A-V regurgitation from the harsher sounds of semilunar stenosis. Note that in contrast to systolic ejection murmurs like AS or VSD, systolic regurgitant murmurs do not increase in intensity after a long diastole (see questions 26 and 68). In fact, if the intensity of a systolic murmur does increase, but only at the base, then it usually reflects the presence of two murmurs: one of ejection (becoming louder at the base) and one of regurgitation (remaining instead unchanged at the apex). Still, in mitral valve prolapse this rule does not hold, since the regurgitation of MVP is dictated by left ventricular volume: with larger volumes (such as those following a long diastole) causing instead reduced regurgitation, and thus a softer murmur.
145 What are the most common “valvular” causes of MR in adults?
 Myxomatous degeneration of the valve (mitral valve prolapse)
Myxomatous degeneration of the valve (mitral valve prolapse)
 Dysfunction of the papillary muscle(s), usually on an ischemic basis. This occurs in 10–20% of acute myocardial infarction cases, usually transiently, but still predicting a less favorable outcome.
Dysfunction of the papillary muscle(s), usually on an ischemic basis. This occurs in 10–20% of acute myocardial infarction cases, usually transiently, but still predicting a less favorable outcome.
 Rheumatic valvular damage (rare in the United States and typically associated with some degree of mitral stenosis)
Rheumatic valvular damage (rare in the United States and typically associated with some degree of mitral stenosis)
149 How is MR detected in adults?
 Asymptomatic patients with primary valve disease (i.e., mitral valve prolapse or a rheumatic sequela) get diagnosed by either detecting the typical systolic murmur or performing an echo for some other reasons.
Asymptomatic patients with primary valve disease (i.e., mitral valve prolapse or a rheumatic sequela) get diagnosed by either detecting the typical systolic murmur or performing an echo for some other reasons.
 Symptomatic patients with primary disease instead come to attention because of heart failure, atrial fibrillation, or endocarditis—often precipitated by some hemodynamic stress, like pregnancy, anemia, or infection.
Symptomatic patients with primary disease instead come to attention because of heart failure, atrial fibrillation, or endocarditis—often precipitated by some hemodynamic stress, like pregnancy, anemia, or infection.
 Finally, patients with secondary MR (i.e., ischemia or endocarditis) get recognized during evaluation of the underlying process.
Finally, patients with secondary MR (i.e., ischemia or endocarditis) get recognized during evaluation of the underlying process.
156 What are the best bedside predictors of MR severity?
The same predictors of murmur severity in general: intensity and length. Hence, the louder (and longer) the MR murmur, the worse the regurgitation. Intensity is a particularly strong predictor of severity, especially in MR due to neither ischemia nor dilated cardiomyopathy. In a study of 170 consecutive patients, none of those with silent disease had a regurgitant volume >50 mL, and only one had a regurgitant fraction >40% (similar data were found for AR patients—see below, questions 236 and 237). Yet, since murmur grading is rather subjective, interobserver variability may be an issue, as can be obesity and emphysema.
158 What are the other bedside signs of severe MR?
 Left ventricular enlargement: This can be determined by palpation, as a downward and lateral displacement (and enlargement) of the apical impulse. A left-lower parasternal impulse also argues strongly in favor of severe disease, usually reflecting a dilated left atrium.
Left ventricular enlargement: This can be determined by palpation, as a downward and lateral displacement (and enlargement) of the apical impulse. A left-lower parasternal impulse also argues strongly in favor of severe disease, usually reflecting a dilated left atrium.
 Widened S2 splitting: This is typical of severe MR and due to early closure of its aortic component. In time, it may become offset by the development of pulmonary hypertension, which, in turn, narrows S2 splitting.
Widened S2 splitting: This is typical of severe MR and due to early closure of its aortic component. In time, it may become offset by the development of pulmonary hypertension, which, in turn, narrows S2 splitting.
 Concomitant S3: This occurs in 90% of severe MR cases, with S3 loudness being directly related to severity of regurgitation. A third sound has 77% specificity for severe MR, but only 41% sensitivity. Note that S4 is rare in MR, unless there is acute regurgitation.
