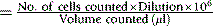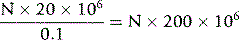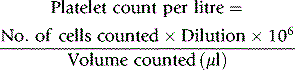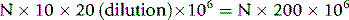Chapter 26 Haematology in under-resourced laboratories
Introduction: types of laboratories
In under-resourced countries, these difficulties are compounded by the high burden of infectious diseases such as HIV/AIDS, tuberculosis and malaria. There are also additional pressures from external agencies for diagnostic and monitoring services which may not accurately reflect local public health priorities. Parasitological diagnosis of malaria is now recommended in all age groups to prevent the over-diagnosis and misuse of new anti-malarial combination drugs. Although it is currently the subject of much debate, WHO guidelines state that the only exception to the requirement for parasitological diagnosis is children aged under 5 years living in stable high-transmission settings where the likelihood of malaria as a cause for fever is high.1 Occasionally, in tertiary centres, the diagnosis of tuberculosis requires aspiration and culture of the bone marrow and trephine biopsy examination, especially in patients who are HIV positive, in whom sputum tests for acid-fast organisms are frequently negative.2 The decision to initiate antiretroviral therapy and the monitoring of therapy require regular haemoglobin concentration (Hb) estimations, CD4-positive lymphocyte counts (or percentages for paediatric care) and, ideally, plasma viral load determinations, although the maintenance of equipment for some of these tests is challenging for low-income countries.3,4
The purpose of this chapter is to provide guidance for an effective haematology service at the different levels of the healthcare system in resource-limited countries. In planning such services, it is necessary to determine what tests are needed at each level and to identify the relevant referral networks for clinical haematology. For example, successful management of haematological malignancies in countries with limited resources might involve partnerships between local institutions and those in more wealthy countries to adapt treatment protocols, to provide training or diagnostic services or to improve local supportive care facilities.5,6
Organization of clinical laboratory services
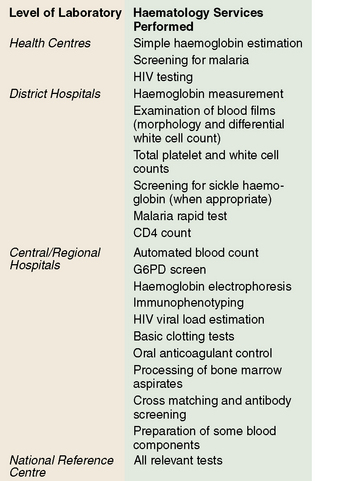
In under-resourced countries clinical laboratory services may be considered at three levels according to their size, staffing and services offered. These are levels: (A) subdistrict facilities including health centres; (B) district and referral hospitals; and (C) central reference and teaching hospitals (Fig. 26.1).
Availability of tests at each level
Level A
Level B
Level C
Microscopes
The microscope is the most important piece of laboratory equipment in under-resourced countries and is essential for the diagnosis of anaemia, malaria and other blood parasitic infections and for performing total and differential white blood cell counts. Reliable assessment of morphological features requires a microscope that is clean and correctly set up with aligned lenses and an electric light source (either inbuilt or reflected light) to ensure clear images, especially at high magnification. Failure to maintain microscopes to a high standard by routine user maintenance or, ideally, with regular professional servicing, can lead to inaccurate diagnoses and inefficient use of technician time.8,9 Routine maintenance of the microscope is described on p. 52.
‘Essential’ haematology tests
Despite the relatively high cost of running a laboratory service and the low per capita healthcare budget in under-resourced countries, there are few data available on which to base rational decisions about ‘essential’ laboratory tests.10,11 In many of these countries, decisions are made at central level by healthcare planners without adequate consultation with laboratory managers and experts. The selection of ‘essential’ tests at each level should be based on the clinical and public health needs of the local community and the availability of qualified clinical and laboratory staff, as well as on the availability of funds. Essential test packages are usually defined as part of national policy and standards, taking into consideration medium- and long-term trends and the requirements of disease control programmes.
