Chapter 436 Heart Failure
Heart failure occurs when the heart cannot deliver adequate cardiac output to meet the metabolic needs of the body. In the early stages of heart failure, various compensatory mechanisms are evoked to maintain normal metabolic function. When these mechanisms become ineffective, increasingly severe clinical manifestations result (Chapter 64).
Pathophysiology
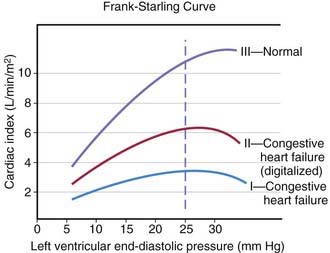
The heart can be viewed as a pump with an output proportional to its filling volume and inversely proportional to the resistance against which it pumps. As ventricular end-diastolic volume increases, a healthy heart increases cardiac output until a maximum is reached and cardiac output can no longer be augmented (the Frank-Starling principle; Fig. 436-1). The increased stroke volume obtained in this manner is due to stretching of myocardial fibers, but it also results in increased wall tension, which elevates myocardial oxygen consumption. Hearts working under various types of stress function along different Frank-Starling curves. Cardiac muscle with compromised intrinsic contractility requires a greater degree of dilatation to produce increased stroke volume and does not achieve the same maximal cardiac output as normal myocardium does. If a cardiac chamber is already dilated because of a lesion causing increased preload (e.g., a left-to-right shunt or valvular insufficiency), there is little room for further dilatation as a means of augmenting cardiac output. The presence of lesions that result in increased afterload to the ventricle (aortic or pulmonic stenosis, coarctation of the aorta) decreases cardiac performance, thereby resulting in a depressed Frank-Starling relationship. The ability of an immature heart to increase cardiac output in response to increased preload is less than that of a mature heart. Thus, premature infants are more compromised by a left-to-right shunt than full-term infants are.
Clinical Manifestations
The clinical manifestations of heart failure depend on the degree of the child’s cardiac reserve. A critically ill infant or child who has exhausted the compensatory mechanisms to the point that cardiac output is no longer sufficient to meet the basal metabolic needs of the body will be symptomatic at rest. Other patients may be comfortable when quiet but are incapable of increasing cardiac output in response to even mild activity without experiencing significant symptoms. Conversely, it may take rather vigorous exercise to compromise cardiac function in children who have less severe heart disease. A thorough history is extremely important in making the diagnosis of heart failure and in evaluating the possible causes. Parents who observe their child on a daily basis may not recognize subtle changes that have occurred over the course of days or weeks. Gradually worsening perfusion or increasing respiratory effort may not be recognized as an abnormal finding. Edema may be passed off as normal weight gain, and exercise intolerance as lack of interest in an activity. The history of a young infant should also focus on feeding (Chapter 416). An infant with heart failure often takes less volume per feeding, becomes dyspneic while sucking, and may perspire profusely. Eliciting a history of fatigue in an older child requires detailed questions about activity level and its course over several months.
In infants, heart failure may be difficult to distinguish from other causes of respiratory distress. Prominent manifestations include tachypnea, feeding difficulties, poor weight gain, excessive perspiration, irritability, weak cry, and noisy, labored respirations with intercostal and subcostal retractions, as well as flaring of the alae nasi. The signs of cardiac-induced pulmonary congestion may be indistinguishable from those of bronchiolitis; wheezing is often a more prominent finding in young infants with heart failure than rales. Pneumonitis with or without atelectasis is common, especially in the right middle and lower lobes, due to bronchial compression by the enlarged heart. Hepatomegaly usually occurs, and cardiomegaly is invariably present. In spite of pronounced tachycardia, a gallop rhythm can frequently be recognized. The other auscultatory signs are those produced by the underlying cardiac lesion. Clinical assessment of jugular venous pressure in infants may be difficult because of the shortness of the neck and the difficulty of observing a relaxed state; palpation of an enlarged liver is a more reliable sign. Edema may be generalized and usually involves the eyelids as well as the sacrum and less often the legs and feet. The differential diagnosis is age dependent (Table 436-1).
