Chapter 67 Hamstring Regeneration Following Harvest for Anterior Cruciate Ligament Reconstruction
A Review of the Current Literature
Anterior cruciate ligament (ACL) reconstruction is one of the most prevalent orthopaedic procedures, with more than 100,000 performed annually.1 The majority of these operations use autograft donor tissue, with hamstring tendons (either semitendinosus [ST] or semitendinosus with gracilis [ST/Gr]) recently gaining approval in relation to the traditionally favored bone–patellar tendon–bone (BPTB) graft. Historically, the BPTB graft has been advocated for a number of reasons, including its well-described regrowth phenomenon, which involves the reconstitution of its central third following harvest. However, supporters of hamstring grafts cite potential patellofemoral pain, patellar tendonitis, tendon rupture, and patellar fracture as possible disadvantages of BPTB use.2–5 In 1992, Cross et al reported the regeneration of the hamstring tendons following harvest, a notion that has been supported with increasing evidence in the literature.6 Overall, as a result of this regeneration of the harvested tendon, hamstring strength has been found to reach near-normal levels 1 year postoperatively. This regeneration is termed the lizard tail phenomenon. The validation of such a phenomenon may offer an additional advantage for the use of hamstrings in ACL reconstruction. This chapter will review and summarize the literature to date examining the morphological and functional regeneration of harvested ST/Gr tendons from a radiographic, functional, and histological standpoint. It must be noted that relevant studies are somewhat difficult to compare due to unavoidable differences in follow-up, rehabilitation protocol, and testing methods found in the literature. Additionally, some studies confer results following ST/Gr harvest, whereas others evaluate those involving only ST harvest.
Radiographic Studies
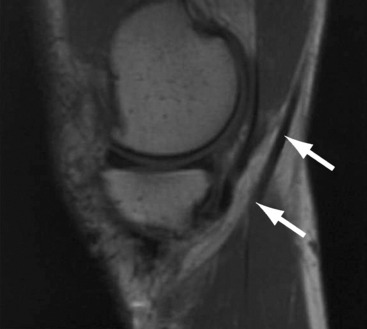
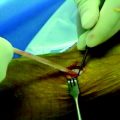
A number of studies have attempted to describe the morphological aspect of regeneration using the radiographic images of the ST and Gr tendons following harvest. These studies have provided information concerning the extent of regeneration, the location of the regenerate tissue, and the presence or absence of muscle atrophy. In the study conducted by Cross noted previously, magnetic resonance imaging (MRI) evaluation of four patients (6 months postoperatively) displayed tendonlike tissue extending from the hamstring muscle bellies to the medial gastrocnemius (Fig. 67-1).6 In this case, insertion appeared to be diffused into the medial popliteal fascia, but electromyographic examination revealed normal muscle activity and innervation patterns in the hamstring. Simonian et al similarly reported proximal insertion of the regenerate ST tendon.7 In contrast with common trends in the literature, however, no compensatory hypertrophy of the biceps femoris, semimembranosus (SM), or sartorius was appreciable when compared with the unaffected side.
A number of papers by Eriksson et al have reported MRI imaging of regenerate hamstring following harvest of the ST tendon. In the first (1999), they reported ST regeneration to the level of the proximal tibia in 8 of 11 patients evaluated 6 to 12 months postoperatively, whereas in the other three patients the remnant ST fused with the SM tendon proximal to the joint line.8 Those patients with regenerate distal ST demonstrated fusion of the ST and Gr approximately 30 mm distal to the joint line with insertion as a conjoint tendon on the pes anserinus. The authors suggested that the precise level of union is insignificant as long as it is distal to the joint line. The proximal cross-sectional area of the ST also differed between groups. This measure averaged 91% of the contralateral tendon in those with distal regeneration and 79% in those without distal regeneration. In a second study (2001), Eriksson et al evaluated six patients who ranged from 7 to 28 months following ACL reconstruction, using only the ST graft.9 MRI imaging displayed regeneration of ST tissue in five of the six patients to the pes insertion site, averaging approximately 30 mm distal to the joint line. The sixth patient had no evidence of a new tendon 24 months after surgery. Concurrently in 2001, Eriksson and Hamberg published another study in which patients underwent MRI between 6 and 12 months after ST harvest.7 In this case, 12 of the 16 patients displayed radiological evidence of ST regeneration in which the new tendon fused with the (nonharvested) Gr 10 to 30 mm below the joint line and inserted on the pes as a conjoined tendon. Although some ST atrophy was notable in all patients, significantly more was appreciable in patients without tendon regeneration. In those patients without regeneration, however, compensatory SM hypertrophy was more extensive.