To ensure cost-effectiveness of the laboratory service, tests with no proven value should be eliminated and new tests for which there is independent evidence of clinical usefulness should be introduced,12 as described in Chapter 24, p. 567. Tests that provide objective qualitative or quantitative information are preferred. Although it is not possible to draw up a list of essential tests that will be applicable to all countries, or even to different regions within a country, the following aspects should be considered.
Cost per Test
Often the cost of a test is calculated from the price of reagents divided by the number of tests performed. However, this oversimplifies the situation and is not accurate enough to form the basis for national policy decisions and budget allocation.11 The factors that need to be taken into account when calculating the total annual costs for a laboratory are given in Chapter 24.
Diagnostic Reliability
The quality of all tests carried out by a laboratory should be regularly monitored; systems for doing this are well-established (see Chapter 24) but are not easily implemented in under-resourced settings. The quality of a test influences its usefulness as well as its utilization by clinicians and community members. For example, if the result of a test in routine practice is correct only 80% of the time, then one in five tests will be wasted, reducing the effectiveness of the test by 20%. Furthermore, the inaccurate test may result in a patient receiving inappropriate treatment. Clinicians and the general public are increasingly aware of the need for accurate diagnosis. Clinicians may not order tests or use the results in patient management decisions if they do not trust the quality of the laboratory results13 and poor quality healthcare may deter patients from using health facilities.
Clinical Usefulness
An assessment of the clinical usefulness of a test should be carried out by an independent clinician who is familiar with local diseases and the diagnostic support services that are available. This assessor needs to compare actual clinical practice with locally agreed ‘best practice’ or, if available, local guidelines.12 From observation of a range of clinical interactions, the percentage of times that ideal practice is followed can be calculated. For example, transfusion guidelines may recommend that transfusions are given routinely to children with an Hb of <5 g/dl. The assessor can record how many children with Hb below this level failed to receive a transfusion and how many transfusions were given without waiting for the Hb result or at an inappropriate Hb level. For each test, the assessor needs to judge whether the test has been appropriately requested and is used to influence patient management or public health decisions. The percentage of tests that are not used to guide clinical decisions will provide a figure for ‘clinical wastage’ of the test that can be entered into the formula (Chapter 24).
Maintaining quality and reliability of tests
Paradoxically, it is in under-resourced laboratories, where equipment and supplies are limited and training and supervision may be minimal, that the level of skills and motivation required to maintain a good-quality service need to be highest. Even the most basic of laboratories should ensure that procedures are in place to monitor quality (see Chapter 25). In addition to monitoring the technical quality of each test, the quality of the whole service must be ensured both within the laboratory and between laboratories. Standard operating procedures (see p. 575) should be drafted for every method performed in the laboratory; these can be adapted from existing standard operating procedure ‘models’. In addition to providing standardized techniques, standard operating procedures are excellent teaching resources and adherence to these procedures will minimize errors. Standard operating procedures need to be regularly reviewed and updated to keep pace with technical developments and changes in local circumstances (e.g. non-availability of reagents, technical limitations).
Quality Control of a Test Method (Technical Quality)
Methods for the control of various haematology tests are described in Chapter 25 but some may need to be adapted to specific local circumstances in resource-poor countries. For example, if commercial controls are not affordable, each batch of sickle cell screening tests should include known positive and negative samples from a previous batch of tests; for monitoring constancy of Hb estimations, a high and a low value sample can be re-tested several times during the day.
External Quality Assessment
The principles of external quality assessment (EQA) are described on p. 595. Poor communications and transport facilities make it difficult, but not impossible, to establish EQA in under-resourced laboratories. Among its other functions, EQA is used to identify poorly performing laboratories that require assistance and to detect the use of inaccurate techniques. Although participation in international, national or local external quality assessment schemes may be beyond the resources of a small rural laboratory, it should be possible for them to exchange samples and results with neighbouring facilities. Rural laboratories can also take advantage of supervisory visits by district officers to exchange samples, including external quality assessment materials and results with other laboratories. Accreditation schemes (see p. 577), either national or local, can be set up to formally recognize laboratories that are performing well and to assist those that are not.14 Step-wise, rather than pass/fail, accreditation schemes provide a better measure of performance and motivate laboratories to improve their services.
