Chapter 92 Haematological malignancies
The treatment of haematological malignancy has been an evolving success story. In recent years, the prognosis for patients with acute leukaemia has changed from death within 1–3 months without effective therapy to long-term survival and cure in many cases.1,2 In some diseases the potential for regular cure of patients has been realised, including Hodgkin’s disease, childhood acute lymphoblastic leukaemia, some high-grade lymphomas and some adult leukaemias.3 These advances have predominantly resulted from the introduction of a wide range of cytotoxic chemotherapeutic regimens which permit obliteration of the disease in conjunction with comprehensive supportive therapy. In some cases, ‘supralethal’ therapy is necessary, with bone marrow transplantation (autologous or allogeneic) being used as marrow ‘rescue’ therapy. The development of ‘engineered’ highly specific and targeted drugs is offering further promise (e.g. monoclonal antibodies, tyrosine kinase inhibitors for chronic myeloid leukaemia).1
CLASSIFICATION AND PATHOPHYSIOLOGY
Most patients with acute myeloid leukaemia present with features of bone marrow failure. Acute promyelocytic leukaemia is a unique subtype of acute myeloid leukaemia which may typically present with disseminated intravascular coagulation (DIC) requiring expert haematological management.4 Acute lymphoblastic leukaemia is the commonest encountered in children. The lymphomas are a complex and heterogeneous group of malignancies ranging from highly malignant disorders through to low-grade indolent disease not requiring therapy.
Dysplasia may affect any of the haemopoietic elements, with the patient presenting with a range of peripheral blood abnormalities, most commonly including anaemia, thrombocytopenia and neutropenia. Diagnosis, classification, understanding of pathophysiology and therapy have undergone enormous changes in recent years and the non-specialist can be excused for feeling confused.5,6 There is a rising prevalence and incidence of the myelodysplastic syndromes. Epidemiological data indicate that they are more common than initially thought and probably on the increase, especially due to the ageing population and their occurrence as a late complication of cytotoxic chemotherapy.
Awareness of these relatively common disorders is important as undiagnosed patients with relatively normal blood counts may present de novo with life-threatening infection or haemorrhage in a postoperative or trauma setting.
COMPLICATIONS OF HAEMATOLOGICAL MALIGNANCY AND ITS THERAPY
METABOLIC DISTURBANCES
At presentation, haematological malignancy may be associated with a range of metabolic derangements.7 Hyperuricaemia and hypercalcaemia are well-recognised complications which may be associated with renal failure.8 The tumour lysis syndrome is a rarer complication which may occur spontaneously or shortly after the initiation of therapy.9,10 There is sudden liberation of intracellular contents in quantities that overwhelm the excretory capacity of the kidneys, resulting in hyperkalaemia, hyperphosphataemia, hypocalcaemia and occasionally lactic acidosis. Pre-empting the development of this syndrome usually allows control of the metabolic effects, especially with the maintenance of high intravenous fluid intake, alkalinising the urine and administration of allopurinol. More recently, intravenous rasburicase – a recombinant uricolytic agent which, in contrast to allopurinol, acts on existing uric acid concentrations – is being used for the management of anticancer therapy-induced hyperuricaemia.11 Rasburicase is contraindicated in patients with methaemoglobinaemia and glucose-6-phosphate dehydrogenase deficiency.
Multiple myeloma may be complicated by renal insufficiency, hypercalcaemia, hyperviscosity and hyperuricaemia. Most of these can be managed conventionally; however, with large amounts of monoclonal protein, fluid management can be difficult due to the hypervolaemia with or without hyperviscosity and plasma exchange may be indicated (see Chapter 90).
COAGULOPATHIES
A range of haemostatic disturbances may occur in association with haematological malignancy and its therapy. Most of these are considered in general terms in Chapter 91 which outlines the general principles of diagnosis and management.
ACUTE RESPIRATORY DISTRESS SYNDROME
Acute (or adult) respiratory distress syndrome (ARDS) remains a potentially lethal complication of autoaggressive inflammation. As most of the mediators (cytokines, neutrophils and endothelial adherence molecules) initiating the disease process are haemopoietic in origin it is not surprising that ARDS may occur in haemopoietic malignancies. However, ARDS is relatively uncommon in the neutropenic septic patient, probably due to the fact that the neutrophil under normal circumstances is one of the central mediators of this syndrome. ARDS and interstitial pneumonitis may be a problem due to hyperleukocytosis, transfusion-related acute lung injury (TRALI; see Chapter 88), cytomegalovirus (CMV) infection, DIC, post marrow transplantation, and sometimes in relation to chemotherapy and radiotherapy. The use of all-trans retinoic acid (ATRA) may be associated with the development of a potentially lethal ARDS/pulmonary leukostasis syndrome (retinoic acid syndrome), usually in association with a rising leukocyte count.12 In all the settings mentioned, early recognition of the symptom complex of fever and dyspnoea (with or without pulmonary infiltrates) is important and therapy with high-dose corticosteroids decreases morbidity and mortality.
