Chapter 47 Hamstring Anterior Cruciate Ligament Reconstruction with IntraFix Tibial Fastener
Introduction
The optimal initial graft fixation technique for hamstring tendon anterior cruciate ligament (ACL) grafts remains controversial.1–6 Biomechanical studies have demonstrated that cross-pin and Endobutton-CL femoral fixation techniques provide excellent initial fixation properties.7,8 However, tibial fixation of hamstring tendon ACL grafts has been more problematic. This is primarily due to the lower bone mineral density of the proximal tibia and the fact that tibial fixation devices must resist tension applied parallel to the axis of the tibial bone tunnel.2,9–11 Extratunnel tibial fixation techniques that anchor to the tibial cortex can provide secure initial fixation; however, the implants are often prominent and cause local skin irritation and pain, requiring a second operation for removal.12 Intratunnel tibial fixation using interference screws eliminates the problem of prominent hardware, but the single interference screw technique has been shown to have somewhat low initial fixation strength and increased slippage under cyclical loading.2,4,9,13
The IntraFix tibial fastener was designed with two goals in mind, one mechanical and one biological. The first goal was to achieve more rigid intratunnel fixation of soft tissue grafts and eliminate or decrease the need for supplemental tibial fixation. The second goal was to maximize bony integration of the soft tissue graft strands into the bone tunnel wall. To achieve these goals, the device was designed with an expandable, four-channel, ridged, 30-mm polyethylene sheath and a tapered Delrin expansion screw. The four channels individually capture and grip each of the four strands of the hamstring tendon graft into separate compartments and directly compress each of the graft strands against cancellous bone. We performed cyclical and single load to failure (LTF) tests comparing the plastic IntraFix and bioabsorbable interference screws in paired young to middle-aged human cadaver tibiae with human doubled gracilis and semitendinosus grafts (DGSTs) (Table 47-1). The plastic IntraFix demonstrated a mean ultimate failure load of 800N and stiffness of 200 N/mm, which was significantly higher than interference screw fixation. In an independent biomechanical study comparing commonly used hamstring tendon graft tibial fixation devices, Kousa et al14 demonstrated that the IntraFix had the highest LTF (1309N) and stiffness (267 N/mm) and the least amount of slippage (1.5 mm) after cyclical loading.
| Graft Diameter | Drill Tunnel | Screw Size |
|---|---|---|
| 7 mm | 8.5 mm | 6–8 mm |
| 8 mm | 9.0 mm | 6–8 mm |
| 9 mm | 10.0 mm | 7–9 mm |
| 10 mm | 11.0 mm | 8–10 mm |
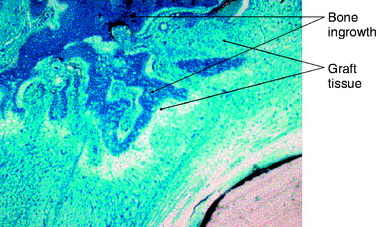
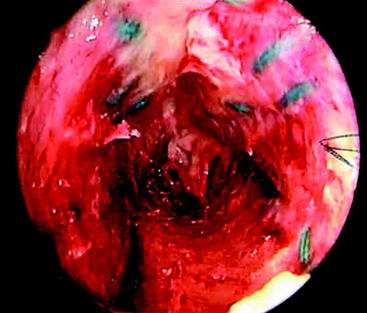
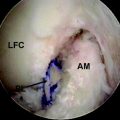
The second goal of this design was to maximize the amount of contact between graft tendons and bone. Fixation outside tunnels and from suspension devices results in a loose fibrous attachment between the tunnel wall and the graft, with little if any bony ingrowth into the graft(Fig. 47-1). In contrast, direct compression of tendon to bone by interference screws within the tunnel leads to bony ingrowth including Sharpey fiber formation.15 However, a single interference screw inserted next to a bundled four-stranded graft definitely leaves a considerable portion of the tunnel filled by the screw and some of the tendons without bony contact. In contrast, the IntraFix has the potential for more extensive bone–graft integration because each strand is pressed against bone and the entire tunnel wall is in contact with graft. A limited histological study performed on the IntraFix in sheep demonstrated early bony integration (Fig. 47-2). Thus extrapolation of the interference screw data to this device seems justified. Further evidence of extensive bony integration when using the IntraFix and Bio-IntraFix comes from direct examination of the tibial tunnel many months after reconstruction. Fig. 47-3 shows an example of the appearance of the tibial tunnel 1 year after ACL reconstruction; it was obtained during a revision case after the sheath had been removed and the arthroscope inserted into the tibial tunnel. It shows a firm surface, apparent integration of the sutured tendons into the bone tunnel wall, capillary ingrowth, and no loose fibrous tissue.