Basic haematology tests
Haemoglobinometry
Various methods for the measurement of Hb are given in Chapter 3. The most accurate method that may be available in under-resourced laboratories is the hemoglobin-cyanide (cyanmethaemoglobin) method. However, this requires a power source and considerable technical expertise to carry out accurate dilutions and to prepare the standard curve. Use of pre-set haemoglobinometers removes the need for calibration curves, but the machine may still need to be set correctly.
Direct Reading Haemoglobinometers
HemoCue Blood Hemoglobin System*
HemoCue Blood Hemoglobin System (see Chapter 3) is a battery- or mains-operated portable, direct read-out machine that uses disposable dry chemistry cuvettes. Measurements are precise and accurate (provided that only the specified cuvettes are used and the reading surfaces are kept clean) and, unlike most other systems, it does not require pre-dilution of the sample. Although the use of the unique disposable cuvettes makes this method relatively expensive, the cuvettes for the new Hb 301 version are cheaper than those for the Hb 201 and are designed for adverse climatic conditions. It is fast and very simple to use so that the cost may be offset by savings on training and supervision time.
Haemoglobin Colour Scale†
Many colour comparison methods have been developed in the past but these have become obsolete because the colours were not sufficiently comparable with blood or were not durable. The haemoglobin colour scale has been developed for anaemia screening where there is no laboratory.15 It consists of a set of printed colour shades representing haemoglobin levels between 4 and 14 g/dl (i.e. 40 and 140 g/l). The colour of a drop of blood collected onto a specific type of paper matrix is compared with that on the chart (Fig. 26.2). It is intended for detecting the presence of anaemia and estimating its severity in 2 g/dl (20 g/l) increments. The scale has been tested in resource-poor settings but more studies are needed to demonstrate whether it is superior to clinical diagnosis.16–18 The instructions should be followed carefully because poor lighting, allowing the blood spot to dry out, and using the incorrect type of paper matrix for the test strips, can have detrimental effects on the results.
Manual Cell Counts Using Counting Chambers
Visual counting of blood cells is an acceptable alternative to electronic counting for white blood cells but it is not recommended for routine red blood cell counts because the number of cells that can be counted within a reasonable time in the routine laboratory (e.g. about 400) will be too few to ensure a sufficiently precise result (see below). Although manual counting is recommended for platelet counts,19 in practice, it is often easier to detect gross reductions or increases in platelet counts from examination of the peripheral blood film.
Counting Chambers
The visibility of the rulings in the counting chamber19 is as important as the accuracy of calibration, so that chambers with a ‘metallized’ surface and Neubauer or Improved Neubauer rulings are recommended. These have nine 1 × 1 mm ruled areas, which, when covered correctly with the special thick coverglass, each contain a volume of 0.1 μl diluted blood (Figs 26.3, 26.4). Coverslips designed for mounting of microscopy preparations must not be used with counting chambers. The sample is introduced between the chamber and the coverglass using a pipette or capillary tube and the preparation is viewed using ×40 objective and ×6 or ×10 eyepieces. With Neubauer and Improved Neubauer chambers, the cells in 4 or 8 horizontal rectangles of 1 × 0.05 mm (80 or 160 small squares) or in 5 or 10 groups of 16 small squares are counted, including the cells that touch the bottom and left-hand margins of the small squares.
