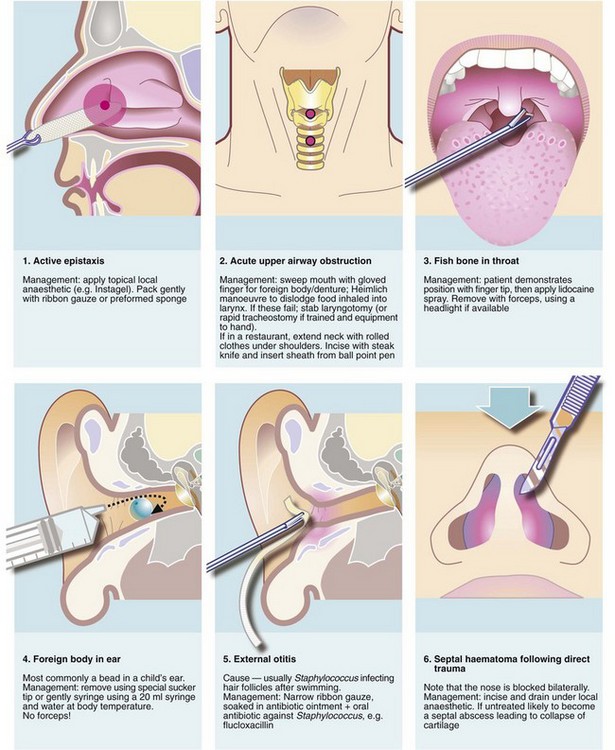
Head and maxillofacial injuries
Head injuries
Introduction
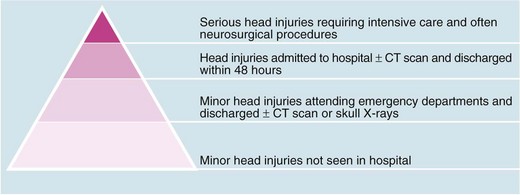
Head injury is a potentially devastating problem with an enormous social and economic cost. Up to a million people attend emergency departments in the UK each year following head injury. Using the Glasgow Coma Scale (GCS, Table 16.1) as a clinical indicator, 90% are classified as mild/minor, with scores of 13–15 respectively, 5% as moderate (score 9–12) and 5% as severe (score 3–8). Head injuries cause approximately 3500 deaths each year in the UK, amounting to about 0.6% of all deaths. Figure 16.1 shows how serious injuries represent the tip of an iceberg of the impact of head injury on health care. The greatest burdens are the acute management of all these cases and dealing with the chronic disability these injuries can cause.
Table 16.1
| Clinical observation | Score* |
| Eye opening: | |
| Spontaneous | 4 |
| To verbal command | 3 |
| To pain | 2 |
| None | 1 |
| Motor response: | |
| Obeys commands | 6 |
| Localises pain | 5 |
| Flexion withdrawal to pain | 4 |
| Abnormal flexion (decorticate) | 3 |
| Extension to pain (decerebrate) | 2 |
| None | 1 |
| Verbal response: | |
| Orientated | 5 |
| Confused conversation | 4 |
| Inappropriate words | 3 |
| Incomprehensible words | 2 |
| None | 1 |
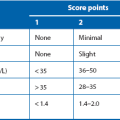
*On this scale, a patient’s Glasgow Coma score is the sum of the scores from all three sections. The worst total score is 3, the best is 15. After the initial score, the observations and scoring are repeated at intervals to look for deterioration
Less than half the head injury patients attending emergency departments require CT scanning or hospital admission and only a small proportion require specialist neurosurgical investigation and care. The difficulty is to recognise those at risk without over-investigating or admitting patients unnecessarily. In order to streamline this process, various triage algorithms have been produced, notably NICE guidelines (http://guidance.nice.org.uk/CG56/Guidance—summarized in Box 16.4, see below). The main focus is detecting clinically important brain injuries (and cervical spine injuries—Box 16.1) whilst avoiding admission of those with low risk of sequelae.