Surgical Technique
Tibial Tunnel
The tibial tunnel must be carefully oriented in both the sagittal and coronal planes for several reasons. Due to the large cross-sectional area of four-strand hamstring tendon grafts, sagittal placement of the tibial tunnel is especially critical.16 The tibial tunnel position in the sagittal plane determines whether the ACL graft impinges against the roof of the intercondylar notch in full knee extension.2,16–19 Roof impingement is associated with effusions, loss of extension, anterior knee pain, quadriceps weakness, and increased anterior laxity. Coronal plane orientation is the primary determinant of placement of the femoral tunnel along the side wall of the intercondylar notch and, to some degree, of the length of the femoral tunnel. A more medial starting position on the tibia allows the femoral tunnel to be drilled closer to the 10- or 2-o’clock position along the sidewall. A femoral tunnel at the 10-o’clock (right knee) or 2-o’clock position (left knee) is important because a single-bundle ACL graft positioned at these locations in the intercondylar notch is more effective at resisting combined rotatory loads than one placed at the 11-o’clock position. Biomechanical studies have demonstrated little difference in coupled anterior tibial translation between this graft and a double-bundle hamstring ACL reconstruction at low degrees of flexion.20
In our surgical technique, a tibial tunnel length of 35 to 45 mm is optimal because this will accommodate the entire 30-mm IntraFix or Bio-IntraFix with no chance of the device protruding into the joint. In general, setting the variable angle tibial aimer between 45 and 55 degrees will allow these tibial tunnel lengths to be achieved. The guidelines of Jackson and Gasser,21 Howell,15 and Simmons et al22 are used for intraarticular placement of the tibial guide pin. If necessary, the tibial guide pin position can be checked by intraoperative radiographs or fluoroscopy with the knee in maximum extension.
Femoral Tunnel and Graft Fixation
Because the IntraFix tibial fastener can be used with any femoral fixation technique, the choice of the femoral fixation is based on the surgeon’s preference. However, we prefer cross-pins or the Endobutton-CL because these fixation techniques have been shown to be strong and stiff and to have the least amount of elongation under cyclical loading.7,8 More importantly, these two femoral fixation techniques permit equal tensioning of all four graft strands. This is an important goal because, as shown by Hamner et al,23 it is necessary to equally tension all four strands of a DGST graft to maximize initial graft strength and stiffness. An equally tensioned DGST graft was stronger and stiffer than a 10-mm, central-third patellar tendon autograft. However, when no attempt was made to equally tension all four graft strands, the ultimate failure load and stiffness of the DGST graft were not statistically different from that of a doubled semitendinosus tendon graft alone. Thus failure to equally tension all four graft strands of a DGST graft negated any contribution from the doubled gracilis tendon graft.