Concomitant S3: This occurs in 90% of severe MR cases, with S3 loudness being directly related to severity of regurgitation. A third sound has 77% specificity for severe MR, but only 41% sensitivity. Note that S4 is rare in MR, unless there is acute regurgitation.
 Diastolic flow murmur: In addition to S3, severe MR is often accompanied by an early diastolic rumble that follows S3 like an extension. The longer and louder the rumble, the worse the MR.
Diastolic flow murmur: In addition to S3, severe MR is often accompanied by an early diastolic rumble that follows S3 like an extension. The longer and louder the rumble, the worse the MR.
164 Can you separate MR of ruptured chordae from MR of dysfunctional papillary muscles?
Yes. Dysfunctional papillary muscle(s) are either asymptomatic or presenting with mild congestive failure. Ruptured chordae (or papillary muscles) present instead much more dramatically, with an intense MR murmur (3/6 or greater), a loud S3, and a flash pulmonary edema. In fact, the large amount of backflow in these patients may even increase atrial pressure so rapidly to eventually slow regurgitation early on in systole. Hence, the MR murmur typically starts immediately after S1 and then softens (and even disappears) at mid-systole. This is the opposite of the murmur of dysfunctional papillary muscle, which starts instead at mid-systole, has a crescendo pattern, and usually ends at S2 (although it also may last into mid-systole or even stay holosystolic). Patterns of radiation also may be unusual in ruptured chordae tendineae (see question 153). Finally, the MR of ruptured chordae is often associated with an S4. This is due to the Starling’s effect of a dilated atrium, which results in greater contractility and thus a stronger (and louder) systole. S4 is instead rare in MR from dysfunctional papillary muscles, and is almost absent in rheumatic disease.
II Mitral Valve Prolapse
171 What is mitral valve prolapse (MVP)?
An entity that throughout its history has received many names, with the most current being mitral valve prolapse syndrome, since this is indeed a syndrome, characterized not only by auscultatory findings, but also arrhythmias, atypical chest pain, abnormal ECG, panic attacks, and, possibly, valve infection. MVP is the most common congenital valvular disease, with a prevalence of 1–2%. In this section, we will focus on its murmur. For the click, refer to Chapter 11, questions 153–163.
181 Do patients with an isolated click necessarily develop regurgitation?
No. When followed for as long as 8 years, they do not develop significant regurgitation. Hence, clickers tend to stay clickers, which may have importance for prophylaxis (see Chapter 11).
184 Can bedside maneuvers identify a murmur of papillary muscle dysfunction?
Yes, since this neither lengthens nor intensifies with sitting, standing, or Valsalva.
III Tricuspid Regurgitant (TR) Murmur
185 What are the most common causes of tricuspid regurgitation?
 Primary TR is usually the result of direct valvular damage, without any preexistent pulmonary hypertension. This is typically acute and due to either endocarditis (almost always from drug abuse) or trauma. Primary TR also can be a congenital anomaly, as in Epstein’s disease.
Primary TR is usually the result of direct valvular damage, without any preexistent pulmonary hypertension. This is typically acute and due to either endocarditis (almost always from drug abuse) or trauma. Primary TR also can be a congenital anomaly, as in Epstein’s disease.
 Secondary TR requires instead either a pressure load to the right ventricle (i.e., pulmonary hypertension) or a volume load (as in atrial septal defect). This is often chronic, eventually causing right ventricular dilation, pulling apart of leaflets, and valvular incompetence.
Secondary TR requires instead either a pressure load to the right ventricle (i.e., pulmonary hypertension) or a volume load (as in atrial septal defect). This is often chronic, eventually causing right ventricular dilation, pulling apart of leaflets, and valvular incompetence.
192 What are the other bedside findings of chronic TR?
90% of patients have distended neck veins, plus peripheral edema and/or ascites.