Primary brain injury
Focal brain injuries
Focal injuries are the result of trauma to localised brain areas and are readily visible on CT scanning. The main lesions are cerebral contusion, laceration or haematoma, all of which may act as space-occupying lesions and are liable to result in secondary brain injury. The site and extent of the primary injury depend on the nature of the damaging force (see Fig. 16.2). Contusions may be small or large and occur either beneath the area of impact (coup) or contralateral to it (contre-coup), caused by rebound of the brain within the skull at the time of impact (Fig. 16.3). The severity of trauma required to cause focal brain injury will usually result in a period of loss of consciousness, followed by confusion.
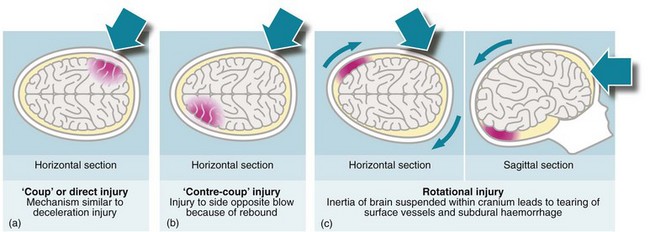
Fig. 16.2 Mechanisms of brain injury
(a) The mechanism of ‘coup’ or direct injury is similar to a deceleration injury (shown in horizontal section). (b) A ‘contre-coup’ injury affects the side opposite to the blow because of rebound (horizontal section). (c) In rotational injury the inertia of the brain suspended within the cranium leads to the tearing of surface vessels and subdural haemorrhage (horizontal and sagittal sections)
Secondary brain injury
Intracranial bleeding
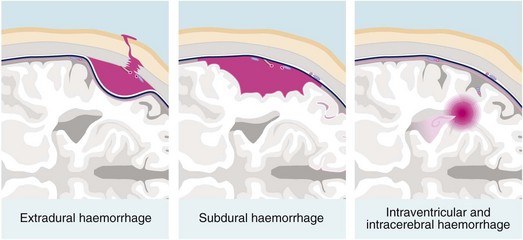
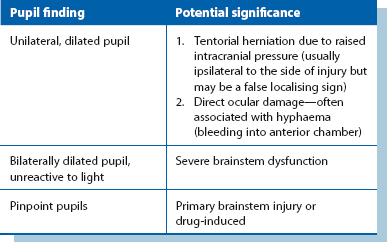
Post-traumatic intracranial bleeding is classified into extradural (epidural), subdural, intracerebral or subarachnoid (see Fig. 16.4). Intracranial bleeding acts as a mass lesion causing a general rise in ICP, whilst local brain compression can cause focal neurological deficit. Untreated, raised ICP may cause ‘coning’. One or both temporal lobes herniate through the tentorium cerebelli, compressing the third nerve and midbrain, whilst herniation of the cerebellar tonsils through the foramen magnum compresses the medulla, causing neurological deterioration and often death. Rising intracranial pressure manifests initially with deteriorating conscious level. Late clinical signs are:
• An enlarging, unresponsive pupil
• Central respiratory depression
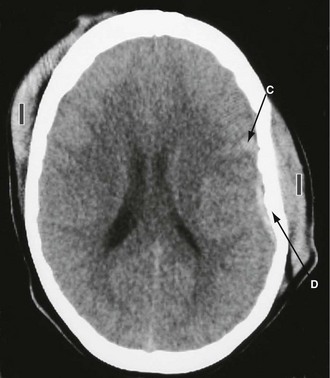
Extradural (epidural) haemorrhage: Extradural haemorrhage occurs when blood accumulates in the space between dura and calvarium. It is most common in children and younger adults, because their dura is less adherent to the skull. Most have a skull fracture, usually in the temporal region (Fig. 16.5). Almost 90% are due to rupture of an artery, usually the middle meningeal or a branch. Immediately after injury causing loss of consciousness, in up to half the patients, there will be a lucid interval, perhaps with no symptoms other than worsening headache. In either group, this is followed by deteriorating conscious level; temporal lobe herniation then leads to compression of the third nerve and pupillary dilatation. Death quickly follows unless the haematoma is evacuated rapidly. Emergency CT scanning is indicated to confirm the diagnosis (typically a lentiform-shaped clot—see Fig. 16.5b) and to show its position. With increased awareness of the condition and widespread availability of CT scanning, emergency ‘blind’ burr hole drainage is almost never appropriate. Urgent transfer to a neurosurgeon for craniotomy is the best course of action, almost without exception.