Graft Passage, Graft Tensioning, and Tibial Fixation
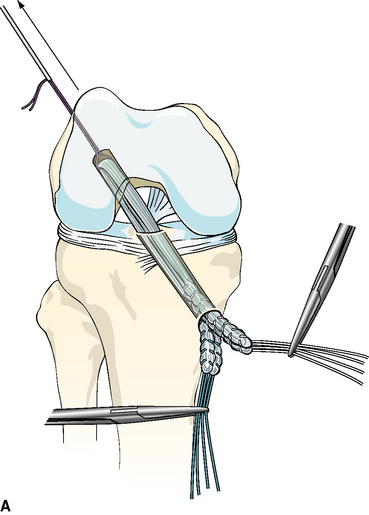
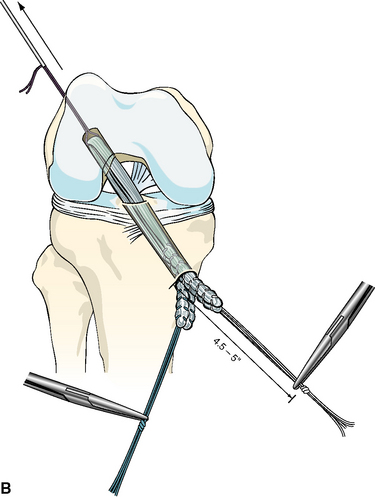
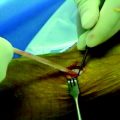
After the femoral side of the DGST or tibialis tendon allograft has been securely fixed in the lateral femoral condyle, the whipstitches from the gracilis tendon or corresponding opposite ends of the tibialis tendon allograft are tied together to create a loop approximately 4.5 to 5 inches from the end of the tibial tunnel. This step is repeated for the semitendinosus tendon and the corresponding opposite ends of the tibialis tendon allograft (Fig. 47-4, A and B).
Device Insertion
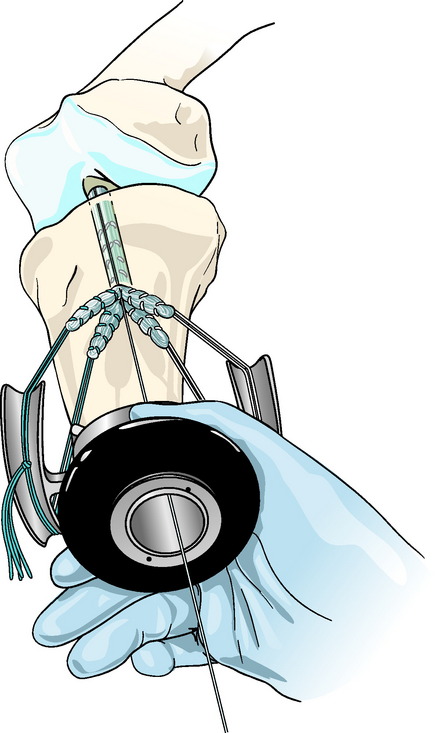
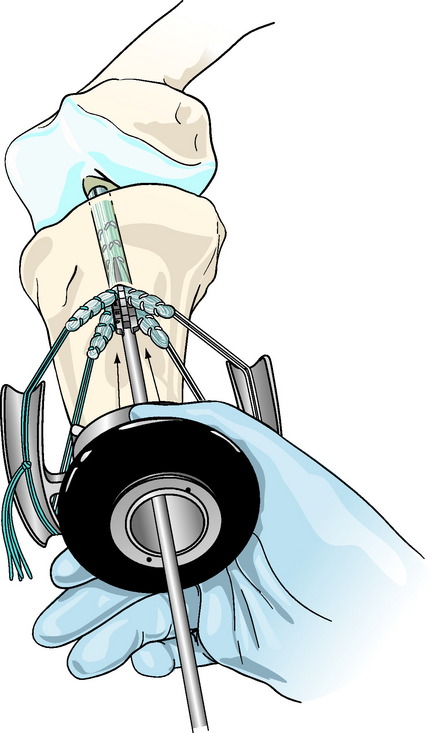
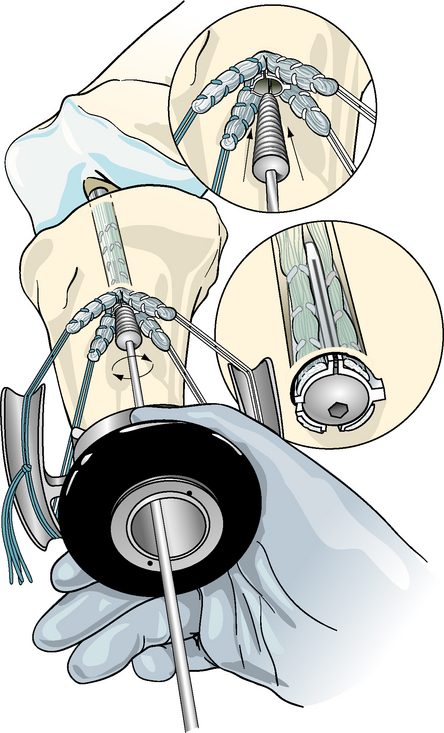
Concentric device placement within the tibial tunnel is critical to the success of the technique. To achieve this, the central axis of the tibial tunnel is identified by passing a stout guidewire or a Trailblazer (Smith & Nephew Endoscopy, Andover, MA) through the center of the tie tensioner and down the center of the four graft strands into the knee joint (Fig. 47-5). Once the central axis of the tibial tunnel is identified, the tie tensioner should be held in this orientation during all the subsequent steps to avoid divergent placement of the IntraFix sheath and screw. The surgeon can improve his or her ability to maintain this orientation by placing several fingers or the entire side of the hand holding the tensioner on the tibia during the next steps. Next, the four-quadrant dilator is inserted down the center of the four graft strands and oriented so that each graft strand sits in its own channel (Fig. 47-6). While maintaining the desired tension on the graft, the four-quadrant dilator is