Chapter 69 Headache and Other Craniofacial Pain
A survey of a sample of 20,000 households estimated 27.9 million migraine patients in the United States. More than 90% of patients report an impaired ability to function during migraine attacks, and 53% report severe disability requiring bed rest. Approximately 31% of patients with migraine missed at least 1 day from work or school in the preceding 3 months due to migraine (Lipton et al., 2001). Indirect costs of migraine related to decreased productivity and lost days of work have been calculated to be $13 billion per year; estimates are that the equivalent of 112 million bedridden days per year are due to migraine (Hu et al., 1999). The World Health Organization declared migraine to be among the most disabling medical conditions experienced worldwide.
Classification
In 1988, the Headache Classification Committee of the International Headache Society introduced a detailed classification of headaches. The classification, revised in 2004, contains 14 main headache types (Box 69.1), and each headache type is further subclassified (Headache Classification Committee, 2004). Careful definition of migraine subtypes and other primary headache disorders is expected to rid future research and clinical publications of the confusing and often poorly defined terminology of earlier work. The 2004 classification has undergone a few revisions since its publication, and further refinement over the next few years is expected.
Box 69.1
Classification of Headache Disorders
Part Two: The Secondary Headaches
5. Headache attributed to head and/or neck trauma
6. Headache attributed to cranial or cervical vascular disorder
7. Headache attributed to non-vascular intracranial disorder
8. Headache attributed to a substance or its withdrawal
9. Headache attributed to infection
10. Headache attributed to disorder of homeostasis
11. Headache or facial pain attributed to disorder of cranium, neck, eyes, ears, nose, sinuses, teeth, mouth, or other facial or cranial structures
Adapted from Headache Classification Committee of the International Society, 2004. International Classification of Headache Disorders, second ed. Cephalalgia 24, 9-195.
Headache Attributed to Nonvascular, Noninfectious Intracranial Disorders
Tumors
Approximately 50% of patients with brain tumors report headaches; in one-third to one-half of these patients, headache is the primary complaint. In a prospective study of over 200 patients with intracranial tumors, 47.6% had headache at the time of presentation (Valentinis et al., 2009). Generalized headache is usual, but in approximately a third of patients, it overlies the tumor and is referred to the scalp near the lesion. Rapidly growing tumors are more likely to produce headache than indolent lesions, but slowly enlarging lesions can eventually produce pain by compromising the ventricular system or exerting direct pressure on a pain-sensitive structure. When the CSF circulation is partially obstructed, headache often becomes generalized and worse in the occipitonuchal area. This type of headache, which is a manifestation of raised intracranial pressure (ICP), is often worse on awakening, aggravated by coughing and straining, and often associated with nausea and vomiting. A prospective study showed that only 5.1% of patients presented with this pattern of headache; in most cases, the headache pattern was nonspecific (Valentinis et al., 2009). In children particularly, the vomiting may be precipitate and without nausea (projectile vomiting).
Supratentorial masses generally produce frontal or temporal head pain because of the trigeminal nerve supply to the anterior and middle cranial fossae. The superior surface of the tentorium cerebelli is supplied by the meningeal branches of the first division of the trigeminal cranial nerve, so an occipital lesion can cause pain referred to the fronto-orbital region. A posterior fossa mass generally causes occipitonuchal pain, because the meningeal nerve supply is largely through the upper cervical nerves which also supply the occipital and cervical dermatomes. CNs VII, IX, and X also provide sensory innervation of the posterior fossa, and therefore pain referral can be more widespread. Posterior fossa tumors result in headache earlier than their supratentorial counterparts, because the greater likelihood of compromise of the ventricular system leads to rapidly developing hydrocephalus and raised ICP (Edmeads, 1997).
Pituitary tumors and tumors near the optic chiasm commonly cause a frontotemporal headache, but they may cause referred pain near the vertex. However, patients with tumors of the sellar and parasellar regions do not often present with headache as the initial symptom, because the visual and endocrine symptoms are typically noted first (Edmeads, 1997).
Features that should serve as warnings that a patient’s headaches may not be of benign origin and raise the possibility of an intracranial mass lesion are (Purdy, 2001): subacute and progressive; new onset in adults; change in pattern; associated with nausea or vomiting; nocturnal or upon awakening in the morning; precipitated or worsened by changes in posture or Valsalva maneuver; associated with confusion, seizures, weakness, and/or abnormal neurological examination.
Obstruction of the Cerebrospinal Fluid Pathways
Congenital obstruction of the foramina of Luschka and Magendie (the Dandy-Walker syndrome) can lead to ballooning of the fourth ventricle and deformity of the cerebellum. Minor degrees of this malformation can remain asymptomatic until later in life and then manifest with obstructive hydrocephalus and headache. Similarly, the Chiari malformation in its various forms can obstruct the free circulation of CSF and lead to hydrocephalus and headache (Taylor and Larkins, 2002). This malformation can result in an occipital-suboccipital headache worsened or even initiated by a Valsalva maneuver during lifting, straining, or coughing. Thus, the Chiari malformation is one of the causes of an exertional or Valsalva maneuver–induced headache.
In communicating hydrocephalus, free communication exists between the ventricular system and the subarachnoid space, but CSF circulation or absorption is impaired. Obstruction in the basal cisterns or at the arachnoid granulations may follow subarachnoid hemorrhage and meningitis. Venous sinus occlusion can impair absorption of CSF. Headache may be a prominent symptom of both obstructive and communicating hydrocephalus, except in the case of normal-pressure hydrocephalus, which is generally painless (see Chapter 59).
Low–Cerebrospinal Fluid Pressure Headache
An identical low–CSF pressure headache can occur when a tear develops in the spinal theca. This is usually in the midthoracic region and may result from lifting or coughing or, at times, occurs spontaneously. It can also occur with a crush injury to the chest or abdomen and in patients with overdraining CSF shunts. When it occurs, in the absence of a significant history of trauma, it may not be considered as a cause of daily headache. A history of a headache rapidly responding to recumbency suggests a tear. However, in some patients whose headaches are long standing, a persistent headache may be noted and the postural feature of the headache may become less prominent. Nausea or emesis, neck pain, dizziness, horizontal diplopia, changes in hearing, photophobia, upper-limb paresthesias, vision blurring, and dysgeusia may also occur, particularly when the headache first develops. As additional cases have been reported in the literature, the clinical picture of CSF leaks has been found to take many forms (Mokri, 2004).
