Heart
Angiocardiography
Indications
1. Congenital heart disease and anomalies of the great vessels – mostly in the paediatric population. Either for diagnosis or therapeutic procedures such as transcatheter closure of patent foramen ovale, atrial septal defect (ASD), ventricular septal defect (VSD) and patent ductus arteriosus.
2. Valve disease, myocardial disease and assess ventricular function. Balloon valvuloplasty and, in selected centres, transcatheter aortic valve implantation (TAVI) can be performed to treat stenotic valvular disease.
Equipment
1. Biplane digital fluoroscopy and cine radiography with C-arms to facilitate axial projections
Technique
1. Right-sided cardiac structures and pulmonary arteries are examined by introducing a catheter into a peripheral vein. In babies the femoral vein may be the only vein large enough to take the catheter. If an atrial septal defect is suspected, the femoral vein approach offers the best chance of passing the catheter into the left atrium across the defect. In adults the right antecubital or basilic vein may be used. The cephalic vein should be avoided as it can be difficult to pass the catheter past the site where the vein pierces the clavipectoral fascia to join the axillary vein. The catheter, or introducer, is introduced using the Seldinger technique. (The NIH catheter must be introduced via an introducer as there is no end hole for a guidewire.)
2. In children it is usually possible to examine the left heart and occasionally the aorta by manipulating a venous catheter through a patent foramen ovale. In adults the aorta and left ventricle are studied via a catheter passed retrogradely from the femoral artery.
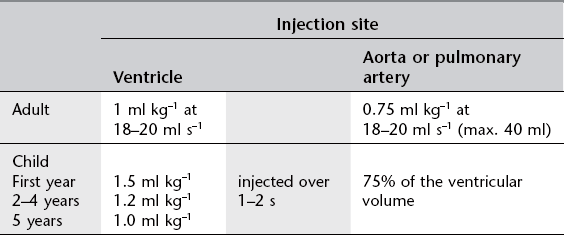
3. The catheter is manipulated into the appropriate positions for recording pressures and sampling blood for oxygen saturation. Following this, angiography is performed.
Image acquisition
1. 40° cranial/40° left anterior oblique (LAO) (hepatoclavicular or four-chamber) view – the beam is perpendicular to the long axis of the heart and aligns the atrial septum and posterior interventricular septum parallel to the beam
2. Long axial 20° right anterior oblique (RAO) (long axial oblique) view – the lateral tube and image intensifier are angled 25–30° cranially, to align with the long axis of the heart, and 20° RAO.
Coronary arteriography
Indications
1. Diagnosis of the presence and extent of ischaemic heart disease
2. After revascularization procedures
4. Therapeutic percutaneous coronary intervention – balloon angioplasty and stenting.
Diagnostic arteriography can be supplemented by intravascular ultrasound (US) to determine the nature and extent of plaque within the vessel wall and, in some centres, with angioscopy. Pressure wire studies to determine fractional flow reduction (FFR) across stenoses prior to angioplasty/stenting can be performed; particularly useful in assessment of intermediate lesions.
Equipment
1. Digital angiography with C-arm
2. Pressure recording device and ECG monitor
3. Selective coronary artery catheters:
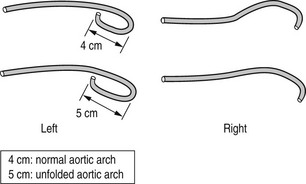
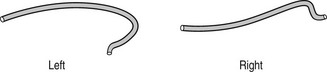
(a) Judkins (Fig. 8.4) or Amplatz (Fig. 8.5) catheters – the left and right coronary artery catheters are of different shape. These can be used for both femoral or radial approaches (usually utilizing smaller Judkins for the left coronary artery)
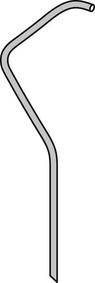
(b) Tiger II catheter (Fig. 8.6) – specifically designed for right radial approach. Single catheter used for both left and right coronary arteries (reduces procedure time, radiation exposure and less manipulation leading to less radial artery spasm)
(c) For assessment of grafts: Judkins, Simmons and others (for both femoral and radial approaches).
Image acquisition
Angiography (7.5 frames s–1) is performed in the following projections:
(* though each projection will show good visualization of various segments of the other coronary artery).