tapped into the tibial tunnel for a distance of 35 mm. This step compresses and separates the four tendon strands, and, in the case of smaller tunnels (7 to 8 mm), notches the bone tunnel wall to accept the sheath. It is important to keep the dilator oriented along the axis of the tibial tunnel as it is impacted because the dilator has a tendency to diverge, as do most tunnel dilators. Because the sheath for the IntraFix and Bio-IntraFix is 9 mm in diameter, the four-quadrant dilator also enlarges the tibial tunnel in the case of smaller tunnels, providing easier insertion of the IntraFix sheath and tapered screw. There are now two sheaths for the Bio-IntraFix and a smaller and larger dilator appropriate to each. The smaller sheath is used for 7- and 8-mm tunnels and the larger for 9- and 10-mm tunnels.
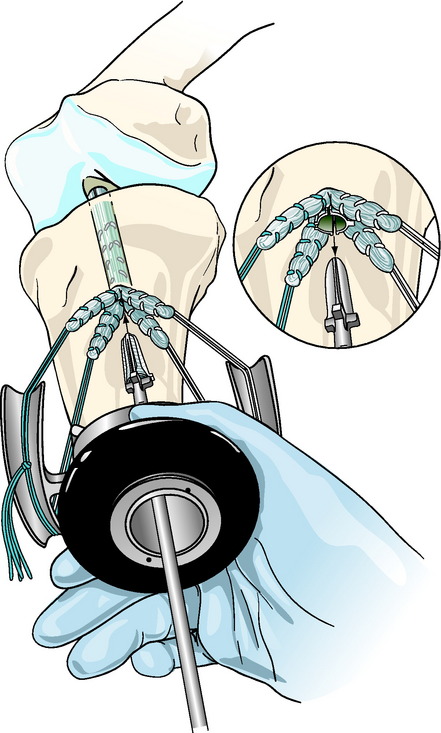
The Intrafix sheath is inserted among the four graft strands, taking care that each graft strand is positioned into a separate channel of the IntraFix sheath. The derotational tab on the sheath is oriented at the 3- or 9-o’clock position (Fig. 47-7). Orienting the derotational tab at these positions allows the IntraFix sheath to be inserted more deeply into the tibia and prevents prominence of the device. The inserter is tapped into the tunnel until the derotational tab is flush with the cortex. As stated earlier, clearing the soft tissue from the bone tunnel opening will allow for better assessment of the depth of insertion and trimming of any protruding tendon or sheath after the screw has been inserted. The sheath inserter is removed, and the 0.042-inch guidewire for the IntraFix tapered screw is inserted through the center of the sheath until a loss of resistance is felt as the tip of the guidewire enters the knee joint.