Rispoli et al performed their study in a different manner, attempting to provide radiographic documentation of tendon regeneration in 21 patients at distinct time intervals ranging from 2 weeks to 32 months following ACL surgery using ST/Gr.5 However, they did not sequentially image any individuals. At the points shortly following surgery, fluid and edema in the tendon tracts were noted, and tendonlike tissue reached the superior patellar pole 6 weeks after surgery. Presence of the tendons below the joint line arose at variable intervals, arising anywhere from 3 to 12 months postoperatively. Papandrea et al also observed similar linear development in a study using sequential ultrasounds of 40 patients at 2 weeks, and 1, 2, 3, 6, 18, and 24 months after surgery using ST/Gr.10 This study documented a course of maturation proceeding from ill-defined hypoechogenic tissue early on to hamstring hypertrophy in the first year, before a distinct, well-defined tendon signal developed between 18 and 24 months. This study noted a more proximal insertion into the medial popliteal fascia than normal.
This process has been further documented by multiple other studies as recently as 2004. Nakamura et al used MRI and three-dimensional computed tomography (3D-CT) scans in a retrospective study assessing ST regeneration in eight patients, each a minimum of 2 years postoperative.11 In five of eight patients, distinct tendonlike tissue was observed running along the same course as the native hamstrings, the most notable difference being distal attachment to the medial popliteal fascia. In the remaining three subjects, the residual ST fused proximally into the SM muscle belly. The study apparently observed similar regeneration of the Gr, but an explicit description was not included. Tadokoro et al also imaged 28 patients with MRI at a minimum of 2 years following ACL reconstruction with ST/Gr.12 This study reported regeneration of 22 of 28 ST tendons and 13 of 28 Gr tendons, with no differences in cross-sectional area between surgical and contralateral knees. More importantly, they found that there was no correlation between morphological regeneration and peak flexion strength in high degrees of flexion (90 and 110 degrees) in both prone and supine positions despite less strength in affected limbs.
Williams et al also performed MRI on eight patients both preoperatively and at the point of return to sports (an average of 6 months after reconstruction using ST/Gr).13 Seven of the eight patients exhibited regenerate tendons; however, the majority of the tendons had not yet inserted on the tibia at the point of imaging. Overall volume of the ST and Gr muscles diminished by an average of 30%, and in contrast with Tadokoro’s study, this extent appeared to correlate well with the extent of tendon regeneration. Additionally, much like Eriksson’s study, compensatory hypertrophy of the SM and biceps femoris muscles was noted.
Nakamae et al also used 3D-CT to gain a better sense of the full-length morphology of the regenerate tissue by imaging 29 patients at various time points.14 A 3D-CT examination was performed in all patients preoperatively, 24 patients at 1 month, 8 patients at 3 months, 21 patients at 6 months, and 20 patients at 12 months postoperatively following ST harvest only. Although no patients had evidence of regeneration at 1 month after surgery, a regenerate tendon was detected in all but two patients at 12 months after surgery, with the regenerate tissue coursing as expected from the muscle bellies to the normal insertion site on the proximal tibia.