Complications
In addition to the general complications discussed in Chapter 9, patients undergoing coronary arteriography may be liable to:
1. Arrhythmias – such as non-sustained atrial fibrillation with right heart catheterization or non-sustained ventricular arrhythmia with left ventriculography
2. Ostial dissection by the catheter
3. Access site complications (more commonly seen with femoral approach) – local haematoma, femoral artery pseudoaneurysm or arterio-venous fistula.
Cardiac CT
Indications
1. Patients with known or suspected chronic coronary artery disease
2. Assessment of suspected anomalous coronary artery anatomy
3. As a screening test in symptomatic patients with typical chest pain but a low clinical probability of significant coronary artery disease
4. As a screening test in asymptomatic but high-risk patients or patients with atypical chest pain
5. Assessment of coronary artery grafts, including left internal mammary artery (LIMA) grafts
6. As an alternative to diagnostic coronary angiography when planning percutaneous angiographic intervention or as post-procedure follow-up.
7. Assessment of left atrium and pulmonary vein anatomy prior to electrophysiological studies and ablation.
Contraindications
Patient preparation
1. Avoid caffeine – this includes tea, coffee and caffeine-containing soft drinks (including ‘decaffeinated’ varieties), ‘energy’ pills and Viagra-type medication.
2. Drugs – patients should take all their normal cardiac medication as usual on the day of the test.
3. Thorough explanation of the procedure to the patient aids compliance, particularly with regard to breath-hold (suggest patient takes a ‘ breath’ when instructed – less likely to move during scan and avoids ‘Valsalva’ manoeuvre affecting contrast bolus entering chest).
breath’ when instructed – less likely to move during scan and avoids ‘Valsalva’ manoeuvre affecting contrast bolus entering chest).
4. 18G cannula in right antecubital fossa – siting cannula in right arm reduces artefact as contrast crosses the mediastinum. For graft studies this avoids streak artefact obscuring origin of LIMA grafts.
5. Assess suitability for administration of β-blockers and sublingual nitrates (see below).
Beta-blockers
• The patient may already be on oral β-blockers (either as regular medication or specifically prescribed by the referrer for the purposes of the examination). In most cases the scan can proceed without further medication.
• If the patient is not on β-blockers, requires heart rate reduction to optimize scan and has no contraindication to β-blocker administration, metoprolol can be given orally or i.v.
(Contraindications to β-blockade include heart failure, significant aortic stenosis, heart block, asthma/COPD, use of other antiarrhythmic medication including calcium channel blockers and digoxin.)
• Metoprolol 100 mg orally 1 h prior to scan. Pulse and blood pressure monitored regularly until heart rate reaches desired level. (If the heart rate does not reach a satisfactory level additional metoprolol can be given intravenously whilst the patient is on the scanner.)
• Metoprolol can be given solely in i.v. form (or to ‘top up’ oral dose where optimal heart rate has not been achieved with oral administration) just before the start of the scan with the patient on the scanner table. 5–30 mg i.v. given in aliquots of 5 mg titrated against heart rate.
Contrast-enhanced cardiac scan
Contrast administration
Bolus timing can be achieved using:
1. Empirical delay of c. 25 s. Unreliable and not recommended
2. ‘Bolus-tracking’ – a selected region-of-interest (ROI) – usually ascending aorta – is scanned every second or so following contrast injection and the full acquisition scan is automatically triggered (after a short delay) once the ROI has enhanced to a preset level e.g. 120 HU
3. ‘Test bolus’ – a small initial injection of contrast (e.g. 20 ml contrast with 40 ml saline ‘chaser’ bolus) is given and the ROI (ascending aorta) is scanned and time-to-peak enhancement is derived. The full scan is then performed using this timing delay. (Usually add 3 s to allow for maximal coronary vessel enhancement). This method is probably the most reliable and accurate, confirms i.v. line patency and familiarizes the patient ready for the final crucial acquisition scan.
Bastarrika, G, Lee, YS, Huda, W, et al. CT of coronary artery disease. Radiology. 2009; 253(2):317–338.
Boxt, LM. Coronary computed tomography angiography: a practical guide to performance and interpretation. Semin Roentgenol. 2012; 47(3):204–219.
Mahesh, M, Cody, DD. AAPM/RSNA physics tutorial for residents: physics of cardiac imaging with multiple-row detector CT. RadioGraphics. 2007; 27:1495–1509.