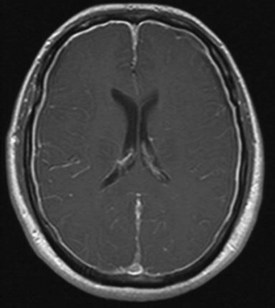
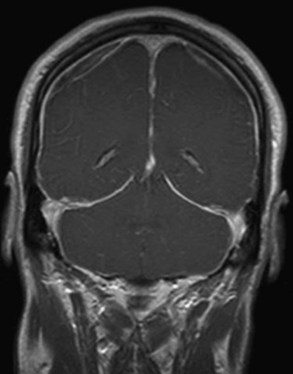
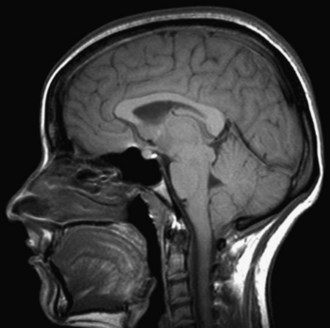
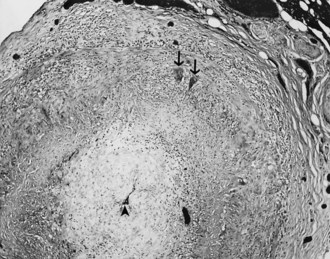
When a CSF leak is possible, the initial diagnostic test is MRI with gadolinium. MRI has become an invaluable diagnostic tool in this syndrome, with the cardinal features being diffuse pachymeningeal thickening with gadolinium enhancement, subdural collections of fluid, and evidence of brain descent (Figs. 69.1 to 69.3). This evidence includes cerebellar tonsillar descent (resembling a Chiari type I malformation); reduction in size or effacement of the prepontine, perichiasmatic, and subarachnoid cisterns; inferior displacement of the optic chiasm; and descent of the iter (the opening of the aqueduct of Sylvius as seen on a midsagittal MRI scan).
If the clinical and MRI findings are typical, determination of the CSF opening pressure may not be necessary. Measurement of the opening pressure is warranted in patients with normal MRI studies. However, in only 50% of patients is the CSF pressure less than 40 mm H2O. Because the opening pressure may be normal, the term CSF volume depletion best identifies the core of the problem in this disorder (Mokri, 2004). These patients may have a variable pleocytosis with up to 50 or more mononuclear cells/mm and a mild to modest increase in CSF protein (Mokri, 2004).
In patients with the typical clinical and radiographic features of low–CSF pressure headache, treatment may be conservative with bed rest and hydration for 1 to 2 weeks. If this is either impractical or ineffective, options include empirical treatment with a blood patch or further studies to identify the site of the CSF leak. In patients with spontaneous leaks, the leak is often at the level of the thoracic spine or cervicothoracic junction. Myelography with CT of the spine is more sensitive than radioisotope cisternography or MRI of the spine at localization of the source of the leak, but the latter procedures may serve as guides for obtaining multiple CT images at the appropriate levels. MR myelography may also be helpful in some patients to reveal a leak that is not demonstrable on conventional MRI sequences (Katramados et al., 2006). Most leaks are stopped with either conservative therapy or blood patches. Although an epidural blood patch is effective in the majority of patients, most require more than one blood patch, and some require as many as four to six blood patches (Mokri, 2004). For resistant leaks, surgical repair of the dural tear may be necessary. Even after a protracted duration, surgical repair of the causative dural tear can be quite effective (Mokri, 2004).
Idiopathic Intracranial Hypertension
Headache, transient visual obscuration, pulsatile tinnitus, and diplopia are the most common presenting symptoms of idiopathic intracranial hypertension (pseudotumor cerebri). The headache is rather nonspecific but tends to be worse on awakening and aggravated by activity. The visual obscurations are direct results of raised ICP leading to papilledema. Indeed, if papilledema is not present, the diagnosis is in doubt. Once determining by MRI that there is no intracranial mass, ventricular system obstruction, or thrombosis of a dural venous sinus, the high CSF pressure can be confirmed by lumbar puncture manometry. Removal of CSF to achieve a normal closing pressure relieves the headache and temporarily prevents visual obscurations. A discussion of long-term management of idiopathic intracranial hypertension is in Chapter 59.
Transient Syndrome of Headache with Neurological Deficits and Cerebrospinal Fluid Lymphocytosis
Although originally termed migrainous syndrome with CSF pleocytosis, several later reports used various terms including headache with neurological deficits and CSF lymphocytosis (Berg and Williams, 1995) and pseudomigraine with temporary neurological symptoms and lymphocytic pleocytosis (Gomez-Aranda et al., 1997). This self-limited syndrome consists of one to several episodes of variable neurological deficits accompanied by moderate to severe headache and sometimes fever. Each episode lasts hours, with total duration of the syndrome being from 1 to 70 days. CSF abnormalities have included a lymphocytic pleocytosis varying from 10 to more than 700 cells/mm, elevation of CSF protein, and in some patients, elevated opening pressure. MRI and CT are normal in the vast majority of reported cases. A single patient had MRI evidence of gray-matter swelling in addition to right temporal and occipital sulci CSF enhancement and hypoperfusion. Similar findings have been reported in hemiplegic migraine (Yilmaz et al., 2009). Another patient was found to have global hemisphere hypoperfusion on MRI perfusion imaging (Vallet et al., 2010). Results of microbiological studies are usually negative. A single patient was reported to have evidence of recent human herpesvirus-6 infection, but its pathogenic role in the syndrome is unclear (Emond et al., 2009). Accordingly, the cause of the syndrome is unclear, although an immune response to a viral infection is speculated. No treatment alters the self-limited course of this disorder. In contrast to this syndrome, episodes of Mollaret meningitis (see Chapters 53B and 73) are separated by months to years and are typically not accompanied by focal neurological symptoms.
Headache Attributed to Head or Neck Trauma
Posttraumatic Headache
Headaches, dizziness, difficulty concentrating, irritability, decreased libido, and fatigue are common complaints after head injury (Keidel and Ramadan, 2005). Many studies suggest that milder injuries are more frequently associated with posttraumatic headache then more severe injuries (Packard, 2005). This counterintuitive observation may result from the fact that seriously injured patients may have other symptoms of such severity that headache may be overshadowed by their other complaints; however, there is no definitive evidence as of yet to support this assumption. In civilian populations, the phenotypical appearance of headache attributed to traumatic brain injuries is most consistent with tension-type headache. In military populations, however, posttraumatic headache more commonly meets criteria for migraine. The underlying mechanisms behind this discrepancy are unclear. The variable latencies between injury and the onset of headache observed in combat veterans suggests the need to revise the diagnostic criteria for posttraumatic headaches; the currently accepted window of 7 days may not adequately capture the number of true cases (Vargas, 2009). Brain imaging rarely reveals abnormalities in the absence of an abnormal neurological examination, although unexpected subdural hematomas are occasionally found. MRI examination tends to be more sensitive for the detection of small occult extracerebral hematomas, cortical contusions, and indeterminate changes in the cerebral parenchyma. Treatment of posttraumatic syndrome and posttraumatic headache is difficult. Encouragement, patience, and a sympathetic attitude on the part of the physician are essential. Undoubtedly, a thorough physical examination and evaluation of appropriate imaging studies are important to reassure the patient that more sinister underlying disorders have been ruled out. As successful treatment can be challenging, reasonable expectations should be established. All the treatments useful for tension-type headaches, migraine, occipital neuralgia, and neck sprains may be needed. Physical therapy, biofeedback, and psychotherapy each have a place in treatment. Drug treatment may include analgesics (nonopioid), nonsteroidal antiinflammatory drugs (NSAIDs), and antidepressants. The tricyclic antidepressants and gabapentin can be particularly helpful.
Recovery from posttraumatic syndrome, including the headache, may be significantly delayed. Most patients who continue to have headaches for more than 2 months after the trauma continue to have them for 1 to 2 years (Ramadan and Láinez, 2005). If the history included a period of unconsciousness or if there is a simple skull fracture, a limited period of bed rest followed by a graduated return to full activity should be advised.