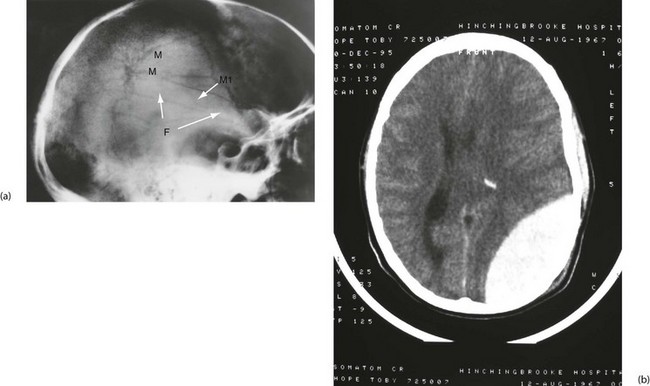
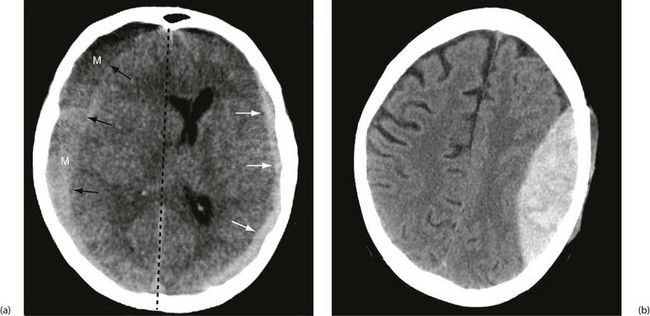
Subdural haematoma: Subdural haematoma usually results from tearing of veins passing between cerebral cortex and dura, or from injury to vessels on the surface of the brain. Blood accumulates in the large potential space between dura mater and arachnoid mater. The haematoma tends to spread laterally over a wide area (Fig. 16.6). In contrast to extradural haemorrhage, there is usually underlying primary brain injury. Acute subdural haemorrhage is more common in older adults because the brain is more mobile within the cranial cavity.
Chronic subdural haematoma: In the elderly, subdural haematomas may develop gradually following trivial, often unrecalled, head trauma. This is due to the relative ease with which their atrophic brains can accommodate blood under venous pressure. Only as the clot lyses and fluid is drawn into the subdural space by osmosis does the condition manifest, some weeks or months later, as non-specific neurological deterioration, chronic headache or coma. At this point the liquid haematoma can be evacuated via burr holes, and the subdural space irrigated with warm saline.
Intracerebral haemorrhage: Haemorrhage into the brain parenchyma is caused by primary brain injury. Multiple small deep lesions are often associated with diffuse axonal injury. Small haematomas should be managed conservatively and monitored for expansion using serial CT scans. A larger haematoma causing ‘mass effect’ should be evacuated early to prevent secondary brain damage.
Skull fractures
The importance of skull fractures
With the advent of NICE guidelines (Box 16.2), CT is the investigation of choice for the diagnosis of clinically significant head injury. Skull radiographs now play little role in the diagnosis, but may be indicated in some instances, for example suspected non-accidental injury in children or lack of access to CT.
Types of skull fracture
Linear fractures: These involve mainly the skull vault, often with little external sign of injury, although there may be some overlying scalp bruising or swelling. Linear fractures rarely exhibit displacement unless there are multiple fracture lines.
Depressed fractures: These are usually caused by blunt injuries and the overlying scalp is usually lacerated or severely bruised. Such fractures rarely produce serious primary brain injury unless they are depressed more than the full thickness of the skull vault. Elevation of closed depressed fractures is usually performed for cosmetic reasons.