Total White Blood Cell Count
Method
Make a 1 in 20 dilution of blood by adding 0.1 ml of well-mixed blood (lack of adequate mixing is a major source of error) to 1.9 ml of diluent* in a 75 × 10 mm plastic (or glass) tube. After sealing the tube with a lid or tightly fitting bung, mix the diluted blood in a mechanical mixer or by hand for at least 2 min by tilting the tube to an angle of about 120° combined with rotation, thus allowing the air bubble to mix the suspension. Fill a clean dry counting chamber, with its coverglass already in position, without delay. This is simply accomplished with the aid of a plastic Pasteur pipette or a length of stout capillary glass tubing that has been allowed to take up the suspension by capillarity. Take care that the counting chamber is filled in one action and that no fluid flows into the surrounding moat.
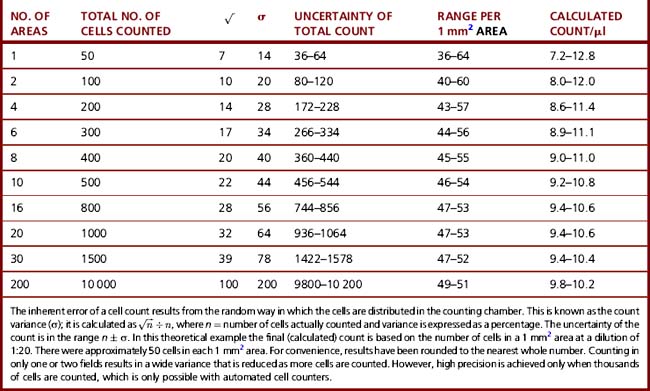
To obtain a coefficient of variation of 5%, it is necessary to count about 400 cells (Table 26.1); in practice, it is reasonable to count 100 white cells. To minimize distribution errors, count the cells in the entire ruled area (i.e. 9 × 0.1 μl areas in an Improved Neubauer counting chamber). In practice, the 100 cell count can be achieved by counting the cells in the four larger corner squares.19
Platelet Count
Automated full blood counters produce platelet counts with a precision that is much superior to that of manual platelet counts. Manual platelet counts may occasionally be needed for blood samples with a significant proportion of giant platelets. Manual platelet counts are best performed on ethylenediaminetetra-acetic acid (EDTA) anticoagulated blood that has been obtained by clean venipuncture. Skin-prick samples are associated with platelet clumping so platelet counts are significantly lower and less constant than those performed on venous blood. Manual platelet counts are performed by visual examination of diluted, lysed whole blood using a Neubauer or Improved Neubauer counting chamber as for total white cell counts.20,21
Method
Examine the preparation with the ×40 objective and ×6 or ×10 eyepieces. The platelets appear under ordinary illumination as small (but not minute), highly refractile particles if viewed with the condenser racked down; they are usually well separated and clumps are rare if the blood sample has been properly collected. To avoid introducing dirt particles into the chamber which might be mistaken for platelets, all equipment must be scrupulously clean. Platelets are more easily seen with the phase contrast microscope. A special, thin-bottomed (1 μm) counting chamber is best for optimal phase contrast effect. The number of platelets in one or more areas of 1 mm2 should be counted. The total number of platelets counted should always exceed 200 to ensure a coefficient of variation of 8–10%.
Calculation
Thus, if N is the number of platelets counted in an area of 1 mm2 (0.1 μl in volume), the number of platelets per litre of blood is:
Errors in Manual Cell Counts
The errors associated with manual cell counts are technical and inherent.
Technical errors can be minimized by avoiding the following:
Standardized Counting Chambers
To reduce errors, it is important to have a good-quality counting chamber. The exact chamber depth depends on the coverglass, which should be free from bowing and sufficiently thick so as not to bend when pressed on the chamber. The coverglass must be free from scratches and even the smallest particle of dust may cause unevenness in its lie on the chamber. The specifications described by WHO19 outline a tolerance of dimensions for counting chambers that provides reasonable accuracy.