Table 436-1 ETIOLOGY OF HEART FAILURE
FETAL
PREMATURE NEONATE
FULL-TERM NEONATE
INFANT-TODDLER
CHILD-ADOLESCENT
Treatment
Diuretics
Furosemide is the most commonly used diuretic in pediatric patients with heart failure. It inhibits the reabsorption of sodium and chloride in the distal tubules and the loop of Henle. Patients requiring acute diuresis should be given intravenous or intramuscular furosemide at an initial dose of 1-2 mg/kg, which usually results in rapid diuresis and prompt improvement in clinical status, particularly if symptoms of pulmonary congestion are present. Chronic furosemide therapy is then prescribed at a dose of 1-4 mg/kg/24 hr given between 1 and 4 times a day. Careful monitoring of electrolytes is necessary with long-term furosemide therapy because of the potential for significant loss of potassium. Potassium chloride supplementation is usually required unless the potassium-sparing diuretic spironolactone is given concomitantly. Chronic administration of furosemide may cause contraction of the extracellular fluid compartment and result in “contraction alkalosis” (Chapter 52.7). Diuretic-induced hyponatremia may become difficult to manage in patients with severe heart failure.
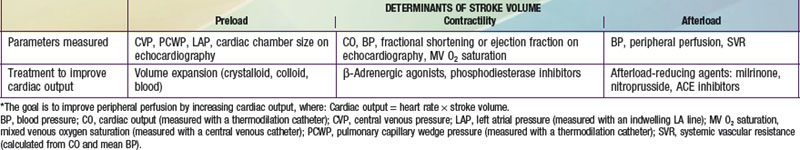
Afterload Reducers, Including Angiotensin-Converting Enzyme Inhibitors (ACEIs) and Angiotensin II Receptor Blockers (ARBs)
Digitalis Glycosides
Rapid digitalization of infants and children in heart failure may be carried out intravenously. The dose depends on the patient’s age (Table 436-2). The recommended digitalization schedule is to give half the total digitalizing dose immediately and the succeeding 2 one-quarter doses at 12-hr intervals later. The electrocardiogram must be closely monitored and rhythm strips obtained before each of the 3 digitalizing doses. Digoxin should be discontinued if a new rhythm disturbance is noted. Prolongation of the P-R interval is not necessarily an indication to withhold digitalis, but a delay in administering the next dose or a reduction in the dosage should be considered, depending on the patient’s clinical status. Minor ST segment or T-wave changes are commonly noted with digitalis administration and should not affect the digitalization regimen. Baseline serum electrolyte levels should be measured before and after digitalization. Hypokalemia and hypercalcemia exacerbate digitalis toxicity. Because hypokalemia is relatively common in patients receiving diuretics, potassium levels should be monitored closely in those receiving a potassium-wasting diuretic in combination with digitalis. In patients with active myocarditis, some cardiologists recommend avoiding digitalis altogether and if used, maintenance digitalis should be started at half the normal dose without digitalization due to the increased risk of arrhythmia in these patients.
Table 436-2 DOSAGE OF DRUGS COMMONLY USED FOR THE TREATMENT OF CONGESTIVE HEART FAILURE
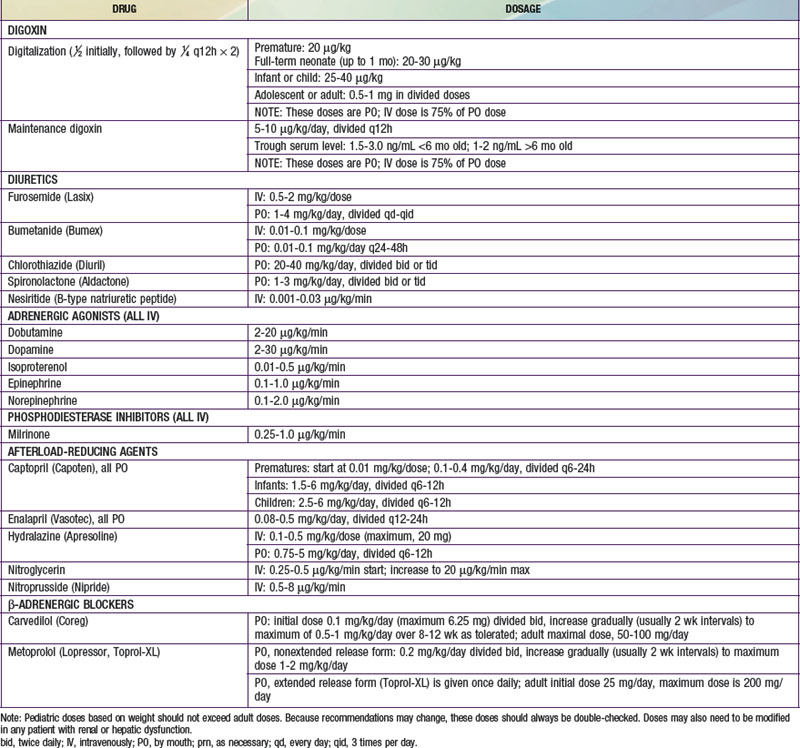
Maintenance digitalis therapy is started ≈12 hr after full digitalization. The daily dosage, one quarter of the total digitalizing dose, is divided in 2 and given at 12-hr intervals. The oral maintenance dose is usually 20-25% higher than when digoxin is used parenterally (see Table 436-2). The normal daily dose of digoxin for older children (>5 yr of age) calculated by body weight should not exceed the usual adult dose of 0.125-0.5 mg/24 hr.