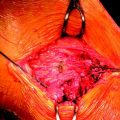
Although there is a perception that regeneration occurs almost universally, it is not an entirely predictable phenomenon. For reasons not fully understood, a small percentage of patients in a number of studies lack regenerate tendons. Notably, two studies by Eriksson and one by Hioki et al report 17%, 18%, and 45% of patients with failed regrowth, respectively.3,9,15,16 Additionally, among the population with evidence of regrown tissue, the regenerate tendon frequently ranges in size and diverges from the expected insertion site, inserting proximally and medially to the pes anserinus (Fig. 67-2). This varied insertion could have important biomechanical consequences that explain varied strength of knee flexion and internal rotation. A more proximal insertion shortens the tendon’s moment arm, inhibiting the muscle’s ability to flex the knee. Additionally, in the case of documented insertion on the medial popliteal fascia, a more lateral insertion hinders the hamstring muscles from generating internal torque and resisting external rotation of the tibia.
Functional Studies
Numerous studies have also examined hamstring strength following tendon harvest. Postoperative leg strength has been a concern since at least 1982, when Lipscomb et al published a retrospective evaluation of 482 cases involving either ST or ST/Gr harvest.17 Impressively, none of the subjects in this study displayed significant loss of knee flexion strength at an average of 26 months postoperatively. Specifically, hamstring strength averaged 99% of the normal knee when ST/Gr was used and 102% when the Gr was not harvested.
More widespread interest in the subject of strength regeneration peaked in the early 1990s, aroused by two noteworthy studies. The aforementioned four-patient study by Cross et al demonstrated minimal decreases (averaging less than 10%) in peak flexion and extension strength in three of the four patients, whereas the fourth patient actually displayed improved strength measurements.6 Concurrently, Marder et al published contradictory results in a study comparing hamstring strength between patients with ipsilateral ST/Gr grafts and BPTB grafts.18 In the group who underwent ST/Gr graft harvest, the study noted a 17% decrease in isokinetic knee flexion strength. Eriksson et al similarly documented vast strength deficiencies relative to the unaffected leg in the peak torque of hamstrings (–25%) and quadriceps (–50%) during both concentric and eccentric testing in 16 patients ranging between 8 and 18 months after surgery.3 This comprehensive, overwhelming weakness may indicate inadequate postoperative rehabilitation. Details of the postoperative protocol were not provided.
Despite these inconsistent findings, more recent studies examining knee flexion strength have generally documented minimal or no deficits in peak torque following both ST and ST/Gr harvest.19,20 Yasuda et al demonstrated this most favorably in their prospective examination of peak torque after ST/Gr harvest from either the ipsilateral or contralateral leg.21 Neither circumstance exhibited significant decrease in flexion strength after the immediate postoperative period was surpassed. More detailed examinations, however, have found slight divergence in torque assessment. A study conducted by Adachi et al evaluated 58 patients with regard to peak torque, peak torque angle, and total work at an average of 2 years after ACL reconstruction using ST, ST/Gr, or allograft tissue.2 Much like their counterparts, they found very little difference in peak strength or total work between groups; however, the autograft groups exhibited peak torque at a significantly shallower angle than in the allograft group. Ohkoshi et al likewise noted no difference in peak torque flexion and quantified the significant decrease in peak torque angle at about 11 to 15 degrees after evaluating 25 patients following ST harvest.22 The study theorized that this decrease in peak torque angles is attributable to compensation by flexor muscles that have peak torque at a shallower angle than the hamstrings. Irie and Tomatsu used cybex testing to assess the flexor strength of 13 patients at 12 to 16 months after ST/Gr or Gr harvest.23 Such testing yielded no difference in maximum torque but demonstrated a 25% deficiency in total work possible at angles of flexion greater than 75 degrees.