For the plastic IntraFix, a tapered screw size 1 mm larger than the tibial tunnel diameter is used. For example, an 8-mm tapered screw is used for a 7-mm tibial tunnel. Given the typical size of DGST grafts, the 7- to 9-mm tapered screw is most commonly used. The IntraFix screw is inserted into the plastic sheath until its inferior aspect is flush with or buried just below the tibial cortex (Fig. 47-8). Because the best bone quality is at or next to the tibial cortex, overly deep insertion of the screw may decrease fixation strength.24 The tension on the graft strands from the tie tensioner should prevent the sheath from rotating during screw insertion in hard bone, but some rotation of the outer sheath is acceptable because the sheath within the tunnel does not move in concert. Protruding or prominent areas of the polyethylene sheath are trimmed flush with the tibial cortex using a 15 blade and a small bone rongeur.
The technique for insertion of the Bio-IntraFix device is identical, but the sizing scheme differs from that just described (Table 47-2). Because the PLLA/TCP sheath is noncompressible and because the insertion torque is higher than with the plastic version, the tunnel should be drilled or dilated 1.0 mm larger than the graft diameter. The Bio-IntraFix sheath adds more than 1 mm to the diameter of the Bio-IntraFix screw, so in effect the fixation device in total is oversized to the tunnel diameter, which is the usual practice with interference screws and with the plastic IntraFix.
| Graft Diameter | Drill Tunnel | Screw Size |
|---|---|---|
| 7 mm | 7 mm | 6–8 mm |
| 8 mm | 8 mm | 7–9 mm |
| 9 mm | 9 mm | 8–10 mm |
| 10 mm | 10 mm | 8–10 mm |
Troubleshooting
Low Bone Density
The fixation strength of any intratunnel fixation device is dependent on the local bone mineral density. If, during the insertion of the tapered screw, the surgeon subjectively feels that there was low insertion torque, or if the patient has soft bone as assessed during drilling and dilation of the tunnel, then we recommend that supplemental tibial fixation be used.13 Depending on the graft length, the tendons can be stapled below the tibial tunnel opening using one or two small barbed staples (Smith & Nephew Orthopaedics, Memphis, TN). Another method of backup is to tie the sutures around a small nonbarbed staple, a screw and washer, or a tibial fixation post (Smith & Nephew Endoscopy).
Closure and Postoperative Dressings
A Hemovac drain can be inserted under the sartorius fascia and into the hamstring harvest site to prevent postoperative hematoma formation and decrease subcutaneous skin ecchymosis along the medial side of the knee.25 This is particularly useful when excessive bleeding is encountered during the hamstring tendon harvest. The sartorius fascia that was preserved during the hamstring tendon graft harvest is closed over the tibial hardware and repaired back to the tibia with a #0 absorbable suture. The subcutaneous tissue is closed in layers with fine absorbable sutures. A running #3–0 Prolene (Ethicon, Sommerville, NJ) subcuticular pullout suture or #4–0 Monocryl produces a very cosmetic closure. A light dressing is applied over the wound, followed by a thigh-length TED antiembolism stocking (Cryocuff, Aircast, Summit, NJ) and knee immobilizer.