Electrophysiologic Approaches to Heart Failure Management
One of the leading causes of sudden death in patients with severe cardiomyopathy (both dilated and hypertrophic) is arrhythmia. Although antiarrhythmic medications can sometimes reduce this risk, for patients at particularly high risk (e.g., those with a condition known to be associated with a high risk of ventricular arrhythmia or those who have already experienced a “missed sudden death” episode), use of an implantable cardioverter-defibrillator (ICD) can be lifesaving (Chapter 429).
436.1 Cardiogenic Shock
Cardiogenic shock (Chapter 64) may occur as a complication of (1) severe cardiac dysfunction before or after cardiac surgery, (2) septicemia, (3) severe burns, (4) anaphylaxis, (5) cardiomyopathy, (6) myocarditis, (7) myocardial infarction or stunning, and (8) acute central nervous system disorders. It is characterized by low cardiac output and hypotension and therefore results in inadequate tissue perfusion.
Sequential evaluation and management of cardiovascular shock are mandatory (Chapter 64). Table 436-3 outlines the general treatment principles for acute cardiac circulatory failure under most circumstances. Treatment of infants and children with low cardiac output after cardiac surgery also depends on the nature of the operative procedure, any intraoperative complications, and the physiology of the circulation after repair or palliation (Chapter 428).
Patients with deteriorating cardiogenic shock may benefit from a left ventricular assist device (LVAD). These devices have been used successfully in children and adolescents as a bridge to cardiac transplantation. Advances have produced devices small enough to support young infants. In some cases, both right and left ventricular assist is necessary (BiVAD). Once implanted, assist devices allow patients to recover sufficiently to be extubated, become ambulatory, and often leave the intensive care setting while awaiting transplantation. However, the morbidity from these devices is not insubstantial, especially in infants, and therefore the benefits and risks of their use should be carefully weighed for each individual patient. Patients with reversible ventricular failure, for example, those in the immediate postoperative state or those with acute myocarditis, may also benefit from extracorporeal membrane oxygenation (ECMO). When ventricular function is not expected to recover, heart transplantation should be considered (Chapter 437.1).
Anker SD, Colet JC, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436-2448.
Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105.
Bristow M. Antiadrenergic therapy of chronic heart failure: surprises and new opportunities. Circulation. 2003;107:1100-1102.
Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30:2102-2108.
Dubin AM, Berul CI. Electrophysiological interventions for treatment of congestive heart failure in pediatrics and congenital heart disease. Expert Rev Cardiovasc Ther. 2007;5:111-118.
Dubin AM, Berul CI, Bevilacqua LM, et al. The use of implantable cardioverter-defibrillators in pediatric patients awaiting heart transplantation. J Card Fail. 2003;9:375-379.
Fried I, Bar-Oz B, Perles Z, et al. N-Terminal pro-B-type natriuretic peptide levels in acute versus chronic left ventricular dysfunction. J Pediatr. 2006;149:28-31.
Gheorghiade M, Braunwald E. Reconsidering the role of digoxin in the management of acute heart failure syndromes. JAMA. 2009;302:2146-2147.
Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996-1002.
Kaufman BD, Shaddy RE, Shirali GS, et al. Assessment and management of the failing heart in children. Cardiol Young. 2008;18(Suppl 3):63-71.
Krum H, Abraham WT. Heart failure. Lancet. 2009;373:941-952.
Law YM, Hoyer AW, Reller MD, et al. Accuracy of plasma B-type natriuretic peptide to diagnose significant cardiovascular disease in children. J Am Coll Cardiol. 2009;54:1467-1475.
Liggett SB. Polymorphisms of beta-adrenergic receptors in heart failure. Am J Med. 2004;117:525-527.
Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-166.
McMurphy JJV. Heart failure. Lancet. 2005;365:1877-1889.
Neubauer S. The failing heart: an engine out of fuel. N Engl J Med. 2007;356:1140-1150.
Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33:887-905.
Rosenthal D, Chrisant MR, Edens E, et al. International Society for Heart and Lung Transplantation: practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23:1313-1333.
Shaddy RE. Optimizing treatment for chronic congestive heart failure in children. Crit Care Med. 2001;29(Suppl):237-240.
Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure. JAMA. 2007;298:1171-1179.
Trani JF. Optimizing management of chronic heart failure. Lancet. 2009;374:1808-1809.
Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.