Chapter 24
Heart Failure with Preserved Ejection Fraction
1. What is diastolic dysfunction?
2. What is diastolic heart failure?
3. What is the prevalence of HFpEF?
4. What is the morbidity and mortality associated with HFpEF compared with heart failure with reduced ejection fraction?
5. Which patients are at the highest risk for developing HFpEF?
Patients with HFpEF are generally elderly and are predominantly women (60% to 70%). Reasons for the female predominance in HFpEF are not entirely clear but may be related to the fact that women have a greater tendency for the left ventricle to hypertrophy in response to load, and lesser predisposition for the ventricle to dilate. Hypertension is the most common cardiac condition associated with HFpEF. Hypertensive heart disease results in LV hypertrophy with resultant impairment in relaxation and increase in LV stiffness. Acute myocardial ischemia results in diastolic dysfunction, although its role in chronic diastolic dysfunction and chronic HFpEF remains uncertain. Valvular heart diseases, including regurgitant and stenotic aortic and mitral valve disease, can also result in the development of HFpEF. Other recognized risk factors associated with HFpEF include obesity, diabetes mellitus, and renal insufficiency. Onset of atrial fibrillation with rapid ventricular rate may precipitate decompensation of HFpEF, and the presence of diastolic dysfunction in general is also a risk factor for the development of this arrhythmia.
6. What are proposed pathophysiologic mechanisms of HFpEF?
7. What factors may precipitate decompensated HFpEF?
In patients with underlying diastolic dysfunction and other abnormalities detailed in Question 6, acute decompensation of heart failure may often be contributed to by uncontrolled hypertension, atrial fibrillation or flutter (especially with rapid ventricular rates), myocardial ischemia, hyperthyroidism, medication noncompliance (especially diuretics and antihypertensives), dietary indiscretion (e.g., high-sodium foods), anemia, and infection.
8. How is the diagnosis of HFpEF made?
9. What common tests are useful in the diagnosis of HFpEF, and what do they often reveal?
 Chest radiographs may demonstrate cardiomegaly (as a result of hypertrophy), pulmonary venous congestion, pulmonary edema, or pleural effusions.
Chest radiographs may demonstrate cardiomegaly (as a result of hypertrophy), pulmonary venous congestion, pulmonary edema, or pleural effusions.
 The electrocardiogram (ECG) may demonstrate hypertrophy, ischemia, or arrhythmia.
The electrocardiogram (ECG) may demonstrate hypertrophy, ischemia, or arrhythmia.
 Echocardiography can be used to assess ventricular function; atrial and ventricular size; hypertrophy; diastolic function and filling pressures (see Question 11); wall motion abnormalities; and pericardial, valvular, or myocardial (hypertrophic or infiltrative) disease. By definition, the LVEF is normal or near normal. Echocardiography often demonstrates LV hypertrophy, enlarged left atrium, diastolic dysfunction, and pulmonary hypertension. Left ventricular volumes are usually normal or even small in HFpEF. Valvular heart disease, such as significant aortic or mitral stenosis and/or regurgitation, can lead to a presentation of HFpEF but needs to be differentiated, as management often requires surgical intervention for the valvular pathology.
Echocardiography can be used to assess ventricular function; atrial and ventricular size; hypertrophy; diastolic function and filling pressures (see Question 11); wall motion abnormalities; and pericardial, valvular, or myocardial (hypertrophic or infiltrative) disease. By definition, the LVEF is normal or near normal. Echocardiography often demonstrates LV hypertrophy, enlarged left atrium, diastolic dysfunction, and pulmonary hypertension. Left ventricular volumes are usually normal or even small in HFpEF. Valvular heart disease, such as significant aortic or mitral stenosis and/or regurgitation, can lead to a presentation of HFpEF but needs to be differentiated, as management often requires surgical intervention for the valvular pathology.
 Stress testing and coronary angiography can identify contributory coronary artery disease.
Stress testing and coronary angiography can identify contributory coronary artery disease.
 Routine laboratory analysis can help identify renal failure or anemia as factors associated with decompensation, electrolyte abnormalities such as hyponatremia seen with heart failure, and transaminase or bilirubin elevation resulting from hepatic congestion. In addition, thyroid function tests can rule out hyperthyroidism (a consideration particularly in patients who develop atrial fibrillation). Studies have shown that B-type natriuretic peptide (BNP) and N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) levels are elevated in HFpEF patients compared with persons without heart failure. However, BNP and NT-proBNP levels in HFpEF patients are usually lower compared with systolic heart failure patients. Of note, increased BNP levels may identify patients with elevation of the LV diastolic pressure, but may not be clinically useful in individual patients to predict preserved versus reduced LVEF. Also, it should be kept in mind that BNP levels increase with age and are higher in women, both of which are common features of HFpEF. Levels of BNP are also higher with worsening renal insufficiency. On the other hand, obesity is associated with lower levels of BNP, making the diagnosis of HFpEF more difficult in this group of patients.
Routine laboratory analysis can help identify renal failure or anemia as factors associated with decompensation, electrolyte abnormalities such as hyponatremia seen with heart failure, and transaminase or bilirubin elevation resulting from hepatic congestion. In addition, thyroid function tests can rule out hyperthyroidism (a consideration particularly in patients who develop atrial fibrillation). Studies have shown that B-type natriuretic peptide (BNP) and N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) levels are elevated in HFpEF patients compared with persons without heart failure. However, BNP and NT-proBNP levels in HFpEF patients are usually lower compared with systolic heart failure patients. Of note, increased BNP levels may identify patients with elevation of the LV diastolic pressure, but may not be clinically useful in individual patients to predict preserved versus reduced LVEF. Also, it should be kept in mind that BNP levels increase with age and are higher in women, both of which are common features of HFpEF. Levels of BNP are also higher with worsening renal insufficiency. On the other hand, obesity is associated with lower levels of BNP, making the diagnosis of HFpEF more difficult in this group of patients.