194 How does acute TR differ from its chronic counterpart?
In many ways. Whenever regurgitation is sudden, its murmur is typically limited to early systole, and often decrescendo because right atrial pressure rises so rapidly to eventually stop backflow at mid-systole—a phenomenon similar to that behind acute MR (see question 166). In fact, whenever regurgitation is so severe that the atrium and ventricle become a single chamber, the murmur of TR (and MR) is absent. This may occur in patients whose chordae rupture. Finally, many of the peripheral manifestations of TR (leg edema, ascites, pulsatile liver), and even some of its jugular findings, may be absent acutely, since pulmonary pressure remains low and TR less prominent.
C. Diastolic Murmurs
(1) Diastolic Atrioventricular Valve Murmurs
203 What are the other physical findings of mitral stenosis?
 Loudness of S1: This is a very important feature, so much so that traditional teaching considers MS unlikely in patients lacking it. The reasons for the increased intensity are primarily two: a higher A-V pressure gradient (which prevents the slow juxtaposition of leaflets) and thickening of the leaflets themselves. Note that patients whose valve becomes calcified, or otherwise less mobile, may paradoxically soften S1—even though their MS is quite severe.
Loudness of S1: This is a very important feature, so much so that traditional teaching considers MS unlikely in patients lacking it. The reasons for the increased intensity are primarily two: a higher A-V pressure gradient (which prevents the slow juxtaposition of leaflets) and thickening of the leaflets themselves. Note that patients whose valve becomes calcified, or otherwise less mobile, may paradoxically soften S1—even though their MS is quite severe.
 Small apical impulse: This is due to the reduced ventricular filling of MS and is also the reason why the arterial pulse may be small in MS patients (and often irregularly irregular, given the predisposition to atrial fibrillation). Hence, a hyperkinetic or downward/laterally displaced PMI argues in favor of a concomitant regurgitant disease (aortic or mitral).
Small apical impulse: This is due to the reduced ventricular filling of MS and is also the reason why the arterial pulse may be small in MS patients (and often irregularly irregular, given the predisposition to atrial fibrillation). Hence, a hyperkinetic or downward/laterally displaced PMI argues in favor of a concomitant regurgitant disease (aortic or mitral).
 Increased central venous pressure and prominent “A” wave: This is common in MS patients with pulmonary hypertension. By the time pulmonary hypertension causes pulmonic and tricuspid regurgitation, other physical findings appear (a giant venous “V” wave, pulsatile liver, right ventricular heave, palpable P2, Graham Steell murmur, and a holosystolic TR murmur).
Increased central venous pressure and prominent “A” wave: This is common in MS patients with pulmonary hypertension. By the time pulmonary hypertension causes pulmonic and tricuspid regurgitation, other physical findings appear (a giant venous “V” wave, pulsatile liver, right ventricular heave, palpable P2, Graham Steell murmur, and a holosystolic TR murmur).
214 How frequently does tricuspid stenosis occur in patients with MS?
In as many as 5%. It is usually detected over the same area of tricuspid diastolic flow murmurs.
(2) Diastolic Semilunar Valve Murmurs
219 What are the other “valvular” causes of AR?
 Calcific aortic degeneration: This is an age-related process that may affect a congenitally bicuspid valve and result in AR, with or without stenosis. Still, only 5% of bicuspid valves develop AR. Moreover, calcific degeneration also can occur with a normal trileaflet valve.
Calcific aortic degeneration: This is an age-related process that may affect a congenitally bicuspid valve and result in AR, with or without stenosis. Still, only 5% of bicuspid valves develop AR. Moreover, calcific degeneration also can occur with a normal trileaflet valve.
 Myxomatous degeneration: This may involve either the aortic or mitral valve, where it presents as mitral valve prolapse. Unrelated to Marfan’s, it may occur in up to 15% of patients with chronic and progressive AR.
Myxomatous degeneration: This may involve either the aortic or mitral valve, where it presents as mitral valve prolapse. Unrelated to Marfan’s, it may occur in up to 15% of patients with chronic and progressive AR.
 Aging tissue prosthesis: This is usually the result of an age-related structural deterioration of a bioprosthetic valve.
Aging tissue prosthesis: This is usually the result of an age-related structural deterioration of a bioprosthetic valve.
 Ventricular septal defect (VSD): This is due to leaflet prolapse into a large VSD.
Ventricular septal defect (VSD): This is due to leaflet prolapse into a large VSD.