Headache Attributed to Infection
Inflammation of pain-sensitive structures such as the meninges and intracranial vessels produces the severe headache frequently associated with both meningitis and meningoencephalitis. Headache is the most common symptom in acute bacterial meningitis, occurring in nearly 90% of cases (van de Beek et al., 2004). Acute bacterial meningitis characteristically produces a severe holocephalic headache with neck stiffness and other signs of meningismus, including photophobia and irritability. Pain may be retro-orbital and may worsen with eye movement. The presence of the classic triad of fever, neck stiffness, and altered mental status has a low sensitivity for the diagnosis of meningitis; however, nearly all patients present with at least two of these symptoms and/or headache (van der Beek et al., 2004). Jolt accentuation of headache has a sensitivity of 100% and a specificity of 54% for meningitis diagnosis (Attia et al., 1999).
Mollaret meningitis is a rare and recurrent aseptic meningitis (see Chapters 53B and 73). The CSF cellular response includes large epithelioid cells (Mollaret cells). The pathogenesis is unknown but may relate to the herpes simplex virus (Jensenius et al., 1998). The condition may recur every few days or every few weeks for months or years. Headache, signs of meningismus, and low-grade fever accompany each attack. Treatment is mainly symptomatic.
Headache Attributed to Cranial or Cervical Vascular Disorders
Aneurysms and Arteriovenous Malformations and Thunderclap Headache
Parenchymal arteriovenous malformations (AVMs) rarely cause pain before rupture. Very large lesions can be associated with ipsilateral or bilateral throbbing cephalalgia, but they rarely cause a migraine-like headache. The presence of a cranial bruit or the classic triad of migraine, seizures, and focal neurological deficits may indicate an AVM. Numerous case reports and retrospective studies suggest migraine may be more prevalent in patients harboring AVMs versus the general population. Among these, the headache frequently occurs ipsilateral to the AVM, highlighting the need for cautious evaluation of patients with side-locked headaches (Monteiro et al., 1993). MR or CT angiography can usually exclude the presence of clinically significant aneurysms and AVMs.
The term thunderclap headache describes a severe headache of instantaneous onset (within seconds) and without warning, like a clap of thunder. Other conditions can also manifest with thunderclap headache: cerebral venous sinus thrombosis, cervicocephalic arterial dissection, pituitary apoplexy, acute hypertensive crisis, spontaneous intracranial hypotension, meningitis, embolic cerebellar infarcts, and reversible cerebral vasoconstriction syndromes (Calabrese et al., 2007; Schwedt et al., 2006). These entities are associated with significant neurological morbidity and not easily seen on the initial CT image, thus underscoring the frequent need for MRI and MRA/magnetic resonance venography (MRV) in this group if results of the initial workup are negative. Finally, for one category of primary thunderclap headache, no underlying cause is established.
Whether an unruptured cerebral aneurysm can cause a thunderclap headache is debated (Schwedt et al., 2006).
Carotid and Vertebral Artery Occlusion and Dissection
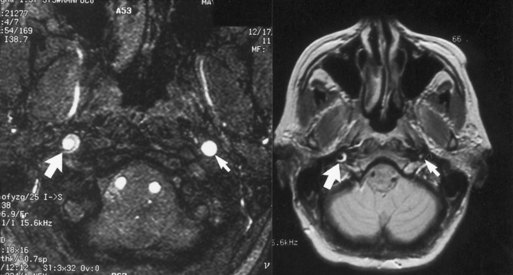
MRI or MRA usually confirm the diagnosis of arterial dissection. At the level of involvement, the lumen of the artery typically appears as a dark circle of flow void of smaller caliber than the original vessel, and the intracranial clot appears as a hyperintense and bright crescent or circle (in both T1- and T2-weighted images) surrounding the flow void (Fig. 69.4). Catheter angiography is rarely required (Mokri, 2002). The pain associated with cervicocephalic dissections is of variable duration and may require treatment with potent analgesics. Patients with evidence of distal embolization are usually treated with either antiplatelet agents or anticoagulation.
Giant-Cell Arteritis
Clinical Symptoms
The clinical manifestations of giant-cell arteritis result from inflammation of medium and large arteries. Table 69.1 summarizes clinical symptoms in 166 patients examined at the Mayo Clinic between 1981 and 1983. Headache was the most common symptom, experienced by 72% of patients at some time and the initial symptom in 33%. The headache is most often throbbing, and many patients report scalp tenderness. Headache is associated with striking focal tenderness of the affected superficial temporal or, less often, occipital artery. One-third of patients with headache may have no objective signs of superficial temporal artery inflammation.
Table 69.1 Symptoms of Giant-Cell Arteritis in 166 Patients*
| Symptom | Patients with Symptom (%) | Patients in Whom It Was Initial Symptom (%) |
|---|---|---|
| Headache | 72 | 33 |
| Polymyalgia rheumatica | 58 | 25 |
| Malaise, fatigue | 56 | 20 |
| Jaw claudication | 40 | 4 |
| Fever | 35 | 11 |
| Cough | 17 | 8 |
| Neuropathy | 14 | 0 |
| Sore throat, dysphagia | 11 | 2 |
| Amaurosis fugax | 10 | 2 |
| Permanent vision loss | 8 | 3 |
| Claudication of limbs | 8 | 0 |
| Transient ischemic attack/stroke | 7 | 0 |
| Neuro-otological disorder | 7 | 0 |
| Scintillating scotoma | 5 | 0 |
| Tongue claudication | 4 | 0 |
| Depression | 3 | 0.6 |
| Diplopia | 2 | 0 |
| Tongue numbness | 2 | 0 |
| Myelopathy | 0.6 | 0 |
* Some patients had coincident onset of more than one symptom.
Data from Caselli, R.J., Hunder, G.G., Whisnant, J.P., 1998. Neurologic disease in biopsy-proven giant cell (temporal) arteritis. Neurology 38, 352-359.
Laboratory Studies
The laboratory abnormality most often associated with giant-cell arteritis is elevation of the erythrocyte sedimentation rate (ESR) (mean, 85 ± 32 mm in 1 hour with the Westergren method), although it may be normal (≥29 mm in 1 hour) in 3% of patients. C-reactive protein levels may be more sensitive than the ESR in some patients and useful to follow disease activity. Patients are usually anemic (mean hemoglobin value 11.7 ± 1.6 g/dL) and show a mild thrombocytosis (mean platelet count 427 ± 116 × 103/µL). As the latter laboratory tests are nonspecific, the confirmatory diagnosis rests on temporal artery biopsy. Contrast-enhanced, high-resolution, temporal artery MRI is an emerging noninvasive tool with a diagnostic accuracy that rivals temporal artery biopsy (Bley et al., 2005).
Pathology
The histopathological features of arterial lesions include intimal proliferation with consequent luminal stenosis, disruption of the internal elastic membrane by a mononuclear cell infiltrate, invasion and necrosis of the media progressing to panarteritic involvement by mononuclear cells, giant-cell formation with granulomata within the mononuclear cell infiltrate, and (variably) intravascular thrombosis (Fig. 69.5). Involvement of an affected artery is patchy, with long segments of the normal unaffected artery flanked by vasculitic foci known as skip lesions which may begin to normalize within days after treatment. For these reasons, biopsy specimens of the superficial temporal artery should be generous (4- to 6-cm-long specimens), multiple histological sections should be taken, and bilateral biopsy considered. Employing these strategies increases the diagnostic yield of temporal artery biopsy up to 86%.