In a more recent study, Nakamura et al evaluated 74 consecutive patients 2 years after ST or ST/Gr surgery and found that peak torque flexion was greater than 90% of that in the unaffected knee.24 Consistent with other studies, peak torque at 90 degrees was again deficient in the ST and ST/Gr groups alike. In one of the only prospective randomized analyses of the subject, Tashiro et al randomly assigned 90 patients to ACL reconstruction with either ipsilateral ST or ST/Gr autograft.25 The authors examined peak flexion torque at 6, 12, and 18 months, finding that the ST and ST/Gr groups did not differ significantly from one another and that torque was only diminished at the 6-month point, after which it returned to preoperative levels. This study also noted significantly less recovery at high angles in both groups, with hamstring strength decreases of up to 30% at angles greater than 70 degrees. In the CT imaging study by Nakamae et al described previously, isokinetic measurements at 60 degrees of knee flexion demonstrated a strength deficiency in which peak torque was an average of 68% of the control side at 6 months and increased to 83% by 12 months.14
All in all, the studies universally demonstrate that deficits in postoperative knee strength, especially in flexion, are minimal in cases of both ST and ST/Gr tendon harvest. The only exception to this conclusion is somewhat diminished strength at high angles of flexion. Due to the limits of strength testing, it cannot be discerned whether the maintenance of flexion strength is a result of hamstring regeneration or the compensatory hypertrophy of other hamstring muscle–tendon units. A few recent studies suggest that internal tibial rotation torque may be a better indication of the extent of regeneration than pure flexion strength. Viola et al found that among 23 patients who had undergone ACL reconstruction with ST/Gr, internal tibial rotation torque was approximately 10% to 15% of the contralateral limb at an average of 51 months postoperatively (observed at all velocities; 60, 120, and 180 degrees/sec).26 Similarly, Armour et al discovered internal rotation deficits in the operated leg measuring between 12% and 15% at a 2-year minimum follow-up.27 This study more specifically targeted the knee joint by limiting motion at the ankle joint during strength testing. Segawa et al also observed comparable results in assessing internal tibial torque in 32 patients with ST grafts and 30 patients with ST/Gr grafts at 1 year postoperative.28 Internal rotation strength was again considerably less than the contralateral leg. The diminished internal rotation strength was more markedly present when the Gr was also harvested, leading the authors to recommend harvest of only the ST whenever possible. Additionally, on account of the overwhelming decrease in strength, the authors encourage a rehabilitation protocol with exercises specifically focused on improving internal rotation strength. Nevertheless, studies have yet to correlate deficiencies in either knee flexion strength or internal rotation strength with functional shortcomings or impaired athletic performance.
Histological Studies
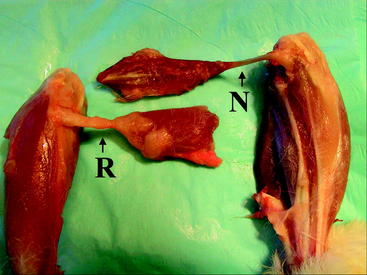
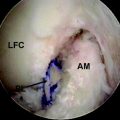
It has long been theorized that neotendons observed on MRI were merely scar tissue. Although radiographic and functional studies have demonstrated conclusively that harvested hamstring tendons regenerate in the same orientation and with the same strength as native tendons, only recently have surgeons performed histological or biomechanical studies to assess the quality of the regenerate tissue. In the Eriksson and Hamberg study assessing MR images of regenerate tissue, biopsies of hamstring muscle tissue preoperatively and postoperatively were also evaluated.3 Evaluation revealed that the cross-sectional area of the muscle fibers trended toward small values in regenerate tissue; however, the fiber composition and citrate synthetase activity remained consistent with native tissue (Fig. 67-3). In another study of the same group, Eriksson at al examined and compared the peripheries of five regenerate ST tendons with normal tendons.9 In this case, the regenerate collagen fibers held the same alignment and breadth as the control tendons and exhibited uniform staining, with the exception of the appropriate forms of collagen. There were also appreciable small areas of irregularity in collagen orientation and increased fibroblast and capillary formation, indicating the presence of scar-like tissue.