225 What are the “central” signs of chronic AR?
 Blood pressure: The hallmark of severe, chronic AR is a marked reduction in diastolic pressure, along with some increase in systolic values due to higher stroke volume. Hence, pulse pressure widens, often exceeding 80 mmHg. At times, determination of diastolic blood pressure may be especially difficult, since Korotkoff sounds can remain audible all the way to 0. Thus, diastolic pressure should be established at point of muffling, and not of disappearance.
Blood pressure: The hallmark of severe, chronic AR is a marked reduction in diastolic pressure, along with some increase in systolic values due to higher stroke volume. Hence, pulse pressure widens, often exceeding 80 mmHg. At times, determination of diastolic blood pressure may be especially difficult, since Korotkoff sounds can remain audible all the way to 0. Thus, diastolic pressure should be established at point of muffling, and not of disappearance.
 Arterial pulse: Typical, with brisk upstroke and downstroke, tall amplitude, and prominent carotid pulsations (“dance of the carotids”). These, in turn, may cause a pulsatile movement of head, larynx, and uvula. Abdominal pulsations may be visible in the suprarenal region.
Arterial pulse: Typical, with brisk upstroke and downstroke, tall amplitude, and prominent carotid pulsations (“dance of the carotids”). These, in turn, may cause a pulsatile movement of head, larynx, and uvula. Abdominal pulsations may be visible in the suprarenal region.
 Precordial impulse: Enlarged, sustained, and downwards/laterally displaced. This is due to the enormous left ventricular dilation that is so typical of regurgitant diseases like AR and MR (volume load). In contrast to MR, AR rarely presents with a palpable S3 (see question 245).
Precordial impulse: Enlarged, sustained, and downwards/laterally displaced. This is due to the enormous left ventricular dilation that is so typical of regurgitant diseases like AR and MR (volume load). In contrast to MR, AR rarely presents with a palpable S3 (see question 245).
229 Is there any way to separate the comitans murmur of AR from the systolic murmur of AS?
The flow murmur of AR tends to be shorter than that of AS, with louder S2 and brisker pulse.
236 What auscultatory characteristics of AR correlate with severity of regurgitation?
The major ones are length (the longer the diastolic murmur, the worse the AR) and intensity.
239 How common is this finding?
Rare in mild disease, but it can occur in more than 50% of moderate to severe AR cases.
243 Which other bedside findings correlate with severity of regurgitation?
Hill’s sign. If >60 mmHg, it argues very strongly in favor of severe regurgitation (see Chapter 2, questions 110–115). So does a diastolic blood pressure <50 mmHg and a pulse pressure >80 mmHg. Conversely, a diastolic blood pressure >70 and a pulse pressure <60 argue strongly against severe disease. Finally, absence of an enlarged or sustained apical impulse also argues against severe disease. Yet, these predictors of severity only apply to patients with chronic AR. Acute AR is very different.
246 What are the “peripheral” signs of AR?
 Increased pulse pressure with systolic hypertension
Increased pulse pressure with systolic hypertension
 Hill’s sign: Exaggerated difference in systolic pressure between the upper and lower extremities
Hill’s sign: Exaggerated difference in systolic pressure between the upper and lower extremities
 Water hammer pulse: Visible, forceful, and bounding peripheral pulses
Water hammer pulse: Visible, forceful, and bounding peripheral pulses
 Corrigan’s pulse: Quickly collapsing pulse, both visible and palpable
Corrigan’s pulse: Quickly collapsing pulse, both visible and palpable
 De Musset’s sign: Bobbing of the head. Lincoln’s sign is a variant (see Chapter 1, questions 53 and 54).
De Musset’s sign: Bobbing of the head. Lincoln’s sign is a variant (see Chapter 1, questions 53 and 54).
 Müller’s sign: Systolic pulsations of the uvula, accompanied by redness and swelling of the velum palati and tonsils. First described in 1889 by the German laryngologist Friedrich von Müller, who, together with Hamman, is actually more famous for his homonymous auscultatory sign: the crunching and rasping precordial sound that occurs in synchrony with each heartbeat in patients with spontaneous mediastinal emphysema (and that was first described by Laënnec).