Epidemiology
The incidence of biopsy-confirmed giant-cell arteritis ranges between 9.5 and 29.1 per 100,000 per year, significantly increases after 50 years of age, and peaks in the eight decade (Gonzalez-Gay et al., 2009). It is the most common vasculitic process in both Europe and North America, appears to be most common among individuals of Scandinavian descent, and is significantly less common among Asians. The reported female-to-male ratio in giant-cell arteritis is as high as 4 : 1.
Treatment And Management
Disease activity must be monitored both clinically and by monitoring the ESR. A flare of symptoms accompanied by an increase in the ESR mandates increasing the corticosteroid dose at least to the last effective higher dose and often boosting it temporarily to a higher level. Relapses generally reflect too rapid a taper, and resumption of a more slowly tapering regimen is indicated after the relapse has stabilized. Some patients may require continuation of low-dose (7.5-10 mg/day) prednisone for several years, although complete withdrawal remains the eventual goal. There is some evidence that treatment with methotrexate 10 mg/week may be an effective adjunctive treatment that allows for more rapid tapering of the prednisone dose (Jover et al., 2001).
Headache Associated with Disorders of Homeostasis
Although no convincing evidence exists to support the notion that headache may be caused by chronic hypertension, conditions such as pheochromocytoma or preeclampsia which predispose to recurrent rapid, fluctuating blood pressures may present with headache. The headache associated with pheochromocytoma is characteristically of abrupt onset, moderate to severe intensity, bilateral distribution, and under 1 hour in duration (Lance and Hinterberger, 1976). Pheochromocytoma will typically present with other symptoms including anxiety, autonomic disturbances, and palpitations. Headache associated with preeclampsia may have migrainous features or present as thunderclap headache (see Chapter 81). Women with migraine history may have a higher risk for gestational hypertension or preeclampsia than non-migraineurs (Facchinetti et al., 2009).
Headache Caused by Disorders of the Cranium, Neck, Eyes, Ears, Nose, Sinuses, Teeth, Mouth, or Other Facial or Cranial Structures
Nasal Causes of Headache and Facial Pain
The occurrence of so-called sinus headaches without an underlying infection remains unsubstantiated. Commonly, migraine headaches are erroneously diagnosed as sinus headaches, because they are associated with cranial autonomic symptoms, have prominent facial involvement, or are triggered (e.g., by a change in altitude/weather, an exposure to pollens, or a seasonal predilection). Most patients with a diagnosis of “sinus headaches” have migraine headaches (Cady et al., 2005).
Temporomandibular Joint Disorders
For the neurologist evaluating head or facial pain, the criteria for identification and localization of TMJ disorders listed in Table 69.2 should be helpful. Bruxism, teeth clenching, and chronic gum chewing are important in the production of pain in the masseter and temporalis muscles. Arthritis and degenerative changes in the TMJ, loss of teeth, ill-fitting dentures or lack of dentures, and other dental conditions can all lead to the TMJ or myofascial pain dysfunction syndrome, which manifests as facial and masticatory muscle pain. Head pain and facial pain, even when associated with the criteria listed in Table 69.2, require full evaluation, which should include a detailed history and examination, appropriate radiographs, and laboratory studies to exclude other more serious causes. If TMJ dysfunction is thought to be the source of pain, further evaluation and treatment are in the province of the appropriate dental specialist. Even when TMJ dysfunction is believed to be responsible for facial or head pain, conservative management with analgesics, antiinflammatory agents, application of local heat, and nonsurgical techniques to adjust the bite generally provide relief. Before using surgical modalities on the TMJ or mandibles, the diagnosis must be secure and other causes of head and facial pain excluded by appropriate investigations.
Table 69.2 Criteria for Identification and Localization of Temporomandibular Joint Disorders
| Temporomandibular Pain | Temporomandibular Dysfunction |
|---|---|
| Pain should relate directly to jaw movements and mastication | Interference with mandibular movement (clicking, incoordination, and crepitus) |
| Tenderness in the masticatory muscles or over temporomandibular joint on palpation | Restriction of mandibular movement |
| Anesthetic blocking of tender structures should confirm presence and location of pain source | Sudden change in occlusal relationship of the teeth |
Headaches and the Cervical Spine
Migraine in particular frequently presents with referred pain to the occipital and nuchal regions, which are innervated by the greater occipital nerve. Furthermore, muscle hypersensitivity and tenderness, restriction of neck movements, and hyperalgesia may accompany the pain. Similarly, pain of cervical origin or cervicogenic headache is confined to the occipital region but may also spread to trigeminal territories. The referral of pain observed in cervicogenic headache and migraine reflects the convergence of trigeminal and cervical afferents onto the same neurons in the trigeminal-cervical complex (Bartsch and Goadsby, 2003). Despite this anatomical overlap, the provocation or exacerbation of the headache by neck movement, a persistent rather than intermittent headache, and lack of photophobia, phonophobia, and nausea are features that may be helpful in distinguishing cervicogenic headache from migraine. Diagnostic blocks performed accurately and under controlled conditions are the only currently available means by which a cervical source of pain can be established. A positive response to occipital nerve block should be interpreted with caution, however, given the fact that many primary headaches, including migraine and cluster headache, have been shown to respond to this procedure. The use of intraarticular steroids and long-acting anesthetics may provide relief that can last several months, and complete relief of headache can occasionally be achieved by radiofrequency neurotomy in patients whose headache stems from the C2-C3 zygohypophysial joint (Bogduk, 2005). Physical therapy may also be helpful in the treatment of cervicogenic headache (Jull et al., 2002).
Migraine
Definition and Classification
The term migraine derives from the ancient Greek word, hemikranios, which means “half head,” underscoring the unilateral distribution of head pain in many sufferers. Box 69.2 shows the classification of migraine (Headache Classification Committee, 2004).
Box 69.2
Migraine
1.2 Probable migraine without aura
1.4 Probable migraine with aura
1.5 Childhood periodic syndromes that are commonly precursors of migraine
From Headache Classification Committee of the International Society, 2004. International Classification of Headache Disorders, second ed. Cephalalgia 24, 9-195.
Clinical Aspects
Although migraine can begin at any age, the initial attack occurs most commonly during adolescence. By the age of 40, 90% of those with the condition have had their first attack. View migraine with suspicion when attacks begin de novo in older patients, because the incidence of serious intracranial disorders that may mimic primary headaches is greater. After puberty, migraine is more common in females, whereas in children there is a small preponderance of males. A family history of migraine is present in up to 90% of patients. In Europe and the United States, the prevalence of migraine is approximately 18% in women and 6% in men (Lipton et al., 2001). Migraine tends to recur with varying frequency throughout life, and attacks tend to get milder and less frequent in later years, although this certainly is not a universal finding.
Although migraine attacks are separable into those with and without aura, the two types are not mutually exclusive, and many patients have attacks of each type. The headache is similar in both types and typically consists of episodic unilateral throbbing head pain of moderate to severe intensity that if untreated persists from 4 hours up to 3 days and tends to worsen with routine physical exertion. Migraine attacks tend to be accompanied by nausea, vomiting, and light or sound sensitivity, although not every patient experiences all of these symptoms (Headache Classification Committee, 2004). Variability exists in the intensity, frequency, and details of the headache from patient to patient and from attack to attack in the same patient.