1 Aglietti P, Giron F, Buzzi R, et al. Anterior cruciate ligament reconstruction: bone-patellar tendon-bone compared with double semitendinosus and gracilis tendon grafts. J Bone Joint Surg. 2004;86A:2143-2162.
2 Brand JC, Weiler A, Caborn DNM, et al. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28:761-774.
3 Brown CH, Sklar JH. Endoscopic anterior cruciate ligament reconstruction using quadrupled hamstring tendons and Endobutton femoral fixation. Tech Orthop. 1998;13:281-298.
4 Brown CH, Wilson DR, Hecker AT, et al. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20:922-935.
5 Cooper DE, Deng XH, Burstein AL, et al. The strength of central third patellar tendon graft. A biomechanical study. Am J Sports Med. 1993;21:818-824.
6 Corry IS, Webb JM, Clingeleffer AJ, et al. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27:444-454.
7 Ahmad CS, Gardner TR, Groh M, et al. Mechanical properties of soft tissue femoral fixation devices for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:635-640.
8 Brown CH, Sklar JH. Endoscopic anterior cruciate ligament reconstruction using doubled gracilis and semitendinosus tendons and Endobutton femoral fixation. Oper Tech Sports Med. 1999;7:201-213.
9 Brand JC, Pienkowski D, Steenlage E, et al. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28:705-710.
10 Steiner ME, Hecker AT, Brown CH, et al. Anterior cruciate ligament graft fixation: comparison of hamstring and patellar tendon grafts. Am J Sports Med. 1994;22:240-242.
11 Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27:35-43.
12 Jansson KA, Harilainen A, Sandelin J, et al. Bone tunnel enlargement with anterior cruciate ligament reconstruction with hamstring autograft and Endobutton fixation technique. Knee Surg Sports Traumatol Arthrosc. 1999;7:290-295.
13 Goble EM, Downey DJ, Wilcox TR. Positioning of the tibial tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 1995;11:688-695.
14 Kousa P, Jarvinen TLN, Vihavainen M, et al. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31:182-188.
15 Howell SM. Roof impingement of ACL grafts: diagnosis, cause, prevention, and late surgical correction. In: Feagin JA, editor. The crucial ligaments. New York: Churchill Livingstone; 1994:637-648.
16 Eriksson K, Anderberg P, Hamberg P, et al. A comparison of quadrupled semitendinosus and patellar tendon grafts in reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 2001;83B:348-354.
17 Howell SM. Principles for placing the tibial tunnel and avoiding roof impingement during reconstruction of a torn anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 1998;6:S49-S55.
18 Howell SM, Clark JA. Tibial tunnel placement in anterior cruciate ligament reconstructions and graft impingement. Clin Orthop. 1992;283:187-195.
19 Howell SM, Taylor MA. Failure of reconstruction of the anterior cruciate ligament due to impingement by the intercondylar roof. J Bone Joint Surg. 1993;75A:1044-1055.
20 Loh JC, Fukuda Y, Tsuda E, et al. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel positions. Arthroscopy. 2003;19:297-304.
21 Jackson DW, Gasser SI. Tibial tunnel placement in ACL reconstruction. Arthroscopy. 1994;10:124-131.
22 Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg. 2003;85A:1018-1029.
23 Hamner DL, Brown CH, Steiner ME, et al. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg. 1999;81A:549-557.
24 Ferrari JD, Ferrari DA. The semitendinosus: anatomic considerations in tendon harvesting. Orthop Rev. 1991;20:1085-1088.
25 Greis PE, Burks RT, Bachus K, et al. The influence of tendon length and fit on the strength of a tendon-bone tunnel complex: a biomechanical and histologic study in the dog. Am J Sports Med. 2001;29:493-497.
Breitfuss H, Frohlich R, Povacz P, et al. The tendon defect after anterior cruciate ligament reconstruction using the mid third patellar tendon—a problem for the patellofemoral joint? Knee Surg Sports Traumatol Arthrosc. 1996;3:195-198.