Müller’s sign: Systolic pulsations of the uvula, accompanied by redness and swelling of the velum palati and tonsils. First described in 1889 by the German laryngologist Friedrich von Müller, who, together with Hamman, is actually more famous for his homonymous auscultatory sign: the crunching and rasping precordial sound that occurs in synchrony with each heartbeat in patients with spontaneous mediastinal emphysema (and that was first described by Laënnec).
 Landolfi’s sign: Systolic contraction and diastolic dilation of the pupil. Named after the Italian Michele Landolfi (1878–1959), professor of semiology at the University of Naples.
Landolfi’s sign: Systolic contraction and diastolic dilation of the pupil. Named after the Italian Michele Landolfi (1878–1959), professor of semiology at the University of Naples.
 Becker’s sign: Retinal arteries’ pulsation; also described in Graves’ disease
Becker’s sign: Retinal arteries’ pulsation; also described in Graves’ disease
 Oliver-Cardarelli sign: Pulsation of the larynx synchronous with ventricular systole. It is elicited by grasping the cricoid cartilage between index finger and thumb while the patient is sitting up with the chin fully uplifted. By applying a gentle upward pressure on the larynx, one would feel a tracheal tug in cases of brisk aortic ejection—as, for example, in patients with AR. More typically, however, the tracheal tug would suggest an aneurysm of the aortic arch—also possibly associated with aortic regurgitation. Yet, the finding also can be due to a mediastinal tumor or simple chronic obstructive lung disease (see Chapter 13, questions 141–144).
Oliver-Cardarelli sign: Pulsation of the larynx synchronous with ventricular systole. It is elicited by grasping the cricoid cartilage between index finger and thumb while the patient is sitting up with the chin fully uplifted. By applying a gentle upward pressure on the larynx, one would feel a tracheal tug in cases of brisk aortic ejection—as, for example, in patients with AR. More typically, however, the tracheal tug would suggest an aneurysm of the aortic arch—also possibly associated with aortic regurgitation. Yet, the finding also can be due to a mediastinal tumor or simple chronic obstructive lung disease (see Chapter 13, questions 141–144).
All these visible (and palpable) signs are enhanced by exercise, as a result of increased pulse pressure. In addition, there are two more peripheral findings that are instead exclusively auscultatory (see Chapter 10, questions 33–36): (1) Traube pistol shot sound(s) and (2) Duroziez double murmur. Both have sensitivity of 37–55% and specificity of 63–98% for AR. Neither predicts severity.
D. Continuous Murmurs
260 If continous murmurs are extracardiac, what conditions are responsible for them?
 Abnormal communication between high-pressure and low-pressure vascular beds. Hence, the continuous flow, such as in patent ductus arteriosus (PDA) or in simple arteriovenous fistulas.
Abnormal communication between high-pressure and low-pressure vascular beds. Hence, the continuous flow, such as in patent ductus arteriosus (PDA) or in simple arteriovenous fistulas.
 Abnormally increased vascular flow. The latter can occur in either veins (venous hum) or arteries (like the mammaries of breast-feeding women or the intercostals of coarctation).
Abnormally increased vascular flow. The latter can occur in either veins (venous hum) or arteries (like the mammaries of breast-feeding women or the intercostals of coarctation).
261 Why are these murmurs continuous?
Because their pressure gradient persists throughout systole and diastole, thus causing a no-stop flow that is always turbulent and noisy. In PDA, this never-ending stream of high-pressure/high-oxygen blood between the aorta and pulmonary artery eventually leads to pulmonary hypertension. This, in turn, gradually decreases the pressure gradient between the two vessels, eventually fading the diastolic component of the murmur. After reversal of the shunt, the murmur will cease altogether, while the patient becomes cyanotic and findings of severe pulmonary hypertension ensue. This is the Eisenmenger’s syndrome (i.e., pulmonary hypertension from an initial left-to-right shunt that has evolved into shunt reversal and cyanosis). First described by the homonymous Austrian physician (1864–1932), Eisenmenger’s from PDA is often associated with clubbing, which, like cyanosis, involves the feet but not the hands since the reversed shunt only affects the dependent portion of the aorta (see “differential clubbing,” question 105 in Chapter 13).