Pannu, HK, Alvarez, W, Fishman, EK. Review: β-blockers for cardiac CT: a primer for the radiologist. Am J Roentgenol. 2006; 186:S341–S345.
Schoepf, UJ, Zwerner, PL, Savino, G, et al. Coronary CT angiography: how I do it. Radiology. 2007; 244:48–63.
Sun, Z, Ng, KH. Prospective versus retrospective ECG-gated multislice CT coronary angiography: a systematic review of radiation dose and diagnostic accuracy. Euro J Radiol. 2012; 81(2):94–100.
Taylor, CM, Blum, A, Abbara, S. Cardiac CT: Patient preparation and scanning techniques. Radiol Clin North Am. 2010; 48(4):675–686.
Cardiac MR
Indications
1. Assessment of chamber dimensions, mass and function
2. Assessment of complex congenital heart disease
3. Diagnosis and evaluation of cardiomyopathies – e.g. sarcoid, amyloid, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy
4. Evaluation of cardiac masses
Technique
Technique will be varied depending on indication, but will consist of elements from the following.
Belloni, E, De Cobelli, F, Esposito, A, et al. MRI of cardiomyopathy. Am J Roentgenol. 2008; 191(6):1702–1710.
Ginat, DT, Fong, MW, Tuttle, DJ, et al. Review: cardiac imaging: Part 1, MR pulse sequences, imaging planes, and basic anatomy. Am J Roentgenol. 2011; 197(4):808–815.
Kramer, CM, Barkhausen, J, Flamm, SD, et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Mag Res. 2008; 10:35.
O’Donnell, DH, Abbara, S, Chaithiraphan, V, et al. Cardiac tumours: optimal cardiac MR sequences and spectrum of imaging appearances. Am J Roentgenol. 2009; 193(2):377–387.
Schwitter, J, Arai, AE. Assessment of cardiac ischaemia and viability: role of cardiovascular magnetic resonance. Euro Heart J. 2011; 32:799–809.
Radionuclide ventriculography
Indications
Gated blood-pool study1
1. Evaluation of ventricular function; particularly left ventricular ejection fraction (LVEF)
2. Assessment of myocardial reserve in coronary artery disease
3. Cardiomyopathy, including the effects of cardio-toxic drugs.
(Previous indications of first pass radionuclide angiography – for evaluation of right ventricular ejection fraction (RVEF) and detection and quantification of intracardiac shunts are now superseded by echocardiography and MR unless for very specialist indications and will not be described here.)
Patient preparation
1. An i.v. injection of ‘cold’ stannous pyrophosphate (20 µg kg–1) is given directly into a vein 20–30 min before the pertechnetate injection. (Avoid injection via long plastic Teflon-coated cannula which may result in a poor label.)
2. Three ECG electrodes are placed in standard positions to give a gating signal.
Technique
2. The ECG trigger signal is connected
3. I.v. injection of 99mTc pertechnetate
4. One minute is allowed for the bolus to equilibrate before computer acquisition is commenced (see ‘Images’ below).
List mode is best, where individual events are stored as their x,y coordinates along with timing and gating pulses. This allows maximum flexibility for later manipulation and framing of data. Around 5 million counts should be acquired. However, MUGA mode is adequate, and is still the most commonly used. In this, the start of an acquisition cycle is usually triggered by the R wave of the patient’s ECG. A series of 16–32 fast frames are recorded before the next R wave occurs. Each of these has very few counts in from a single cycle, so every time the R wave trigger arrives another set of frames is recorded and summed with the first. The sequence continues until 100–200 kilo-counts per frame have been acquired in about 10 min. Some degree of arrhythmia can be tolerated using the technique of ‘buffered bad beat rejection’, where cardiac cycles of irregular length are rejected and the data not included in the images. The length of time to acquire an image set increases as the proportion of rejected beats rises.
Images
A number of views may be recorded, depending on the clinical problem:
Additional techniques
1. Gated blood-pool imaging can be carried out during controlled exercise with appropriate precautions to assess ventricular functional reserve. Leg exercise using a bed-mounted bicycle ergometer is the method of choice. Shoulder restraints and hand grips help to reduce upper body movement during imaging. For patients unable to exercise effectively, stress with dobutamine infusion is used.2 Under continuous monitoring, the dose is incrementally increased from 5 to 20 µg kg–1 min–1, infusing each dose for 3 min. The infusion is stopped when S-T segment depression of >3 mm, any ventricular arrhythmia, systolic blood pressure >220 mmHg, attainment of maximum heart rate, or any side effects occur.