Likewise, Ferretti performed histological analysis of regenerate tissue from three patients at 6, 24, and 27 months after surgery at the time of routine hardware removal.29 At the histological level, the neotendon is initially (at 6 months) a fibrous structure with fibroblastic proliferation and capillaries but few collagen fibers. However, over time (at the latter two time points) the neotendon acquires qualities consistent with a healthy tendon demonstrating thicker, longitudinally oriented fibers. However, small focal regions of scar tissue and irregular collagen orientation often persist long after harvest. This compositional inconsistency may alter biomechanical properties of the reconstituted tendon.
Animal Models
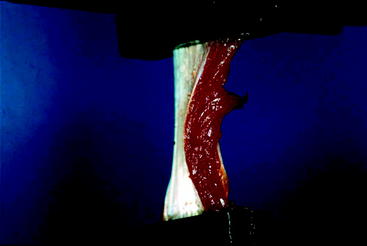
Animal models of tendon regeneration are quite useful in offering trends in regeneration for a large data source. Two studies by Miller et al used MRI as well as histological and biomechanical evaluation of New Zealand white rabbits to evaluate tendon regeneration. In the first study, each of 10 rabbits demonstrated radiologic evidence of some regenerate tissue at 16 or 28 weeks.30 MRI indicated a process from wavy regenerate tissue stopping short of the tibia at 16 weeks, becoming taut and inserting on the ST insertion site by 28 weeks. This course of maturation was mirrored in histological examination. Microscopic examination at 16 weeks displayed wavy collagen fibers turning into healthy tendinous fibers by 28 weeks. The tendons also appeared to strengthen with maturation as indicated by tensile biomechanical testing (Fig. 67-4). However, at both time points the regenerate tissue withheld significantly less maximum load to failure than native tissue (by 77% at 16 weeks and 48% at 28 weeks).
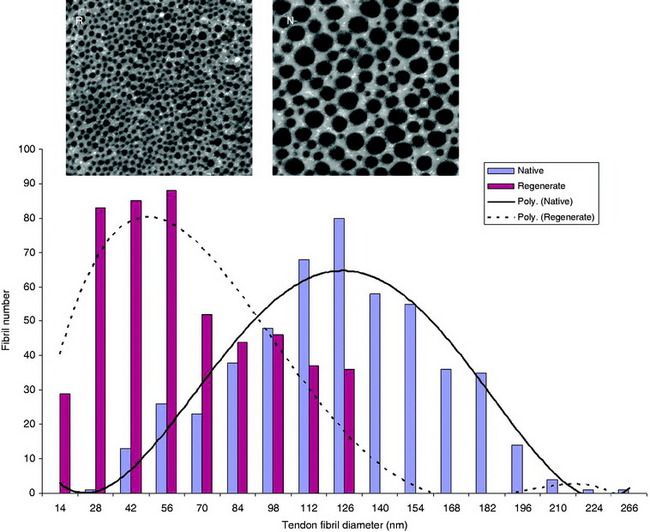
A more comprehensive analysis of regenerate rabbit tendons was reported in a follow-up study conducted by Miller and Gill et al.31 In follow-up, ST tendons appeared to regenerate at 9 to 12 months after harvest in 26 of 31 of the rabbits evaluated. However, the neotendon was highly variable in size and tibial insertion site. The tissue resembled native tendon in cellularity and immunolocalization of type I collagen, fibril size was markedly smaller, fibril orientation was more irregular, and regenerate tendon composition contained significantly lower levels of proteoglycan than native tissue. Detailed biomechanical examination in the same study demonstrated significantly lower maximum loads to failure and structural stiffness (approximately 25% of control tendons) in regenerate tissue. The regenerate tendons’ inferior biomechanical qualities strongly suggest inferior material properties of the tissue. However, because measures were not precisely taken, it may also be at least partly attributable to smaller cross-sectional areas of the regenerate tissue.