Open (compound) fractures: An open fracture indicates a communication exists between underlying brain and external environment. The communication may be overt (e.g. a penetrating injury), or result from a skull base fracture. Linear and depressed skull fractures can both be compound. There is a high risk of infection in a depressed fracture if the dura is torn, so early debridement and dural closure are indicated. Compound fractures of the base of the skull are diagnosed clinically (Box 16.3) +/− CT. Fluid may be analysed for beta transferrin.
Management of head injuries
Clinical assessment
History: The most important factors that indicate potential brain injury and a risk of future complications are unconsciousness and amnesia for events before the impact (retrograde amnesia). The duration of unconsciousness and amnesia are roughly proportional to the severity of brain injury. If the patient was travelling in a motor vehicle, the extent of injuries to other passengers may give an indication of the energy transfer in the accident. Likewise, knowing the use of seatbelts and helmets can be useful.
Examination: In addition to general examination, a systematic neurological examination must be performed, however trivial the head injury. Particular attention should be paid to:
The findings should be recorded periodically on a standard head injury proforma, and the Glasgow Coma Scale (GCS) calculated for each set of observations. If there is a deep scalp laceration or a history of penetrating injury, the scalp should be assessed carefully for the presence of a bony defect or step. Because of scalp mobility, any underlying bone injury may not lie directly beneath the scalp wound.
1: Level of consciousness This is the most important single observation in head injury patients. The GCS (see Table 16.1) is used worldwide to standardise assessment and monitoring of head injuries. Level of consciousness can be categorised simply and reproducibly by this method. Formal assessment should take place after resuscitation and before intubation if possible. Aggressive behaviour in a patient smelling of alcohol or having taken illicit drugs must not be assumed to result from intoxication (i.e. removal of social inhibition) because this behaviour can also be a manifestation of brain injury or hypoxia. A thorough examination and CT scan should be performed to exclude significant cerebral injury. In addition to eye opening and verbal responses, the motor response is an important observation. In a patient with impaired conscious level, pressure over the supraorbital nerve at the orbital rim is usually employed to elicit pain. To be scored as being able to localise the pain, the patient’s hand should rise above the clavicle.
An assessment of the severity of the head injury can be made on the elicited GCS following resuscitation. The probability of there being an intracranial haematoma likely to need surgery in GCS groups is shown in Table 16.2.
Table 16.2
Probability of intracranial haematoma requiring surgery according to the severity of head injury as assessed by the Glasgow Coma Scale (GCS)
| GCS score | Severity of head injury | Probability of haematoma |
| 3–8 | Severe | 1 in 7 |
| 9–12 | Moderate | 1 in 50 |
| 13–14 | Mild | 1 in 3500 |
2: Pupil size and reactivity Pupillary size and response to light should be assessed, along with the full range of eye movements and, if possible, the visual fields. Normal pupillary size and response to light require the integrity of both 2nd and 3rd cranial nerves. Pupillary changes are a late indicator of developing intracranial hypertension (Table 16.3).
3: Limb movements and responses In a fully conscious patient, tone, power and coordination can be readily assessed. If a subtle abnormality is suspected, the patient should be asked to close the eyes and hold the arms outstretched with palms upwards. Pronation or downward drift on one side indicates brain injury. For the semiconscious or unconscious patient, the pattern of limb response to painful stimuli provides a useful indication of the conscious level, as indicated in the GCS (see Table 16.1).
Imaging for suspected head injuries
The published NICE guidelines for head and cervical spine imaging are summarised in Box 16.2.