2. Gated single photon emission computed tomography (SPECT) blood pool acquisition can be used for measurement of left as well as the right ventricular function using special software (Autoquant+) which calculates the left and right ventricular volumes.
3. With gated SPECT using the myocardial perfusion imaging agents 99mTc-MIBI and 99mTc-tetrofosmin (see ‘Radionuclide myocardial perfusion imaging’), it is possible to combine ventriculography and perfusion scans in a single study.3,4
When acquiring gated SPECT studies ventricular parameters are usually measured during rest study only because of the length of the examination (20 min).
References
1. Metcalfe, MJ. Radionuclide ventriculography. In: Sharp PF, Gemmell HG, Murray AD, eds. Practical Nuclear Medicine. London: Springer, 2005.
2. Konishi, T, Koyama, T, Aoki, T, et al. Radionuclide assessment of left ventricular function during dobutamine infusion in patients with coronary artery disease: comparison with ergometer exercise. Clin Cardiol. 1990; 13:183–188.
3. Fukuoka, S, Maeno, M, Nakagawa, S, et al. Feasibility of myocardial dual-isotope perfusion imaging combined with gated single photon emission tomography for assessing coronary artery disease. Nucl Med Commun. 2002; 23(1):19–29.
4. Anagnostopoulos, C, Underwood, SR. Simultaneous assessment of myocardial perfusion and function: how and when? Eur J Nucl Med. 1998; 25:555–558.
Radionuclide myocardial perfusion imaging
Radiopharmaceuticals1,2
1. 99mTc-methoxyisobutylisonitrile (MIBI or sestamibi), up to 800 MBq (8 mSv ED) for planar imaging, 800 MBq (8 mSv ED) for SPECT (or 1600 MBq max. for the total of two injections in single day rest/exercise protocols). Cationic complex with myocardial uptake in near proportion to coronary blood flow but minimal redistribution. There is also, normally, liver uptake and biliary excretion, which can cause inferior wall artifacts on SPECT if care is not taken. Separate injections are required for stress/rest studies, but image timing is flexible due to minimal redistribution.
2. 99mTc-tetrofosmin (Myoview) (activity and radiation dose as for MIBI). Similar uptake characteristics and diagnostic efficacy to MIBI, but with easier preparation.
3. 201Tl-thallous chloride, 80 MBq max. (18 mSv ED). Thallium is a potassium analogue with initial rapid myocardial uptake in near proportion to coronary blood flow, and subsequent washout and redistribution. Hence, unlike the 99mTc agents, same-day stress/rest redistribution studies can be performed with a single injection. With principal photon energies of 68–72 and 167 keV and T1/2 of 73 h, it is not ideal for imaging and gives a higher radiation dose than the newer 99mTc alternatives. It is increasingly being replaced by 99mTc agents. However, many still consider 201Tl for assessment of myocardial viability and hibernation, with either re-injection at rest or a separate day rest-redistribution study giving the greatest sensitivity.
4. 18FDG + blood flow PET. The radio-isotope gold standard for viability assessment, but not widely available.3
5. Rubidium-82 PET. This study can be used to assess myocardial perfusion. Not widely available.
Equipment
1. SPECT-capable gamma-camera, preferably dual-headed
2. Low-energy, high-resolution, general purpose or specialized cardiac collimators
3. Pharmacological stressing agent (adenosine or dobutamine [dipyridamole now rarely used]) or exercise (bicycle ergometer or treadmill)
4. Nitroglycerin (tablets or sublingual spray) to enhance resting uptake of ischaemic, but viable segments
6. Resuscitation facilities including defibrillator
7. Lidocaine to reverse serious arrhythmias caused by dobutamine infusion.
Technique
The principle of the technique is to compare myocardial perfusion under conditions of pharmacological stress or physical exercise, with perfusion at rest. Diseased but patent arterial territories will show lower perfusion under stress conditions than healthy arteries, but will show relatively improved perfusion at rest. Infarcted tissue will show no improvement at rest. Hence, prognostic information on the likelihood of adverse cardiac events and the benefits of revascularization can be gained.4
Stress regimen
Pharmacological stress has become increasingly widely used instead of physical exercise.5 The optimal stress technique aims to maximize coronary arterial flow. The preferred pharmacological stressing agent is adenosine infusion (0.14 mg kg–1 min–1 for 6 min).6 Adenosine is a potent coronary vasodilator. It reproducibly increases coronary artery flow by more than maximal physical exercise (which often cannot be achieved in this group of patients). It has a short biological half-life of 8–10 s, so most side effects are reversed simply by discontinuing infusion. Stressing with adenosine has now almost completely replaced dipyridamole and will not be discussed here.