Microscopy Artefacts
Inherent errors result from uneven distribution of cells in the counting chamber and no amount of mixing will minimize this inherent variation in numbers between areas. Inherent error can only be reduced by counting more cells in a preparation. In theory, the count varies in proportion to the square root of the number of cells counted, i.e. if four times the number of cells are counted, the variation is halved (Table 26.1). For example, when performing a manual white cell count, 95% of the observed counts in a sample of true value 5.0 × 109 cells/l would lie within the range 4.0–6.0. In practice, the difference between 5.0 and 6.0 × 109 cells/l for a white cell count is of little clinical significance.
It is also important for the observer not to bias the count by foreknowledge of what result might be expected or by selecting certain areas in the chamber for counting.22
Peripheral Blood Morphology
Examination of the peripheral blood film is one of the most important and useful examinations in the peripheral laboratory but it requires a great deal of skill and experience as well as a good-quality microscope (see Chapter 5). In addition to providing information about quantitative changes in blood cells, careful analysis of the qualitative changes may help in elucidating the underlying reasons for clinical problems. These observations may identify the cause of anaemia or undiagnosed fever or the presence of a haemoglobinopathy (see Chapter 14). Problems that might occur when preparing blood films relate to the slides themselves and to the quality of fixing of the films and the quality of staining reagents. When glass slides are in short supply, laboratories sometimes find it necessary to wash and reuse slides (see p. 623). Traces of detergent can result in misleading appearances of the red cells (Fig. 26.5), as can residual stain and scratches on the slide. In humid conditions, particularly during the rainy season, water may be absorbed by the methanol used for fixing slides and this can cause gross artefactual changes in red cell appearance (see Fig. 4.2). To avoid this problem, stock bottles of methanol should be kept tightly closed after use; small amounts should be aliquoted into a bottle with a tightly fitting cap for daily use and replaced regularly from the stock bottle.
Modified (One-Tube) Osmotic Fragility Test
A variety of concentrations of buffered saline have been used. A concentration of 0.36% in buffered saline (see p. 622 for preparation) is recommended to ensure a high sensitivity with an acceptable specificity.23 Because the false-positive rate is around 10%, confirmation of a positive result requires referral of a sample to a laboratory able to quantitate haemoglobin A2. The test can also be used to screen for α0 thalassaemia trait, with positive samples being referred to a reference centre for DNA analysis. About 50% of samples containing haemoglobin E also give a positive result; this is an advantage rather than a disadvantage because detection of haemoglobin E is important in predicting the possibility of thalassaemia major or intermedia in compound heterozygotes with β thalassaemia.
Haemoglobin E Screening Test
Ideally, the diagnosis of haemoglobin E heterozygosity or homozygosity should be by haemoglobin electrophoresis at alkaline pH or high-performance liquid chromatography with a second method being used to confirm the provisional identification (see Chapter 14). When these facilities are not available, a screening test using the blue dye 2,6-dichlorophenolindophenol (DCIP), can be used. Samples containing haemoglobin E become faintly turbid when incubated with DCIP.24,25
Tests for Paroxysmal Nocturnal Haemoglobinuria
When facilities permit, flow cytometry for glycosylphosphatidylinositol-anchored proteins is the test of choice to detect paroxysmal nocturnal haemoglobinuria (PNH) clone. If positive, a sample of urine should be examined for haemosiderin. The Ham test and sucrose lysis test (see Chapter 13) can be used as tests for PNH. They are less sensitive and less quantitative than flow cytometry, but nevertheless are clinically useful.