Practical management of head injuries
• Patients with a GCS of 15 and no risk factors demanding inpatient observation (alcohol intoxication, etc.) can be discharged safely to the care of a responsible adult with standard head injury advice. Patients with risk factors requiring admission should be observed in a ward with experience in managing head injuries (Box 16.4)
• Patients with reduced scores require early CT scanning, following NICE guidelines (Box 16.2)
• Patients with normal scans in whom GCS returns to normal can be discharged, unless other injuries or social circumstances make admission necessary (see Box 16.4)
• Patients admitted with uncomplicated minor head injuries who are fully alert can safely be allowed to go home after 24 hours of neurological observation
• Severely head-injured patients require urgent discussion with the local neurosurgery unit, resuscitation and transfer once resuscitated and stabilised
Head injury observations: The necessary observations for patients admitted to hospital are shown in Table 16.4. These are sufficiently sensitive to give early warning of developing complications. The frequency of observation depends on the state of the patient. If there is a skull fracture or any suggestion of reduced consciousness, confusion, disorientation, alcohol or drug effects, observations should be made at 30-minute intervals until a GCS of 15 is maintained, then hourly for 4 hours, then 2 hourly after that. Observations are recorded or plotted on a special head injury proforma so that deterioration will be immediately obvious and can be reported to medical staff at once. Note that transient unconsciousness or amnesia with full recovery is not necessarily an indication for admission of an adult, but may be so in a child, and patients with head injuries may have other serious internal injuries that are easily overlooked.
Table 16.4
Essential observations for head injury patients (findings should be recorded on a standard head injury proforma)
| Observation | Sign of neurological deterioration |
| Conscious level (GCS) | Falling score |
| Pupil size and light response | Dilatation, loss of light reaction or developing asymmetry |
| Respiratory pattern and rate | Irregularity, slowing or reduced depth of breathing |
| Developing neurological signs | Focal signs point to localised intracranial damage |
| Pulse rate | Falling pulse rate (late sign) |
| Blood pressure | Rising blood pressure (late sign) |
Indications for prompt neurosurgical referral after head injury: The care of all patients with new, surgically significant abnormalities on imaging should be discussed with a neurosurgeon and, where possible, the imaging linked electronically. Other criteria for referral include:
• Persisting coma (GCS less than or equal to 8) after resuscitation
• Unexplained confusion for more than 4 hours
• Deterioration in GCS after admission (greater attention should be paid to deterioration of motor response)
• Progressive focal neurological signs
• A seizure without full recovery
Management of moderate and severe head injuries: Most trauma deaths result from head injuries or from multiple injuries involving chest, abdomen and limbs. Some head injuries are so severe as to preclude survival, whilst others require urgent recognition and surgical decompression, e.g. extradural haemorrhage. The report of the Working Party on Head Injuries (Society of British Neurological Surgeons, 1998) recommended a maximum delay of 4 hours between the injury and neurosurgical intervention.
Initial management: Any patient with focal neurological signs, whose conscious level is moderately depressed (GCS 14 or less) or who is unconscious, must be considered to have a significant head injury. Patients should be resuscitated along ATLS lines, focusing on secondary brain injury prevention, prioritisation of other injuries and the timing of CT scanning. If the receiving hospital does not have CT scanning facilities, the patient may need to be transferred to a regional centre after resuscitation. Patients requiring urgent neurosurgery should be transferred promptly, but only after adequate resuscitation.
Continuing care: Management of severe head injury ideally requires specialist care from neurosurgeons and neurointensivists, and transfer to a neurosurgical unit should be seriously considered. Continuing care of the patient with a stable serious brain injury (usually in a neurological critical care unit) involves some or all of the following procedures:
• Intensive monitoring of vital signs and neurological status
• Endotracheal intubation and artificial ventilation
• Nasogastric aspiration for any unconscious patient to prevent inhalation of gastric contents
• Monitoring of fluid and electrolyte balance
• Monitoring of intracranial pressure using a surgically implanted ICP monitoring device
• Measures for control of raised intracranial pressure—escalating protocols are employed for incremental rises, including controlled hyperventilation (reducing PCO2 causes cerebral vasoconstriction, reduced cerebral oedema and hence reduced intracranial pressure), CSF drainage, mannitol or hypertonic saline infusion (for its osmotic effect in reducing cerebral oedema), hypothermia, barbiturates and decompressive craniectomy
• Measures to maintain cerebral perfusion pressure—(volume expansion and inotropic support)
Rehabilitation: The risk of long-term disability following severe head injury is high, and significant cognitive dysfunction may persist in patients after good physical recovery. Even patients with apparently minor brain injury may be moderately or severely disabled a year after injury (post-concussion syndrome). Problems include headache, dizziness, mental deficits, slowness of thought, poor concentration, difficulty communicating, inability to work, poor performance at school and difficulties with self-care.