There are circumstances where adenosine is contraindicated, e.g. asthma, second-degree heart block or systolic blood pressure <100 mmHg. Dobutamine stress may be employed in these circumstances.7 Dobutamine acts as a ß1 receptor agonist, increasing contractility and heart rate. Under continuous monitoring, the dose is incrementally increased from 5 to 20 µg kg–1 min–1, infusing each dose for 3 min. The infusion is terminated when S-T segment depression of >3 mm, any ventricular arrhythmia, systolic blood pressure >220 mmHg, attainment of maximum heart rate, or any side effects occur. Dobutamine is contraindicated in patients with aortic aneurysm. New, more specific targeted agents such as regadenoson (A2A adenosine receptor agonist) are being developed which could be used in asthmatic patients rather than dobutamine.8
99mTc-MIBI or tetrofosmin rest/stress test
Because MIBI and tetrofosmin have minimal redistribution, separate injections are needed for stress and rest studies. Two-day protocols are optimal, but it is often more convenient to perform both studies on the same day. A number of groups have shown that this is possible without significantly degrading the results, most effectively when the resting study is performed first.9
2-Day protocol (stress/rest)
1. Initiate pharmacological stress or exercise
2. Up to 800 MBq MIBI or tetrofosmin is administered i.v. at maximal stress, continuing the stress protocol for 2 min post injection to allow uptake in the myocardium
3. 10–30 min post injection, a milky drink or similar is given to promote biliary clearance; high fluid intake will dilute bowel contents
4. Images are acquired 15–30 min after tetrofosmin or 60–120 min after sestamibi injection. If there is excessive liver uptake or activity in small bowel close to the heart, imaging should be delayed by a further 60 min
5. Depending on the clinical situation, if the stress scan is completely normal the patient may not need to return for the rest scan10
6. Preferably 2–7 days later, the patient returns for a resting scan
7. Glyceryl trinitrate (GTN) (two 0.3 mg tablets) or equivalent sublingual spray may be given to improve blood flow to ischaemic, but viable segments11
8. Immediately, up to 800 MBq MIBI or tetrofosmin i.v., is administered and proceed as for stress imaging.
1-Day protocol (stress/rest)
1. Initiate pharmacological stress or exercise; up to 400 MBq MIBI or tetrofosmin i.v. is administered at maximum stress
2. Image as per 2-day protocol
3. A minimum of 2 h after first tetrofosmin injection and minimum of 4 h after sestamibi injection, GTN is administered as above, followed by up to 1200 MBq MIBI or tetrofosmin (with longer period between injections, the activity of the second injection may be reduced)
201Tl stress/rest test
1. Initiate pharmacological stress or exercise
2. Administer 80 MBq 201Tl i.v. at maximal stress, continuing the stress protocol for 1 min post injection to allow uptake in the myocardium
4. Image at rest 3–4 h after redistribution period, during which time patients should not eat
5. If fixed defects are present in exercise and rest images and assessment of viability and hibernation is required, either:
Images
SPECT
1. Position patient as comfortably as possible with their arms above their head (or at least the left arm) if possible. SPECT images may be severely degraded by patient movement, so attention should be paid to keeping the patient very still.
2. For the 99mTc agents, check for activity in bowel loops close to the inferior wall of the heart. This can cause artifacts in the reconstructed images, so if significant activity is seen, delay the imaging to give greater time for clearance.
3. 180° orbit from RAO 45° to LPO 45°, elliptical if possible. With modern dual-headed systems, this can be achieved with the heads at 90° to each other to minimize the amount of camera rotation required.
4. Matrix size and zoom to give a pixel size of 6 mm.
5. 60 projections, with a total imaging time of about 30–40 min for single and 15–25 min for dual-head systems.