Reagents for Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT)
Rabbit Brain Thromboplastin for Prothrombin Time
Laboratory support for management of hiv/aids: cd4-positive t-cell counts
The WHO recommends that district hospitals and higher level facilities should be able to provide a CD4+ cell count to aid identification of patients who would benefit from antiretroviral drugs and to monitor their progress. Several methods are available but their effectiveness in developing country settings is very variable.26 Some of the low-cost methods that have good correlation with flow cytometric enumeration of CD4+ cells include the FACS count system (Becton Dickinson Sciences, CA), the Guava Easy CD4 assay (Guava technologies, Hayward, CA), Cyflow (Partec, Germany) and the panleucogating (PLG) CD4 technique. Manual methods such as Dynabeads (Dynal) and Cytospheres (Coulter) can also be used to measure CD4+ T-cell count but the cost, time and skill required make them unsuitable in many settings when other options are available.27 Dried spots of serum or blood can be used to assess HIV-1 viral load and for resistance genotyping.28
Laboratory management
Specimen Transport
The need to ensure appropriate conditions for keeping specimens after they have been collected and other aspects of specimen transport to the laboratory are described in Chapter 1. The special problem of transporting specimens from remote clinics to laboratories and reference centres in low-resource countries requires further consideration. Peripheral laboratories can assist in rural development initiatives by creating employment opportunities for bicycle riders, motorcyclists, taxi operators and formal and informal courier companies. Commercial or private air services can be used to deliver samples to referral centres. The location of clinics is helped if Global Positioning System (GPS) coordinates are available for each clinic.29
Staff Training
Access to up-to-date information is important for adapting and enhancing laboratory performance. Many medical and technical journals now publish their articles in full on the internet and by an arrangement between the main international publishers and WHO, they can be accessed free of charge or at a significantly discounted cost in most under-resourced countries (see www.who.int/hinari/en).
Clinical Staff Interaction
A major delay in test turnaround time (see p. 574) and the failure of clinicians to use test results is often due to the slow delivery of reports within the health facility after the test has been performed. Appropriate clinical use of the laboratory has a direct impact on the cost-effectiveness of the service12. Laboratory tests may be initiated by nurses, health field workers and public health officers, as well as doctors. Many of these individuals have little or no training in how to request appropriate tests, how to provide timely and suitable samples and how to use the results for maximal benefit to patients. Training for laboratory users needs to be incorporated into laboratory training programmes and be closely monitored. In poorer countries, there is often a dearth of clinicians with both clinical and laboratory experience who are qualified to provide such training. However, the relationship between the laboratory and clinical staff can be enhanced and use of the laboratory can be optimized by using a clinician/scientist team to provide joint training in both disciplines. Various publications are now available to guide laboratory users on which test to select, how to interpret the results and how to select and collect the correct samples.
Health and Safety
Details of laboratory safety issues are described in Chapter 24. Awareness of these issues should be constantly promoted within all laboratories,30 and the working environment needs to be made as safe as possible. A ‘Code of Safe Laboratory Practice’ should be prepared that is affordable and relevant to the local circumstances. It should include the following:
1 WHO. Guidelines for the Treatment of Malaria, 2010. Available at: www.who.int/malaria/publications/atoz/9241546948/en/index.html Accessed 25 01
2 Akpek G., Lee S.M., Gagnon D.R., et al. Bone marrow aspiration, biopsy and culture in the evaluation of HIV-infected patients for invasive mycobacteria and histoplasma infections. Am J Hematol. 2001;67:100-106.
3 Larsen C. The fragile environment of inexpensive CD4+ T-cell enumeration in the least developed countries: strategies for accessible support. Clinical Cytometry Society. 2008;74B(Suppl 1):S1007-S1116.
4 WHO. Consultation on Laboratory Services for Monitoring HIV Anti-retroviral Therapy in Resource-limited Settings. Available at: www.who.int/diagnostics_laboratory/events_hivlab/en/, 2004. Accessed 25 01 2010
5 Magrath I Shanta V., Advani S., et al. Treatment of acute lymphoblastic leukaemia in countries with limited resources; lessons from use of a single protocol in India over a twenty year period. Eur J Cancer. 2005;41:1570-1583.
6 Parkins E., Owen R.G., Bedu-Addo G., et al. UK-based real-time lymphoproliferative disorder diagnostic service to improve the management of patients in Ghana. Journal of Hematopathology. 2009;2(3):143-149.