E. Systolic-Diastolic Murmurs/Sounds
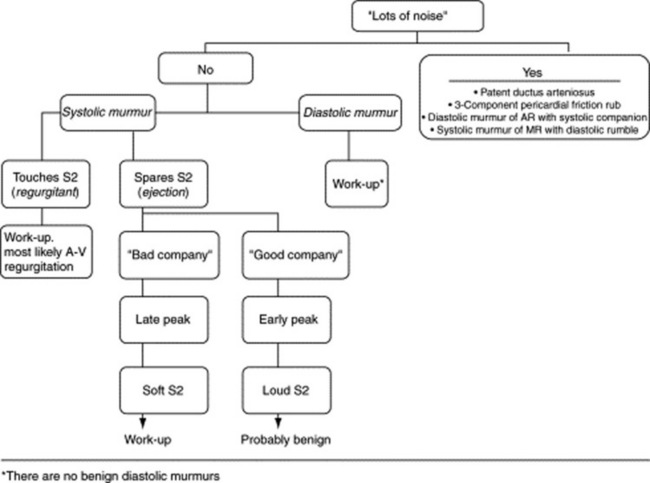
263 What is the differential diagnosis of “lots of noise” throughout the cardiac cycle?
“Noise” throughout systole and diastole usually reflects four possible processes (see Fig. 12-3):
 The to-and-fro murmur of AR with its companion systolic flow murmur (or, alternatively, a combined murmur of aortic regurgitation and stenosis)
The to-and-fro murmur of AR with its companion systolic flow murmur (or, alternatively, a combined murmur of aortic regurgitation and stenosis)
 A three-component pericardial friction rub (see Chapter 11, questions 164–174)
A three-component pericardial friction rub (see Chapter 11, questions 164–174)
 Less commonly, “lots of noise” may indicate a holosystolic murmur of severe MR accompanied by its high-flow diastolic rumble.
Less commonly, “lots of noise” may indicate a holosystolic murmur of severe MR accompanied by its high-flow diastolic rumble.
1 Aronow WS, Kronzon I. Correlation of prevalence and severity of aortic regurgitation detected by pulsed Doppler echocardiography with the murmur of aortic regurgitation in elderly patients in a long-term health care facility. Am J Cardiol. 1989;63:128-129.
2 Barlow JB, Bosman CK, Pocock WA, et al. Late systolic murmurs and nonejection (“mid-late”) systolic clicks: An analysis of 90 patients. Br Heart J. 1968;30:203-218.
3 Burch GE, Phillips JH. Murmurs of aortic stenosis and mitral insufficiency masquerading as one another. Am Heart J. 1963;66:439-442.
4 Cha SD, Gooch AS. Diagnosis of tricuspid regurgitation. Arch Intern Med. 1983;143:1763-1768.
5 Cha SD, Gooch AS, Maranhao V. Intracardiac phonocardiography in tricuspid regurgitation: Relation to clinical and angiographic findings. Am J Cardiol. 1981;48:578-583.
6 Chun PKC, Dunn BE. Clinical clue of severe aortic stenosis: Simultaneous palpation of the carotid and apical impulses. Arch Intern Med. 1982;142:2284-2288.
7 Cohn KE, Hultgren HN. The Graham Steell murmur re-evaluated. N Engl J Med. 1966;274:486-489.
8 Constant J, Lippschutz EJ. Diagramming and grading heart sounds and murmurs. Am Heart J. 1965;70:326-332.
9 Danielsen R, Nordrehaug JE, Vik-Mo H. Clinical and haemodynamic features in relation to severity of aortic stenosis in adults. Eur Heart J. 1991;12:791-795.
10 Dennison AD. Aortic regurgitation: Multiple eponyms, physical signs and etiologies. J Ind State Med Assoc. 1959;52:1283-1289.
11 DePace NL, Nestico PF, Morganroth J. Acute severe mitral regurgitation: Pathophysiology, clinical recognition, and management. Am J Med. 1985;78:293-306.