Future Directions
The precise mechanism of regeneration and the desirable qualities for strong biomechanical performance are not well understood. It is hypothesized that the regeneration process likely begins at the more proximal vascular areas and extends distally as extrasynovial hematoma collects along fascial planes and acts as a scaffold for fibroblast precursor cells. Carofino et al notes, however, that the fascial planes could not possibly constrict a regenerate tendon to dimensions and shape similar to the original because the medial knee does not form a well-defined tubular structure necessary for precise reconstitution.15 Despite the primitive state of our knowledge, surgeons have begun to propose the use of regenerate tendon for revision surgeries. Yoshiya et al describe the reharvesting of hamstrings for revision ACL reconstruction, 8 months following the index reconstruction in a 2004 case study.32 At the time of surgery, Yoshiya obtained a biopsy of the regenerate tissue that displayed significant smaller diameter of collagen fibrils compared with normal tendons. Although it is premature to make an overarching assessment, the patient was reportedly doing well at 6 months postoperatively. The authors appropriately note that it is too early to make a determination on the viability of regenerate grafting on a routine basis.
1 Battaglia TC, Miller MD. Strength and regrowth of hamstring tendons after hamstring autograft anterior cruciate ligament reconstruction. Tech Orthop. 2005;20:1-5.
2 Adachi N, Ochi M, Uchio Y, et al. Harvesting hamstring tendons for ACL reconstruction influences postoperative hamstring muscle performance. Arch Orthop Trauma Surg. 2003;123:460-465.
3 Eriksson K, Hamberg P, Jansson E, et al. Semitendinosus muscle in anterior cruciate ligament surgery: morphology and function. Arthroscopy. 2001;17:808-817.
4 Graham SM, Parker RD. Anterior cruciate ligament reconstruction using hamstring tendon grafts. Clin Orthop. 2002;402:64-75.
5 Rispoli DM, Sanders TG, Miller MD, et al. Magnetic resonance imaging at different time periods following hamstring harvest for anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:2-8.
6 Cross MJ, Roger G, Kujawa P, et al. Regeneration of the semitendinosus and gracilis tendons following their transection for repair of the anterior cruciate ligament. Am J Sports Med. 1992;20:221-223.
7 Simonian PT, Harrison SD, Cooley VJ, et al. Assessment of morbidity of semitendinosus and gracilis tendon harvest for ACL reconstruction. Am J Knee Surg. 1997;10:54-59.
8 Eriksson K, Larsson H, Wredmark T, et al. Semitendinosus tendon regeneration after harvesting for ACL reconstruction. A prospective MRI study. Knee Surg Sports Traumatol Arthrosc. 1999;7:220-225.
9 Eriksson K, Kindblom LG, Hamberg P, et al. The semitendinosus tendon regenerates after resection: a morphologic and MRI analysis in 6 patients after resection for anterior cruciate ligament reconstruction. Acta Orthop Scand. 2001;72:379-384.
10 Papandrea P, Vulpiani MC, Ferretti A, et al. Regeneration of the semitendinosus tendon harvested for anterior cruciate ligament reconstruction. Evaluation using ultrasonography. Am J Sports Med. 2000;28:556-561.
11 Nakamura E, Mizuta H, Kadota M, et al. Three-dimensional computed tomography evaluation of semitendinosus harvest after anterior cruciate ligament reconstruction. Arthroscopy. 2002;20:360-365.
12 Tadokoro K, Matsui N, Yagi M, et al. Evaluation of hamstring strength and tendon regrowth after harvesting for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:1644-1649.
13 Williams GN, Snyder-Mackler L, Barrance PJ, et al. Muscle and tendon morphology after reconstruction of the anterior cruciate ligament with autologous semitendinosus-gracilis graft. J Bone Joint Surg. 2005;86A:1936-1946.
14 Nakamae A, Deie M, Yasumoto M, et al. Three-dimensional computed tomography imaging evidence of regeneration of the semitendinosus tendon. J Comput Assist Tomogr. 2005;29:241-245.