Maxillofacial injuries
Mandibular fractures
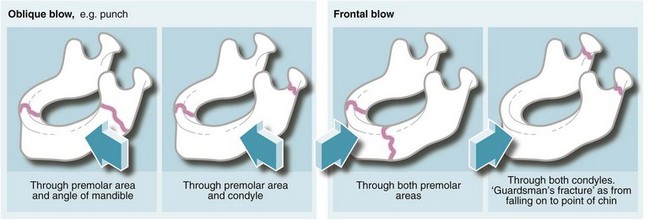
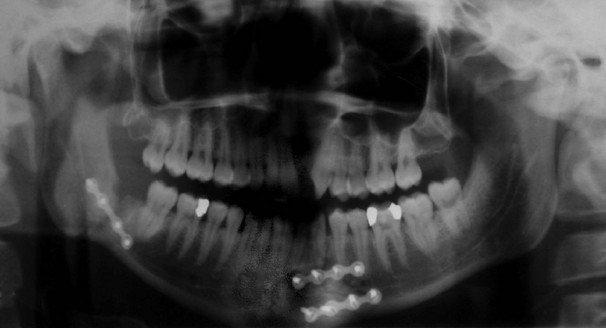
The common sites of mandibular fractures are shown in Figure 16.9. Because of the effects of oblique trauma, a fracture on one side is often accompanied by a fracture on the other side in a different position, e.g. body of mandible on one side and condylar neck on the other. Fracture lines tend to occur through points of weakness, e.g. mental foramina, unerupted third molar teeth or condylar necks. Most undisplaced mandibular fractures need no active intervention but displaced fractures require fixation. This can be achieved by wiring the lower teeth to the upper teeth, by direct wiring of the bones or (more often these days) by internal plate fixation (see Fig. 16.10). Any fracture passing through a tooth socket defines the fracture as ‘compound’ and prophylactic antibiotics should be administered.
Fractures of the orbit and zygoma
Depressed fractures of the zygoma
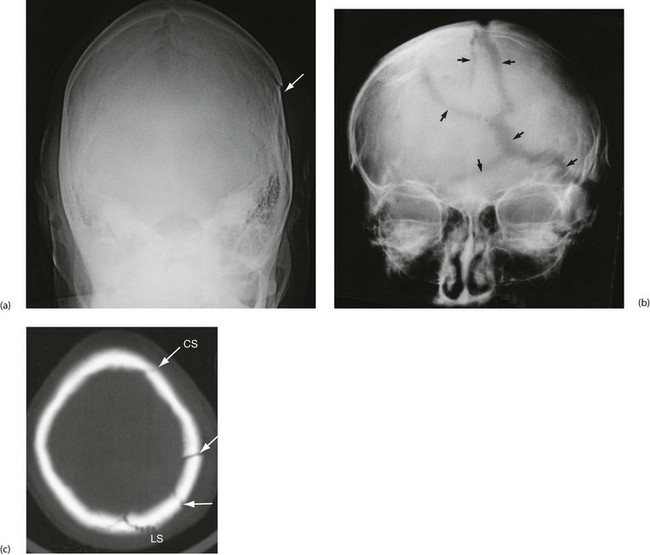
A depressed fracture of the zygoma (see Fig. 16.11) is the most common orbital fracture and results from a blow to the cheek. The fracture line usually passes through the infraorbital foramen and causes a palpable step in the inferior orbital margin. The infraorbital nerve becomes compressed with any substantial degree of depression, causing paraesthesia or numbness in the upper lip, upper teeth and buccal mucosa on that side. Diagnosis may be suspected by flattening of the cheek contour; this is best seen from above and behind the patient. Overlying oedema may obscure a depressed fracture, and these patients warrant radiological examination. An associated fracture of the lateral orbital wall may produce enough bleeding for it to track forward under the conjunctiva. This type of subconjunctival haemorrhage has no visible posterior limit and is the characteristic sign of an orbital wall fracture (Fig. 16.12).