Migraine with Aura
Weakness of the limbs or facial muscles on one side of the body occurs only rarely as a motor aura in migraine, now classified as hemiplegic migraine (Headache Classification Committee, 2004). When it occurs, it usually affects the upper limb and is sometimes accompanied by mild dysphasia if the dominant hemisphere is involved (see Complications of Migraine, later).
Basilar-Type Migraine
Basilar-type migraine is usually first encountered during childhood or adolescence. It appears to occur in men almost as commonly as in women (Peatfield and Welch, 2000). Basilar migraine is recognizable only in the classic form, because only the dramatic constellation of brainstem symptoms allows its recognition. The headache is usually occipital and severe. The aura, which lasts 10 to 45 minutes, usually begins with typical migrainous disturbances of vision such as teichopsia, graying of vision, or actual temporary blindness. The visual symptoms are bilateral. Numbness and tingling of the lips, hands, and feet often occur bilaterally. Ataxia of gait and ataxic speech, vertigo, dysarthria, and tinnitus are also features of basilar migraine.
Ophthalmoplegic Migraine
Ophthalmoplegic migraine is a very uncommon condition that almost always has its onset in childhood. Recurrent attacks are stereotypical. Each episode begins with a unilateral orbital and retro-orbital headache, often accompanied by vomiting, that lasts 1 to 4 days. During the painful stage or as the headache is subsiding, ipsilateral ptosis occurs and within a few hours progresses to a complete paralysis of cranial nerve III. Rarely, cranial nerves IV or VI may be involved. The neural deficit can last from hours to several months. In the past, the focal nature of the deficit often led to major investigations including angiography to rule out the presence of an internal carotid or posterior communicating artery aneurysm. Usually, no abnormality is found. MRI scans have shown thickening and contrast enhancement of the nerve as it exits the midbrain, which may persist after the third nerve palsy has disappeared (Daroff, 2002). Lance and Zagami (2001) speculated that this represents a recurrent demyelinating/inflammatory neuropathy, although pathological evidence has not been available for confirmation. Whether cases with no identifiable cause (or cranial nerve enhancement on MRI) and spontaneously remitting episodes represent a form of migraine or not remains to be determined (Friedman, 2009). In any case, this disorder is probably a cranial neuropathy. The prognosis is favorable for recovery unless attacks occur very often.
Laboratory Findings
Case series extending back to the early 1990s have reported an increased occurrence of small areas of increased MRI T2 signal within the subcortical white matter. A meta-analysis of seven MRI-based case-control series by Swartz and Kern (2004) indicated a significantly increased risk for asymptomatic white-matter lesions in migraineurs, with an odds ratio (OR) of 3.9 (95% confidence interval [CI], 2.26-6.72). The clinical significance of the findings is unknown. A recent population-based cross-sectional MR study comparing 295 migraineurs (161 with aura, 134 without aura) and 140 nonmigraineurs (Kruit et al., 2004), which controlled for vascular risk factors, found that female migraineurs both with and without aura had an increased number of deep white-matter lesions compared with nonmigraine controls. In addition, this study reported that migraineurs carried an increased risk for subclinical infarct-like lesions within the cerebellum. The occurrence of these lesions positively correlates with attack frequency and the presence of aura (Kruit et al., 2004). We emphasize that the findings were subclinical, that the study was cross-sectional rather than longitudinal, and that at this time assessment for unexplained cerebellar infarcts is not a reliable diagnostic tool in migraine.
Migraine Genetics
Familial hemiplegic migraine (FHM) is a rare autosomal dominant subtype of migraine with aura in which, in the context of otherwise typical migraine attacks, patients experience hemiplegia. The hemiparesis of FHM in many patients extends beyond the customary time limit of 1 hour usually associated with migraine aura. Ataxia, nystagmus, and coma occur in the context of FHM. In 1993, FHM was mapped to chromosome 19p13 in linkage studies that were inspired by the clinical association with migraine and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). About 50% of tested families have mutations in CACNA1A, a gene located on chromosome 19p13 that codes for the α1-subunit of a brain-specific voltage-gated P/Q-type calcium channel (Ophoff et al., 1996). Mutations in the same gene occur in some pedigrees with hereditary paroxysmal cerebellar ataxia, indicating that these two disorders that share several features are allelic channelopathies (see Chapter 64). Gardner et al. (1997) reported another locus for FHM on chromosome 1q31 in a 39-member four-generation pedigree showing a clear FHM phenotype. This region on chromosome 1 contains a neuronal calcium channel α1E-subunit gene. Recent reports (Terwindt et al., 2001) not only implicate CACNA1A in FHM, but it may be overrepresented in patients with migraine without hemiplegia. Subsequent studies in other families have found two additional mutations linked to FHM. One that mapped to chromosome 1q21-q23 (De Fusco et al., 2003) is located in a gene coding for a subunit of a sodium/potassium pump (ATP1A2). Another mutation identified on chromosome 2q24 is located in a gene that encodes a sodium channel subunit (Dichgans et al., 2005). Although FHM is a rare genetic subtype of migraine with aura, the clinical similarities (with typical migraine, with and without aura) suggest at least the possibility of a shared pathophysiology. The discovery of a genetic locus for FHM has generated considerable interest and prompted a large effort in the field of molecular genetics to find the fundamental defect in the more common forms of migraine. However, studies testing the 19p13 region for linkage to typical migraine have produced conflicting results. One study implicated chromosome 19 mutations, either in the CACNL1A4 gene or in a closely linked gene in some pedigrees with familial typical migraine, and concluded that the disorder is genetically heterogeneous (Nyholt et al., 1998). The numerous sites reporting linkage to migraine underscores the likelihood of genetic heterogeneity in the common form of migraine. Published association sites include 1p13.3 (Kusumi et al., 2003), 4q31.2 (Tzourio et al., 2001), 9q34 (Lea et al., 2000), 11p15 (Mochi et al., 2003), 11q23 (Del Zompo et al., 1998; Peroutka et al., 1997, 1998), 17 q11.1-q12 (Ogilvie et al., 1998), 19p13.3/2 (McCarthy et al., 2001), and 22q11.2 (Emin Erdal et al., 2001).
A recent study based on a genome-wide screen of 50 multigenerational clinically well-defined Finnish families showing intergenerational transmission of migraine with visual aura yielded significant evidence of linkage between the migraine with aura phenotype and marker D4S1647 located on chromosome 4q24 (Wessman et al., 2002). Genomic and proteomic techniques will likely lead to further unraveling of the precise molecular defects that underlie migraine.