7 WHO. Learning Bench Aids Series 1–9. 3rd ed., 2008. Available at: www.tht.ndirect.co.uk/2009orderform.htm Accessed 25 01 2010
8 Mundy C., Ngwira M., Kadawele G., et al. Evaluation of microscope condition in Malawi. Transactions of the Royal Society of Medicine and Hygiene. 2000;94:583-584.
9 Opoku-Okrah C., Rumble R., Bedu-Addo G., et al. Improving microscope quality is a good investment for under-resourced laboratories. Transactions of the Royal Society of Medicine and Hygiene. 2000;94:582.
10 World Health Organization. Laboratory Services for Primary Heath Care: Requirements for Essential Clinical Laboratory Tests. Geneva: WHO, 1998. Document: LAB/98.1
11 Mundy C.J., Bates I., Nkhoma W., et al. The operation, quality and costs of a district hospital laboratory service in Malawi. Trans R Soc Trop Med Hyg. 2003;97:403-408.
12 Mepham S., Squire S.B., Chisuwo L., et al. Utilization of laboratory services by health workers in a district hospital in Malawi. J Clin Pathol. 2009;62:935-938.
13 Bates I., Mundy C., Pendame R. Use of clinical judgement to guide administration of blood transfusions in Malawi. Trans R Soc Trop Med Hyg. 2001;95:510-512.
14 WHO. Guidelines for the Establishment of Accreditation of Health Laboratories, 2010. Available at: www.searo.who.int/LinkFiles/Publications_SEA-HLM-394.pdf Accessed 24 01
15 Lewis S.M., Stott G.J., Wynn K.J. An inexpensive and reliable new haemoglobin colour scale for assessing anaemia. J Clin Pathol. 1998;51:21-24.
16 Critchley J., Bates I. Haemoglobin colour scale for anaemia diagnosis where there is no laboratory. Int J Epidemiol. 2005;34:1425-1434.
17 Van den Broek N.R., Ntonya C., Mhanga E., et al. Diagnosing anaemia in pregnancy in rural clinics: assessing the potential of the haemoglobin colour scale. Bull World Health Organ. 1999;77:15-21.
18 Montresor A., Ramsan M., Khalfan N., et al. Performance of the haemoglobin colour scale in diagnosing severe and very severe anaemia. Trop Med Int Health. 2003;8:1-6.
19 WHO. Recommended Methods for the Visual Determination of White Blood Cell Count and Platelet Count. Geneva: World Health Organization, 2000. Document WHO/DIL/00.3
20 Brecher G., Cronkite E.P. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950;3:365-377.
21 Lewis S.M., Wardle J., Cousins S., et al. Platelet counting – development of a reference method and a reference preparation. Clin Lab Haematol. 1979;1:227-237.
22 Sanders C., Skerry D.W. The distribution of blood cells on haemocytometer counting chambers with special reference to the amended British Standards Specification 748 (1958). J Clin Pathol. 1961;48:298-304.
23 Chow J., Phelan L., Bain B.J. The role of a single-tube osmotic fragility for thalassemia screening in under-resourced laboratories. Am J Hematol. 2005;79:198-201.
24 Fucharoen G., Sanchaisuriya K., Sae-ung N., et al. A simplified screening strategy for thalassaemia and haemoglobin E in rural communities in south-east Asia. Bull World Health Organ. 2004;82:364-372.
25 Chapple L., Harris A., Phelan L., et al. Reassessment of a simple chemical method using DCIP for screening for haemoglobin E. J Clin Pathol. 2005;59:74-76.
26 Kimmel A.D., Losina E., Freedberg K.A., et al. Diagnostic tests in HIV management: a review of clinical and laboratory strategies to monitor HIV-infected individuals in developing countries. Bull World Health Organ. 2006;84:581-588.
27 Taiwo B.O., Murphy R.L. Clinical applications and availability of CD4+ T cell count testing in sub-Saharan Africa. Cytometry B. Clin Cytom. 2008;74(suppl 1):S11-S18.