15 Carafino B, Fulkerson J. Medial hamstring tendon regeneration following harvest for anterior cruciate ligament reconstruction: fact, myth, and clinical implication. Arthroscopy. 2005;21:1257-1264.
16 Hioki S, Fukubayashi T, Ikeda K, et al. Effect of harvesting the hamstring tendon for anterior cruciate ligament reconstruction on the morphology and movement of the hamstring muscle: a novel MRI technique. Knee Surg Sports Traumatol Arthrosc. 2003;11:223-227.
17 Lipscomb AB, Johnston RK, Snyder RB, et al. Evaluation of hamstring strength following use of semitendinosus and gracilis tendons to reconstruct the anterior cruciate ligament. Am J Sports Med. 1982;10:340-342.
18 Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19:478-484.
19 Maeda A, Shino K, Horibe S, et al. Anterior cruciate ligament reconstruction with the multistranded autogenous semitendinosus tendon. Am J Sports Med. 1991;19:478-484.
20 Marcacci M, Zaffagnini S, Ianoco F, et al. Arthroscopic intra- and extra-articular anterior cruciate ligament reconstruction with gracilis and semitendinosus tendons. Knee Surg Sports Traumatol Arthrosc. 1998;6:68-75.
21 Yasuda K, Tsujino J, Ohkoshi Y, et al. Graft site morbidity with autogenous semitendinosus and gracilis tendons. Am J Sports Med. 1995;23:706-716.
22 Ohkoshi Y, Inoue C, Yamane S, et al. Changes in muscle strength properties caused by harvesting of autogenous semitendinosus tendon for reconstruction of contralateral anterior cruciate ligament. Arthroscopy. 1998;14:580-584.
23 Irie K, Tomatsu T. Atrophy of semitendinosus and gracilis and flexor mechanism function after hamstring tendon harvest for anterior cruciate ligament reconstruction. Orthopedics. 2002;25:491-495.
24 Nakamura N, Horibe S, Sasaki S, et al. Evaluation of active knee flexion and hamstring strength after anterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. 2002;18:598-602.
25 Tashiro T, Kurosawa H, Kawakami A, et al. Influence of medial hamstring tendon harvest on knee flexor strength after anterior cruciate ligament reconstruction: a detailed evaluation with comparison of single- and double-tendon harvest. Am J Sports Med. 2003;31:522-529.
26 Viola RW, Sterett WI, Newfield D, et al. Internal and external tibial rotation strength after anterior cruciate ligament reconstruction using ipsilateral semitendinosus and gracilis tendon autografts. Am J Sports Med. 2000;28:552-555.
27 Armour T, Forwell L, Litchfield R, et al. Isokinetic evaluation of internal/external tibial rotation strength after the use of hamstring tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:1639-1653.
28 Segawa H, Omori G, Koga Y, et al. Rotational muscle strength of the limb after anterior cruciate ligament reconstruction using semitendinosus and gracilis tendon. Arthroscopy. 2002;18:177-182.
29 Ferretti A, Conteduca F, Morelli F, et al. Regeneration of the semitendinosus tendon after its use in anterior cruciate ligament reconstruction: a histologic study of three cases. Am J Sports Med. 2002;30:204-207.
30 Leis HT, Sanders TG, Larsen KM, et al. Hamstring regrowth following harvesting for anterior cruciate ligament reconstruction: the lizard tail phenomenon. Am J Knee Surg. 2003;16:159-164.
31 Gill SS, Turner MA, Battaglia TC, et al. Regeneration of the semitendinosus tendon after its use in anterior cruciate ligament reconstruction: a histologic study of three cases. Am J Sports Med. 2002;30:204-207.
32 Yoshiya S, Matsui N, Matsumoto A, et al. Revision anterior cruciate ligament reconstruction using the regenerated semitendinosus tendon: analysis of ultrastructure of the regenerated tendon. Arthroscopy. 2004;28:532-535.