Brown CH, Sklar JH, Darwich N. Endoscopic anterior cruciate ligament reconstruction using autogenous doubled gracilis and semitendinosus tendons. Tech Knee Surgery. 2004;3:215-237.
Butler DL. Evaluation of fixation methods in cruciate ligament surgery. AAOS Instruct Course Lect. 1987;36:173-178.
Ejerhad L, Kartus J, Sernert N, et al. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? A prospective randomized study with two-year follow-up. Am J Sports Med. 2003;31:19-25.
Harvery AR, Thomas NP, Amis AA. The effect of screw length and position on fixation of four-stranded hamstring grafts for anterior cruciate ligament reconstruction. Knee. 2002;10:97-102.
Hill PF, Russell VJ, Salmon LJ, et al. The influence of supplementary tibial fixation on interior laxity measurements after anterior cruciate ligament reconstruction with hamstring tendons in female patients. Am J Sports Med. 2005;33:94-101.
Howell SM, Clark JA, Farley TC. A rationale for predicting anterior cruciate graft impingement by the intercondylar roof: an MRI study. Am J Sports Med. 1991;19:276-281.
Howell SM, Gittens ME, Gottlieb JE, et al. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29:567-574.
Jannson KA, Linko E, Sandelin J, et al. A prospective randomized study of patellar versus hamstring tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:12-18.
Karahan M, Erol B, Bekiroglu N, Uyan D. Effect of drain placed in the donor site in the early postoperative period after arthroscopically assisted cruciate ligament reconstruction with quadrupled hamstring tendons. Am J Sports Med Preview. 2005;33:900-906.
Kartus J, Magnusson L, Stener S, et al. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2–5 year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:2-8.
Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17:971-980.
Laxdal G, Kartus J, Hannson L, et al. A prospective randomized comparison of bone-patellar tendon-bone and hamstring grafts for anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:34-42.
Levy M, Prudhomme J. Anatomic variations of the pes anserinus: a cadaver study. Orthopedics. 1993;16:601-606.
Levy M. Surgical technique for harvesting the gracilis and semitendinosus tendons. Contemp Orthop. 1993;26:369-372.
Muneta T, Yamamoto H, Ishibashi T, et al. The effects of tibial tunnel placement and roofplasty on reconstructed anterior cruciate ligament knees. Arthroscopy. 1995;11:57-62.
Pagnani MJ, Warner JJ, O’Brien SJ, et al. Anatomic considerations in harvesting the semitendinosus and gracilis tendons and a technique of harvest. Am J Sports Med. 1993;21:565-571.
Pinczewski LA, Deehan DJ, Salmon LJ, et al. Five-year comparison of patellar tendon versus four-strand hamstring tendon autograft for arthroscopic reconstruction of the anterior cruciate ligament. Am J Sports Med. 2002;30:523-535.
Romano VM, Graf BK, Keene JS, et al. Anterior cruciate ligament reconstruction: the effect of tibial tunnel placement on range of motion. Am J Sports Med. 1993;21:415-418.
Soloman CG, Pagnani MJ. Hamstring tendon harvesting. Reviewing anatomic relationships and avoiding pitfalls. Orthop Clin North Am. 2003;34:1-8.
Warren LF, Marshal JL. The supporting structures and layers on the medial side of the knee. J Bone Joint Surg. 1979;61A:56-62.
Woo SL-Y, Hollis JM, Adams DJ, et al. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effect of specimen age and orientation. Am J Sports Med. 1991;19:217-225.
Yamamoto Y, Hsu W-H, Woo SL-Y, et al. Knee stability and graft function after anterior cruciate ligament reconstruction. A comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32:1825-1832.
Yasuda K, Onkoshi Y, Tanabe Y. Graft site morbidity with autogenous semitendinosus and gracilis tendons. Am J Sports Med. 1994;23:705.