28 Hamers R.L., Smit P.W., Stevens W., et al. Dried fluid spots for HIV type-1 viral load and resistance genotyping: a systematic review. Antivir Ther. 2009;14:619-629.
29 Medical Research Council of South Africa. Geographic Information Systems in Health, 2001. Available at: www.mrc.ac.za/mrcnews/june2001/geoinformation.htm
30 WHO. Laboratory Quality Management System Training Toolkit. http://www.who.int/ihr/training/laboratory_quality/en/, 2011.
Laboratory Organization and Management
Houng L., El-Nageh M.M. Principles of management of health laboratories. WHO Regional Publications, 1993. Eastern Mediterranean Series No. 3
World Health Organization. Johns W.L., El-Nageh M.M., editors. Selection of basic laboratory equipment for laboratories with limited resources. Regional Office for the Eastern Mediterranean, 2000.
World Health Organization. Health laboratory services in support of primary healthcare in South East Asia. WHO South East Asia Regional Office (SEARO), 1999. Publication No. 24. 2nd ed
World Health Organization. Health laboratory facilities in emergency and disaster situations. WHO Regional Publications, 1994. Eastern Mediterranean Series No. 6
World Health Organization. Maintenance and repair of laboratory, diagnostic imaging and hospital equipment. Geneva: WHO Distribution and Sales, 1994.
Carter J.Y., Lema O.E. Practical laboratory manual for health centres in Eastern Africa. Nairobi, Kenya: African Medical and Research Foundation, 1994. PO Box 31025
Cheesbrough M. District Laboratory Practice in Tropical Countries: Vols 1 and 2. Cambridge: Cambridge University Press, 2006.
World Health Organization. Manual of basic techniques, Revised 2nd ed., Geneva: WHO Distribution and Sales, 2000.
World Health Organization. Production of basic diagnostic laboratory reagents. WHO Regional Publications, 1995. Eastern Mediterranean Series No. 2
World Health Organization. Methods Recommended for Essential Clinical Chemical and Haematological Tests for Intermediate Hospital Laboratories. Geneva: WHO, 1986. WHO/LAB/86.3
Lewis S.M. Quality Assurance in Haematology. WHO Document: LAB/98.4. Geneva: WHO, 1998.
World Health Organization. Quality Systems for Medical Laboratories: Guidelines for Implementation and Monitoring. WHO Regional Publications, 1995. Eastern Mediterranean Series No. 14
World Health Organization. Quality Assurance Related to Health Laboratory Technology. Geneva: WHO, 1993.
Sources of Teaching Material and Equipment for Under-resourced Laboratories
Bridge House, Worlington Road, Barton Mills, IP28 7DX, UK
Tel: +44 (0) 1603 416058; Fax: +44 (0) 1603 416066; e-mail: dht@gordon-keeble.co.uk;
PO Box 50, Fakenham, Norfolk NR21 8XB, UK
Tel: +44 (0) 1328 855805; Fax: +44 (0) 1328 853799; e-mail: thtbooks@tht.ndirect.co.uk;
Teaching Aids at Low Cost (TALC)
PO Box 49, St Albans, Herts AL1 5TX, UK
Tel: +44 (0) 1727 853869; Fax: +44 (0) 1727 846852; e-mail: Info@talcuk.org;
Institute of Child Health, 30 Guilford Street, London WC1N 1EH, UK
* A bench-aid on morphology is available from Tropical Health Technology.7
* Available from HemoCue AB, Angelholm, Sweden; www.hemocue.com (Accessed 25 January 2010).
† Available from Teaching Aids at Low Cost; www.talcuk.org/accessories/haemoglobin-colour-scale.htm (Accessed 25 January 2010).
* Or a proportionately smaller volume: 0.02 ml (20 μl) of blood in 0.38 ml of lysing fluid.