6. View the projections as a cine before the patient leaves the department. If available, perform software motion correction. If there is significant movement that cannot be corrected, repeat imaging. Beware ‘diaphragmatic creep’, particularly on 201Tl patients breathless after exercise, where the average position of the diaphragm changes as they recover.
Additional techniques
1. Gated SPECT is now recommended,12 and with special software (e.g. Cedars Sinai QGS package, Emory cardiac tool box) can provide additional information on ventricular wall motion, ejection fraction and chamber volume. It can also improve specificity by reducing artifactual defects caused by regional myocardial motion and wall thickening.
2. Bull’s-eye maps can be compared to normal databases and displayed quantitatively in terms of severity and extent of relative underperfusion.13
3. Attenuation and scatter correction using scanning transmission line sources or CT are now available on most modern dual-headed systems. This technique can improve diagnostic specificity by correcting attenuation artifacts and thereby increasing the normalcy rate. However, algorithms are still being improved, so at present it is wise to view the attenuation-corrected and uncorrected images together to identify possible introduced artifacts.14
4. The combination of rest 201Tl and exercise 99mTc MIBI or tetrofosmin can be used to assess both ischaemia and viability.15
References
1. Reyes, E, Loong, CY, Harbinson, M, et al. A comparison of Tl-201, Tc-99m sestamibi, and Tc-99m tetrofosmin myocardial perfusion scinitgraphy in patients with mild to moderate coronary stenosis. J Nucl Cardiol. 2006; 13(4):488–494.
2. Kapur, A, Latus, KA, Davies, G, et al. A comparison of three radionuclide myocardial perfusion tracers in clinical practice: the ROBUST study. Eur J Nucl Med Mol Imaging. 2002; 29(12):1608–1616.
3. Nandalur, KR, Dwamena, BA, Choudhri, AF, et al. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol. 2008; 15(4):444–451.
4. Travin, MI, Bergmann, SR. Assessment of myocardial viability. Semin Nucl Med. 2005; 35(1):2–16.
5. Travain, MI, Wexler, JP. Pharmacological stress testing. Semin Nucl Med. 1999; 29:298–318.
6. Takeishi, Y, Takahashi, N, Fujiwara, S, et al. Myocardial tomography with technetium-99m-tetrofosmin during intravenous infusion of adenosine triphosphate. J Nucl Med. 1998; 39:582–586.
7. Verani, MS. Dobutamine myocardial perfusion imaging. J Nucl Med. 1994; 35:737–739.
8. Iskandrian, AE, Bateman, TM, Belardinelli, L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007; 14(5):645–658.
9. Tadehara, F, Yamamoto, H, Tsujiyama, S, et al. Feasibility of a rapid protocol of 1-day single-isotope rest/adenosine stress Tc-99m sestamibi ECG-gated myocardial perfusion imaging. J Nucl Cardiol. 2008; 15(1):35–41.
10. Lavalaye, JM, Shroeder-Tanka, JM, Tiel-van Buul, MM, et al. Implementation of technetium-99m MIBI SPECT imaging guidelines: optimizing the two-day stress-rest protocol. Int J Card Imaging. 1997; 13:331–335.
11. Thorley, PJ, Sheard, KL, Wright, DJ, et al. The routine use of sublingual GTN with resting 99mTc-tetrofosmin myocardial perfusion imaging. Nucl Med Commun. 1998; 19:937–942.
12. Travin, MI, Heller, GV, Johnson, LL, et al. The prognostic value of ECG-gated SPECT imaging in patients undergoing stress Tc-99m sestamibi myocardial perfusion imaging. J Nucl Cardiol. 2004; 11(3):253–262.
13. Schwaiger, M, Melin, J. Cardiological applications of nuclear medicine. Lancet. 1999; 354:661–666.
14. Banzo, I, Pena, FJ, Allende, RH, et al. Prospective clinical comparison of non-corrected and attenuation- and scatter-corrected myocardial perfusion SPECT in patients with suspicion of coronary artery disease. Nucl Med Commun. 2003; 24(9):995–1002.
15. Kim, Y, Goto, H, Kobayshi, K, et al. A new method to evaluate ischemic heart disease: combined use of rest thallium-201 myocardial SPECT and Tc-99m exercise tetrofosmin first pass and myocardial SPECT. Ann Nucl Med. 1999; 13:147–153.