10. What is the clinical approach to further evaluate patients with HFpEF?
A diagnostic algorithm, based on the 2006 Heart Failure Society of America Heart Failure Practice Guidelines, provides a systematic approach for the clinical workup and classification of HFpEF (Fig. 24-1). This framework addresses the common clinical conditions presenting as HFpEF, including hypertensive heart disease, hypertrophic cardiomyopathy, ischemic heart failure, valvular heart disease, infiltrative (restrictive) cardiomyopathy, pericardial constriction, high-cardiac-output state, and right ventricular dysfunction.
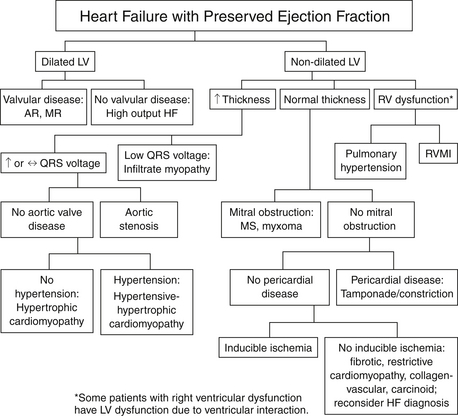
Figure 24-1 Diagnostic considerations in patients with heart failure with preserved ejection fraction. (From Adams, KF, Lindenfeld J, Arnold JMO, et al: Executive summary: HFSA 2006 Comprehensive heart failure practice guideline. J Cardiac Failure 12:10-38, 2006.) AR, Aortic regurgitation; HF, heart failure; LV, left ventricle; MR, mitral regurgitation; MS, mitral stenosis; RV, right ventricle; RVMI, right ventricular myocardial infarction.
11. What tests are available to evaluate diastolic function?
Cardiac catheterization with a high-fidelity pressure manometer allows for precise intracardiac pressure measurements. This information can be used to estimate the rate of LV relaxation by calculation of indices such as peak instantaneous LV pressure decline (-dP/dt max) and the time constant of LV relaxation, tau. Estimation of LV myocardial stiffness requires simultaneous assessment of LV volume and pressure to evaluate the end-diastolic pressure-volume relationship. However, these measurements are invasive and cannot be made on a routine basis. Therefore, noninvasive markers of diastolic dysfunction are more commonly used in clinical practice.
Doppler measurements of mitral and pulmonary venous flow, as well as Doppler tissue imaging (DTI), allow for determination of ventricular and atrial filling patterns and estimation of LV diastolic filling pressures. The normal transmitral filling pattern consists of early rapid filling (E wave) and atrial contraction (A wave). The contribution of each of these stages of diastole is expressed as the E/A ratio. Mitral annular tissue Doppler velocities (which measure tissue velocities rather than the conventional Doppler, which measures blood flow velocities) are relatively independent of preload conditions. As such, the early diastolic filling annular tissue velocity (E′) is a marker of LV relaxation and correlates well with hemodynamic catheter-derived values of tau. The ratio of the transmitral early filling velocity to the annular DTI early filling velocity (E/E′) has been shown to accurately estimate mean LA pressure. Using these various echocardiographic parameters, the severity of diastolic dysfunction and of elevated LV diastolic pressures can be assessed. These issues are also discussed in Chapter 5 on echocardiography.
12. How do you treat acutely decompensated HFpEF?
13. How do you treat patients with chronic HFpEF?
Large clinical trials have not shown consistent improvement in morbidity or mortality with ACE inhibitors and/or angiotensin receptor blockers (ARBs), or with digoxin. Large trials evaluating β-blockers specifically in HFpEF are not available. A large trial examining the efficacy of spironolactone, an aldosterone receptor blocker, in patients with HFpEF is ongoing. Current therapeutic recommendations for HFpEF are aimed at symptom management and treatment of concomitant comorbidities, as outlined in Box 24-1.
Bibliography, Suggested Readings, and Websites
1. Ahmed, A., Rich, M.W., Fleg, J.L., et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403.
2. Ather, S., Chan, W., Bozkurt, B., et al. Impact of non-cardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1006.
3. Borlaug, B.A., Paulus, W.J. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679.
4. Cleland, J.G.F., Tenderar, M., Adamus, J., et al. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345.
5. Deswal, A. Treatment of heart failure with a positive ejection fraction. In: Mann D.L., ed. Heart failure: a companion to Braunwald’s Heart Disease. St Louis: Saunders, 2011.
6. Hogg, K., Swedberg, K., McMurray, J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327.
7. Lindenfeld, J., Albert, N.M., Boehmer, J.P., et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e126–e133.
8. Massie, B.M., Carson, P.E., McMurray, J.J., et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467.
9. Paulus, W.J., Tschöpe, C., Sanderson, J.E., et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550.
10. Yusuf, S., Pfeffer, M.A., Swedberg, K., et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781.
11. Zile, M.R., Baicu, C.F. 2011 Alterations in ventricular function: diastolic heart failure. In: Mann D.L., ed. Heart failure: a companion to Braunwald’s Heart Disease. St Louis: Saunders, 2011.