Pathophysiology
Genesis Of Migraine Syndrome
Clinical and experimental evidence supports the concept of abnormal intracranial and extracranial vascular reactivity in migraine and other vascular headaches. Dilatation of the scalp arteries causes increased scalp blood flow and large-amplitude pulsations during attacks of migraine. Radioactive xenon cerebral blood flow studies show significantly reduced regional flow through the cortex during the aura stage of migraine with aura. At first sight, these studies seem to support the long-held theory of cerebral vasoconstriction during the aura and increased external carotid flow during the headache phase. However, the vasoconstriction-vasodilatation model has several difficulties. First, there is solid evidence from functional MRI studies that a phase of focal hyperemia precedes the phase of oligemia during the migraine aura (Hadjikhani et al., 2001). Second, headache may begin while cortical blood flow remains reduced, thereby rendering obsolete the theory that vasodilatation is the sole mechanism of the pain. The oligemia that spreads across the cerebral cortex at a rate of 2 to 3 mm/min does not conform to discrete vascular territories, making it also unlikely that vasospasm of individual cerebral arteries, with subsequent cerebral ischemia, is the source of the aura. The headache after an aura is often on the inappropriate side. In other words, an ipsilateral headache can follow a right-sided visual field or somatosensory aura, despite the fact that the cerebral blood flow changes occurred in the opposite hemisphere. Finally, migraine is also associated with a premonitory phase in up to 60% of patients, which would be incompatible with a vascular or ischemic hypothesis. This premonitory phase consists of mood changes, thirst, food cravings, excessive yawning, and drowsiness. Although brainstem and hypothalamic generators have been proposed (Weiller et al., 1995; Zurak, 1997), the brain location initiating a migraine attack is still unclear.
The spreading depression noted by Leao’s and Lashley’s observations led to the hypothesis that the aura of migraine is primarily a neuronal event that causes the cortical circulation to close down in response to decreased metabolic requirements. Although spreading depression is undocumented in human cortex, functional MRI studies strongly support this hypothesis. There is also a body of evidence suggesting the presence of a disturbance in energy metabolism in both the brain and extraneural tissues of patients with migraine. Based on abnormalities identified in the mitochondrial respiratory chain and matrix enzyme activities from the muscle and platelets of patients with migraine, a defect in brain energy metabolism due to abnormal mitochondrial oxidative phosphorylation is proposed (Welch et al., 1995). In support of these findings, interictal phosphorus-31 magnetic resonance spectroscopy (MRS) studies have shown reduced phosphocreatine levels and phosphorylation potential and increased adenosine diphosphate levels in the occipital lobes of migraineurs. Phosphorus-31 MRS studies done during the ictal phase reveal depletion of high-energy phosphates without an accompanying change in intracellular pH, indicating that the energy failure results from defective aerobic metabolism rather than from vasospasm with ischemia.
In addition, there is evidence to support the presence of both systemic and brain magnesium deficiency in migraineurs, particularly in the occipital lobes (Welch et al., 1995). Magnesium normally maintains a strongly coupled state of mitochondrial oxidative phosphorylation. Magnesium also plays an important role in “gating” N-methyl-d-aspartate (NMDA) receptors. A magnesium deficit can therefore result in an abnormality of mitochondrial oxidative phosphorylation and lead to a gain in NMDA receptor function, thereby causing instability of neuronal polarization because of a loss of ionic homeostasis. This would then lead to a state of neuronal hyperexcitability and a lower threshold for spontaneous depolarization.
Spreading depression might therefore be more aptly described as spreading activation followed by a wave of spreading depression. This would support the clinical observation of “positive” visual “scintillations” followed by a “negative” visual scotoma or by the march of positive sensory (paresthesia) symptoms. This theory may also explain why focal hyperemia may precede spreading oligemia. These findings taken together suggest that the changes in blood-vessel caliber and blood flow may be due to a primary neuronal event triggered by enhanced neuronal excitability and susceptibility to spontaneous depolarization, resulting in prolonged hypometabolism because of impaired energy metabolism caused by mitochondrial dysfunction. The finding of increased interictal lactate levels in the occipital cortex of migraineurs using proton MRS supports this hypothesis (Watanabe et al., 1996). Strengthening the theory that the migraine aura is a primary neuronal event was another study that demonstrated no change in the apparent diffusion coefficient on diffusion-weighted MRI, despite a reduction of regional cerebral blood flow during spontaneous migraine aura. Because diffusion-weighted MRI is very sensitive to tissue ischemia, the researchers concluded that the reduction in cerebral blood flow was not of sufficient magnitude to cause tissue ischemia (Cutrer et al., 1998).
Platelets And Serotonin
The role of serotonin in migraine has yet to be fully defined. It constricts large arteries and is a dilator of arterioles and capillaries. It is also—perhaps more importantly—a neurotransmitter. Serotonin-containing neurons are especially concentrated in the brainstem raphe, the projections of which have a widespread distribution to other neuronal centers and cerebral microvessels. The importance of the brainstem in migraine is still uncertain. Highlighting its role is the presence of binding sites for specific antimigraine drugs and the demonstration of persistent brainstem activation during and after a migraine attack, as imaged by PET (Weiller et al., 1995). Moreover, recurrent migraine headaches were precipitated in a nonmigraineur after a stereotactic procedure produced a lesion in the dorsal raphe and periaqueductal gray matter, which is part of the endogenous antinociceptive system. Welch et al. (2001) found evidence for simultaneous activation of red nucleus, substantia nigra, and occipital cortex during provoked attacks with a visual stimulation paradigm. This was demonstrated using blood oxygen level–dependent functional MRI, which revealed hyperoxia in these regions. Furthermore, serotonergic circuits are believed involved in the modulation of sleep cycles, pain perception, and mood, all of which are important factors in migraine.
Mechanism Of The Headache
Supporting the importance of neurogenic inflammation in the production of the pain of migraine is the fact that medications that block neurogenic inflammation and mediate vasoconstriction are successful in aborting a migraine attack. Neurogenic plasma extravasation can be inhibited by the ergot alkaloids, indomethacin, acetylsalicylic acid, valproic acid, and the new highly selective serotonin (5-HT1) receptor agonists; these are discussed later under Symptomatic Treatment. However, neurogenic inflammation by itself is probably not the sole mechanism of pain, because treatment with selective inhibitors of neurogenic inflammation has uniformly failed in the clinic (May et al., 2001). Ergotamine compounds and the “triptans” also act centrally to inhibit the activity of neurons within the TNC, which may be important in the termination of an attack. Whether these drugs act on the postsynaptic neuron in the TNC is unclear.
Activation of the trigeminal sensory system is reflected by the development of cutaneous allodynia in most patients during migraine attacks. The underlying mechanism is sensitization of central trigeminal neurons (Burstein et al., 2001). Triptans can prevent but not reverse cutaneous allodynia in patients and central sensitization in animals, and the presence or absence of cutaneous allodynia can be used as a marker to predict whether triptans would be able to abort a given migraine attack (Burstein et al., 2004). Repeated quantitative sensory testing of patients with migraine was done early in a migraine attack (triptans given before cutaneous allodynia) and late in a migraine attack (triptans administered after cutaneous allodynia). In attacks without allodynia, triptans completely relieved the headache and blocked the development of allodynia. In 90% of attacks with established allodynia, triptans provided little or no headache relief and did not suppress allodynia. However, late triptan therapy abolished the throbbing quality of the pain and its worsening upon bending over (peripheral sensitization) in 90% of attacks in which pain relief was not complete and allodynia was not suppressed.
A link between the migraine aura and headache has now established that cortical spreading depression (CSD), implicated in migraine visual aura, activates trigeminovascular afferents and evokes a series of cortical meningeal and brainstem events consistent with the development of headache (Bolay et al., 2002). By using laser speckle-contrast imaging, CSD was shown to cause long-lasting blood flow enhancement selectively within the middle meningeal artery, dependent upon trigeminal and parasympathetic activation. CSD has also been shown to lead to plasma protein leakage within the dura mater. This neuroinflammatory response to CSD may be due in part to an up-regulation of inducible nitric oxide synthase and inflammation (Reuter et al., 2002). These findings provide a neural mechanism by which extracerebral cephalic blood flow and neurogenic inflammation couple to a cortical neuroelectrical event.