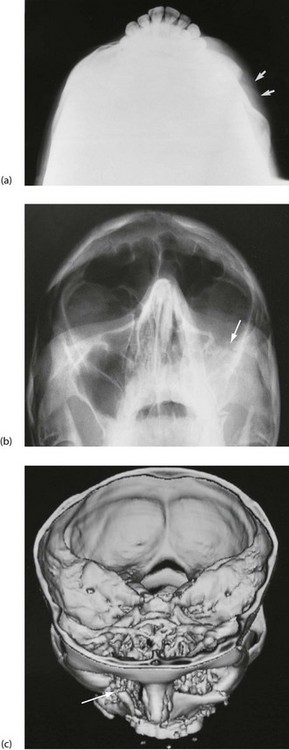
Fig. 16.11 Depressed zygomatic fractures
(a) Submento-vertical projection of a 43-year-old man who had been punched on the left cheek showing a depressed fracture of the zygomatic arch (arrowed). (b) 30° Occipito-mental radiograph after a similar injury in a different patient. This patient had a depressed ‘tripod’ fracture of the zygoma manifest by discontinuity of the lower orbital margin (arrowed). Note that since the roof of the maxilla is involved, the maxillary sinus (the antrum) has typically filled with blood and is rendered radiopaque. (c) 3D reconstruction of CT scans showing a severely depressed fracture of right lower orbital rim involving maxilla and zygomatic body (arrowed). The left lower orbital rim is also fractured and displaced
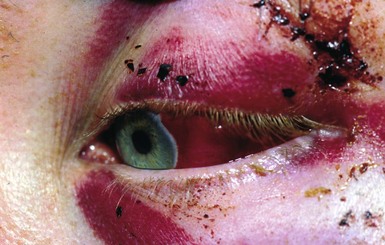
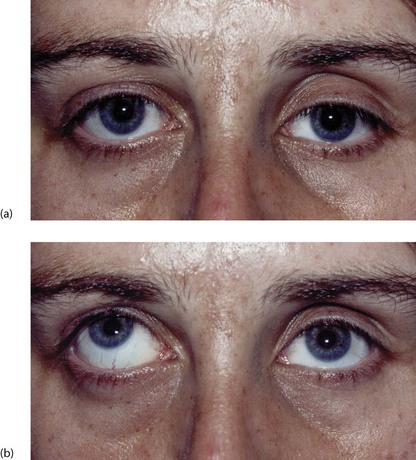
Fig. 16.12 Subconjunctival haematoma following a head injury
This 14-year-old boy fell off his bicycle and momentarily lost consciousness. This photograph shows a subconjunctival haematoma with no posterior limit indicating a fracture of the orbital wall, in this case the petrous temporal bone of the base of the skull
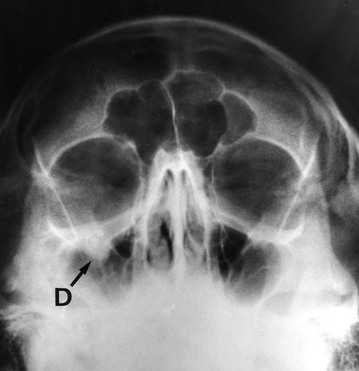
Blow-out fractures of the orbit
A direct frontal blow to the orbit from an object about the size of a squash ball (3–4 cm) may act like a plunger, causing a ‘blow-out’ fracture of the orbital floor without damaging the orbital margin. Blow-out fractures can also occur after a blow to the inferior orbital rim which then causes a ripple effect, fracturing the orbital floor whilst the rim remains intact. The blow-out most commonly involves the floor of the orbit where the bony walls are thinnest. This causes herniation of peribulbar fat into the maxillary sinus and disrupts the function of the extraocular muscles, causing diplopia and restricted upward gaze (see Figs 16.13 and 16.14). Hence it is important to test eye movements in any patient with a facial injury. Diagnosis is suggested by finding an antral opacity (haematoma) on occipito-mental X-ray, but CT scanning of the orbit is required if the bony defect needs to be demonstrated. Treatment involves exploring the orbital floor and may require a bone graft or silicone implant.