12 Desjardins VA, Enriquez-Sarano M, Tajik AJ, et al. Intensity of murmurs correlates with severity of valvular regurgitation. Am J Med. 1996;100:149-156.
13 Devereux RB, Perloff JK, Reichek R, et al. Mitral valve prolapse. Circulation. 1976;54:3-14.
14 Emi S, Fukuda N, Oki T, et al. Genesis of the Austin Flint murmur: Relation to mitral inflow and aortic regurgitant flow dynamics. J Am Coll Cardiol. 1993;21:1399-1405.
15 Etchells E, Glenns V, Shadowitz S, et al. A bedside clinical prediction rule for detecting moderate or severe aortic stenosis. J Gen Intern Med. 1998;13:699-704.
16 Etchells E, Bell C, Robb K. Does this patient have an abnormal systolic murmur? JAMA. 1997;277:564-571.
17 Folland ED, Kriegel BJ, Henderson WG, et al. Implications of third heart sounds in patients with valvular heart disease. The Veterans Affairs Cooperative Study on Valvular Heart Disease. N Engl J Med. 1992;327:458-462.
18 Fontana ME, Wooley CF, Leighton RF, et al. Postural changes in left ventricular and mitral valvular dynamics in systolic click-late systolic murmur syndrome. Circulation. 1975;51:165-173.
19 Forssell G, Jonasson R, Orinius E. Identifying severe aortic valvular stenosis by bedside examination. Acta Med Scand. 1985;218:397-400.
20 Frank S, Braunwald E. Idiopathic hypertrophic subaortic stenosis: Clinical analysis of 126 patients with emphasis on the natural history. Circulation. 1968;37:759-788.
21 Freeman AR, Levine SA. The clinical significance of the systolic murmur: A study of 1000 consecutive “noncardiac” cases. Ann Intern Med. 1933;6:1371-1385.
22 Grayburn PA, Smith MD, Handshoe R, et al. Detection of aortic insufficiency by standard echocardiography, pulsed Doppler echocardiography, and auscultation: A comparison of accuracies. Ann Intern Med. 1986;104:599-605.
23 Henein MY, Xiao HB, Brecker SJ, et al. Bernheim “a” wave: Obstructed right ventricular inflow or atrial cross talk? Br Heart J. 1993;69:409-413.
24 Leach RM, McBrien DJ. Brachioradial delay: A new clinical indicator of the severity of aortic stenosis. Lancet. 1990;335:1199-1201.
25 Lembo NJ, Dell’Italia LJ, Crawford MH, et al. Bedside diagnosis of systolic murmurs. N Engl J Med. 1988;318:1572-1578.
26 McCraw DB, Siegel W, Stonecipher HK, et al. Response of heart murmur intensity to isometric (handgrip) exercise. Br Heart J. 1972;34:605-610.
27 McGee S. Evidence-Based Physical Diagnosis. Philadelphia: WB Saunders, 2001.
28 Nellen M, Gotsman MS, Vogelpoel L, et al. Effects of prompt squatting on the systolic murmur in idiopathic hypertrophic obstructive cardiomyopathy. Br Med J. 1967;3:140-143.
29 Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142-147.
30 Otto CM. Clinical practice: Evaluation and management of chronic mitral regurgitation. N Engl J Med. 2001;345:740-746.
31 Otto CM. Valvular aortic stenosis: Which measure of severity is best? Am Heart J. 1998;136:940-942.
32 Perloff JK. Auscultatory and phonocardiographic manifestations of pulmonary hypertension. Prog Cardiovasc Dis. 1967;9:303-340.
33 Perloff JK. Clinical recognition of aortic stenosis: The physical signs and differential diagnosis of the various forms of obstruction to left ventricular outflow. Prog Cardiovasc Dis. 1968;10:323-352.
34 Roldan CA, Shively BK, Crawford MH. Value of the cardiovascular physical examination for detecting valvular heart disease in asymptomatic subjects. Am J Cardiol. 1996;77:1327-1331.
35 Sutton GC, Craige E. Clinical signs of severe acute mitral regurgitation. Am J Cardiol. 1967;20:141-144.