Treatment and Management
Persons with migraine may note that stress is a trigger for attacks, but helping them deal with or avoid stress is difficult. Long-term stress management may require the help of a psychologist or other appropriately trained professional. Useful techniques include biofeedback, relaxation training, hypnosis, and cognitive behavioral training (Campbell et al., 2000).
Pharmacotherapy
Symptomatic Treatment
Triptans
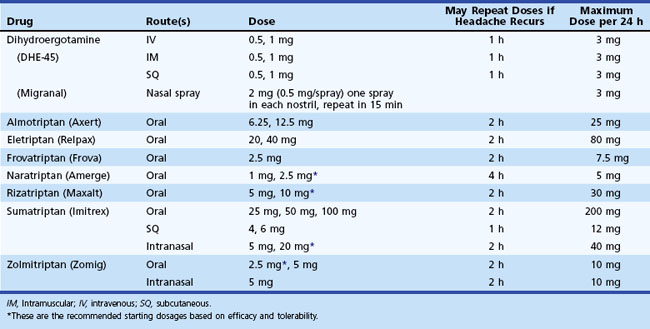
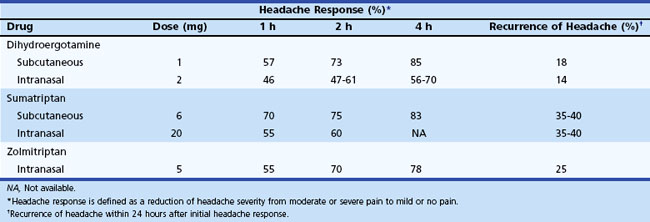
Administration of sumatriptan can be orally, intranasally, and by subcutaneous injection (Tables 69.3 to 69.5). Self-administered as a 6-mg subcutaneous injection, either using the manufacturer’s auto-injector device or conventional subcutaneous injection, sumatriptan results in significant pain relief at 1- and 2-hour time points after drug administration (see Table 69.4). For patients who had no significant pain relief after 1 hour, administration of a second dose of 6 mg provided little further benefit. Zolmitriptan is available as an oral and intranasal preparation.
Table 69.5 provides a comparison of the currently available oral triptans. The potential side effects are quite similar: tingling, flushing, and a feeling of fullness in the head, neck, or chest. In general, the indications and contraindications for all 5-HT1 agonists are similar. They are not safe when administered within 24 hours of ergot preparations or other members of the triptan class.
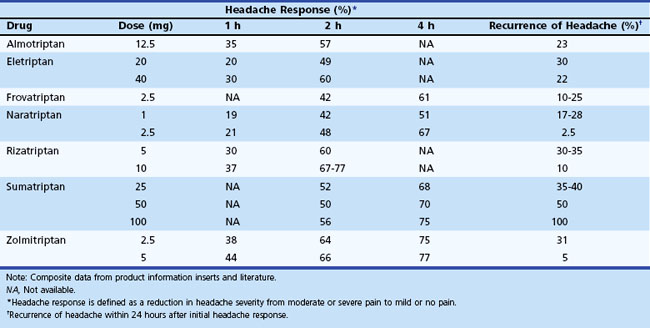
At this time, no evidence exists to allow accurate prediction of which of these agents will be most effective in a given patient. A few practical guidelines are available, based on the clinical situation and knowledge about available agents. If severe nausea or vomiting occurs early in an attack, the parenteral or intranasal routes should be used. Some patients may prefer nasal or injectable routes (sumatriptan and zolmitriptan). For patients with benign but intolerable side effects from this group of medications, consider naratriptan or almotriptan, given their favorable side-effect profiles. Recurrence of headache after initial relief may necessitate a repeat dose. With future attacks, a higher dose (if available) may be used, or the triptan can be combined with an NSAID and/or metoclopramide. If one agent fails, it seems reasonable, barring major side effects, to try another agent in the class. Since there is evidence that some of these agents have a lower oral bioavailability when taken by patients with migraine, both during an attack and interictally (Aurora et al., 2006), it is logical to consider combining them with metoclopramide to improve gastric emptying. Coadministration with an NSAID might be helpful for individuals whose headache responded only partially or who tend to have a headache recurrence after initial relief (Peroutka, 1998).
Ergots
Dihydroergotamine (DHE) has been a treatment for migraine since the 1940s. Its poor oral bioavailability limits its administration to the parenteral and intranasal routes (see Tables 69.3 and 69.4). Patients can self-administer this drug by each of these routes. This medication should be considered when nausea and vomiting limit the use of oral medications or when other medications are ineffective. Although DHE’s effects are slower than sumatriptan (see Table 69.4), efficacy after 2 hours is similar, and the drug is associated with a lower recurrence of headache in 24 hours. Increased nausea in some patients may require combination with an antiemetic agent. When given intravenously (IV) in an acute medical care setting, use of an antiemetic is mandatory. A new inhaled formulation of DHE is currently being evaluated for U.S. Food and Drug Administration (FDA) approval. This new formulation may provide a means of administering DHE without IV infusion.
For many patients, an attack of migraine becomes a harrowing experience. After a variable period, they go to an emergency room or physician’s office expecting relief. These patients pose a difficult problem for the physician, but there are several treatment options. One can use neuroleptic agents acutely, with or without DHE. DHE (0.5-1 mg) with metoclopramide (10 mg) by IV injection is an effective treatment for acute headache and provides an alternative to the use of an opioid. Similarly, prochlorperazine, 10 mg IV over 3 to 4 minutes, alone or combined with DHE, can be effective. Sumatriptan, 6 mg subcutaneously, may provide relief of both the headache and the associated symptoms. Evidence is mixed with respect to the efficacy of magnesium sulfate as an acute treatment for migraine; IV infusion of 1 g of magnesium sulfate results in relief of headache pain in some patients (Bigal et al., 2002). Alternatively, chlorpromazine, 5 mg injected IV every 10 minutes to a maximum of 30 mg, is also an effective agent when used acutely. The latter agent often produces hypotension, and patients should first receive a bolus of 250 to 500 mL of 5% dextrose in one-half normal saline. (Dehydrated patients should always receive appropriate IV hydration.) Some patients develop acute extrapyramidal symptoms after treatment with neuroleptic agents. These are treatable with parenteral diphenhydramine, 25 to 50 mg. The neuroleptic agents do produce sedation, and patients should be advised not to operate a motor vehicle after treatment. Injectable ketorolac, 60 mg given intramuscularly, is another alternative to the narcotic or sedative agents. The use of this NSAID in elderly patients, those who are dehydrated, or those having any history of renal insufficiency should be avoided. A single dose of dexamethasone combined with other parenteral antimigraine agents is useful for the emergency room treatment of attacks of intractable migraine. Although not a first-line treatment, combination of an opioid and an agent for nausea, such as chlorpromazine (25-50 mg), promethazine hydrochloride (12.5-25 mg), or prochlorperazine (5-10 mg) is an effective treatment if the physician is sure the patient genuinely has a headache of major proportions.
Prophylactic Treatment
β-Adrenergic Blockers
β-Adrenergic antagonists are widely used for prophylaxis of migraine headaches (Silberstein, 2000). Propranolol in doses of 80 to 240 mg/day, if tolerated, should be given a trial of 2 to 3 months. Compliance increases with the use of a long-acting form of propranolol given once daily. Side effects are not usually severe. Lethargy or depression may occur and may be a reason for discontinuation of the medication. Hypotension, bradycardia, impotence, insomnia, and nightmares can all occur. As with all β-adrenergic blocking agents, propranolol should be discontinued slowly to avoid cardiac complications. It is contraindicated in persons with a history of asthma or severe depression and should be used with caution in patients using insulin or oral hypoglycemic agents, because it may mask the adrenergic symptoms of hypoglycemia. The benefit of propranolol in migraine may be separate from its action as a β-adrenergic blocking agent, but its exact mechanism of action is unknown.
Antidepressants
Amitriptyline and other tricyclic antidepressants can be helpful in migraine prophylaxis (Silberstein, 2000). The benefit seems to be independent of their antidepressant action. Blockade of noradrenaline uptake at catecholamine terminals and inhibition of serotonin reuptake may be related, but the action of antidepressants in migraine is unclear at present. Used in doses of 10 to 150 mg at night, amitriptyline, imipramine, desipramine, or nortriptyline may all provide some reduction in attacks of migraine, although evidence of efficacy in clinical trials is available only for amitriptyline. Side effects can be rather troublesome. Morning drowsiness, dryness of mouth, weight gain, tachycardia, and constipation are common. The anticholinergic side effects may decrease with time. If tolerated, give the tricyclic agents a trial of at least 3 months after reaching a therapeutic dose. The optimal dose for migraine prophylaxis is determined by titration to the effective or maximum tolerated dose within the therapeutic range (usually 40-150 mg).
Anticonvulsants
In the early 1990s, several blinded placebo-controlled studies showed a beneficial effect of valproate in the prophylactic treatment of migraine; 50% of patients showed a response with a 50% or better reduction in migraine incidence (Silberstein, 2000). Valproic acid, given in the form of divalproex sodium, is generally effective at a range of 500 to 1750 mg/day taken in divided doses. Side effects include sedation, dizziness, increased appetite, increased bleeding time, increased fragility of hair, and an asymptomatic increase in liver function test values. Valproate is contraindicated in women who are at risk for becoming pregnant, because it is associated with an increased risk for neural tube defects. Gabapentin is effective in the reduction of migraine incidence (Mathew et al., 2001). It also has beneficial effects in somatic pain and may be a good choice if a patient has neck pain, back pain, or painful peripheral neuropathy as well as migraine. It appears relatively well tolerated, although dizziness and sedation may limit its use in some patients. The usual therapeutic dose range for gabapentin is 900 to 2400 mg/day. Topiramate is a recent addition to the antimigraine armamentarium; its efficacy for migraine was demonstrated in pivotal large randomized trials (Brandes et al., 2004). Topiramate has effects not only on γ-aminobutyric acid (GABA) but also on non-NMDA glutamate and carbonic anhydrase activity. It may have prominent sedating and cognitive side effects, making a slow gradual titration of the drug (15-25 mg/wk initially) to the therapeutic range of 75 to 200 mg/day the most successful strategy. Other side effects include paresthesia and weight loss, the latter making topiramate a particularly attractive choice for many patients. It is also associated with a mildly increased risk for kidney stones.
Other Prophylactic Agents
Riboflavin administered orally in a dose of 400 mg/day has been shown to be effective in migraine prophylaxis in a prospective randomized controlled study that enrolled a relatively small number of subjects. Its effect on the frequency of attacks was not statistically significant until the third month of the trial (Schoenen et al., 1998). There are minimal side effects associated with this agent.
Evidence is mixed regarding the efficacy of magnesium in migraine prophylaxis. Oral magnesium supplementation with 600 mg of a chelated or slow-release preparation is the recommended dosage. Magnesium-induced diarrhea and gastric irritation are the most common side effects (Mauskop and Altura, 1998). Aspirin, 325 mg, taken every other day for the prevention of cardiovascular disease slightly reduces the incidence of migraine. The NSAIDs have some benefit for migraine prophylaxis.
Onabotulinumtoxin A injection in the treatment of migraine is supported by an increasing body of anecdotal evidence from small series and a controlled study (Silberstein et al., 2000a). The pooled data from two large multicenter placebo-controlled trials indicates efficacy for onabotulinumtoxin A in the treatment of chronic migraine (Dodick et al., 2010). The treatment may exert its effect by reducing the release of proinflammatory and vasodilating neuropeptides from nociceptive terminals. Botulinum toxin blocks the release of glutamate from nociceptive terminals and therefore may reduce or inhibit the development of peripheral and central trigeminal sensitization. Doses of approximately 150 units are injected in muscles of the forehead, as well as the temporalis, splenius capitis, and trapezius. The effect, when it occurs, usually appears within 7 to 10 days and persists for up to 3 months. Side effects are minimal when avoiding lateral forehead injection. For patients who do not tolerate or do not comply with daily drug usage or who may prefer an injectable agent rather than chronic oral therapy, onabotulinumtoxin A may be a viable option.
Hormones and Migraine
Menstrual Migraine
Migraine attacks are associated with menses in one of three ways. The attacks may occur exclusively during menstruation and at no other time during the cycle. This association is referred to as true menstrual migraine (TMM), and it has been proposed that TMM be defined as attacks that occur between days −2 and +3 of the menstrual cycle (MacGregor, 1996). The prevalence of TMM according to this definition is about 7%. More commonly, migraine attacks occur throughout the cycle but increase in frequency or intensity at the time of menstruation. This association occurs in up to 60% of female migraineurs. Finally, premenstrual migraine can occur between days −7 and −3 before menstruation as part of premenstrual syndrome or late luteal phase dysphoric disorder. A cluster of symptoms in the luteal phase—depression, irritability, fatigue, appetite changes, bloating, backache, breast tenderness, and nausea—characterize the disorder. These different relationships between migraine and the menstrual cycle can be determined by reviewing headache diaries, and their distinction is important because pathophysiology may differ, as would the therapeutic approach.
Management of Menstrual Migraine
Acute Menstrual Migraine Therapy
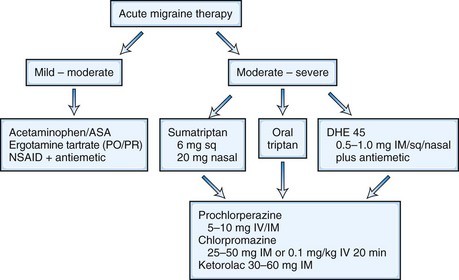
The goal of acute menstrual migraine therapy is to decrease the severity and duration of pain as well as the associated symptoms of an individual migraine attack, including nausea, vomiting, photophobia, and phonophobia. Some women may control attacks of menstrual migraine quite adequately with abortive therapy only (Fig. 69.6).
Prophylactic Menstrual Migraine Therapy
Prophylaxis may either be perimenstrual (cyclic) or continuous (noncyclic) (Box 69.3). Many of the regimens suggested for perimenstrual migraine prophylaxis depend on regular menstruation. Perimenstrual prophylaxis commences a few days before the period is expected and continues until the end of menstruation. In women whose cycles are difficult to predict, continuous prophylaxis with standard migraine prophylactic agents such as tricyclic antidepressants and beta-blockers can be quite effective if taken continuously.