BONE MARROW FAILURE
Neutropenia
Neutropenia should be considered in the following terms:
For practical purposes neutropenia has been divided into the following groups:
Figure 92.1 illustrates the numerous interacting factors to be considered when managing patients with neutropenia in association with haematological malignancy and its therapy. Table 92.1 lists the organisms which may be associated with various defects in the host defence system.
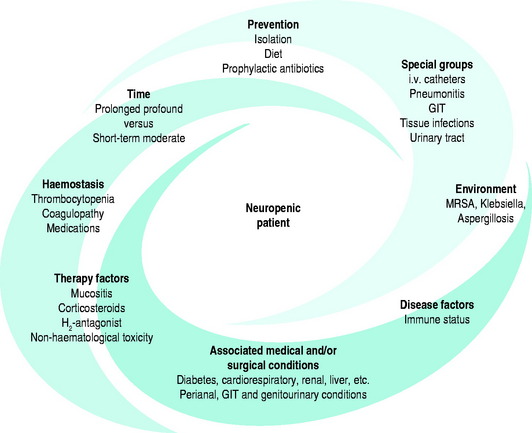
Figure 92.1 The neutropenic patient. GIT, gastrointestinal tract; MRSA, meticillin-resistant Staphylococcus aureus.
Table 92.1 Organisms associated with immune deficiency states
| Neutropenia |
| Bacteria |
PRINCIPLES OF MANAGEMENT OF HAEMATOLOGICAL MALIGNANCIES
ICU ADMISSION
Patients with haematological malignancy may be critically ill from their disease or therapy, for brief or long periods. At times during therapy they may require intensive supportive therapy and can develop a range of life-threatening complications. Rarely, it is necessary to admit critically ill patients with haematological disorders to the ICU. Under normal circumstances, admission of such patients is to be avoided as they have severely impaired host defences and poor tolerance for invasive procedures. However, mechanical ventilation may be required for severe respiratory infections or pneumonitis. Such patients may present specific problems for the ICU staff. Most patients have severe marrow failure requiring intensive transfusion support. Severe neutropenia is the main, and most life-threatening, defect in the host defence system. Nutrition can be a challenge in these patients due to a multitude of factors. The anorexia, nausea and vomiting with chemotherapy, oral mucositis, hypermetabolism, malabsorption and diarrhoea are only some of the factors mitigating against maintaining an adequate nutritional state, and parenteral nutrition may be needed if the period of therapy is prolonged.
INFECTION PREVENTION
Other preventive measures include sterile techniques for invasive procedures by experienced staff, maintaining a clean environment, cooked patient meals, and limited use of prophylactic antibiotics. Needless to say, excessive use of antibiotics is a major factor in the emergence of resistant strains and the susceptibility to colonisation with hospital-acquired organisms. Many would argue that the efficacy of empirical therapy in the neutropenic patient is such that prophylaxis may be unnecessary. Reverse barrier nursing is instituted in order to minimise exposure to exogenous infection. This can be difficult in a critical care setting and will not prevent infections of endogenous origin.
BLOOD COMPONENT THERAPY
Regular platelet concentrates are required. In the presence of sepsis platelets are consumed rapidly and transfusions may be necessary on a daily basis, otherwise every second day is usually adequate. The patient should be examined daily for evidence of haemostatic failure (e.g. purpura, ecchymoses, mouth, fundoscopy). Granulocyte transfusion may have a role in patients with proven unresponsive bacterial sepsis.13
ANTIMICROBIAL THERAPY
The febrile neutropenic patient
The role of prophylactic antibiotic therapy has been controversial due to concerns related to the development of antibiotic resistant micro-organisms, but there is evidence to support their use.14,15 Early empirical antibiotic therapy, without microbiological proof of cause, may be life-saving.16 With most patients fever resolves, especially if marrow function returns in the short term. Ultimate proof of infection is frequently not established. Patients with agranulocytosis will not show the typical features of inflammation. For example, lung consolidation may not occur, cellulitis or sputum may not be clinically obvious, and urinary tract symptoms may be minimal. The role of steroids in suppressing temperature and clinical features of inflammation must also be considered. Non-infectious fever should be borne in mind (e.g. malignancy or drugs).
The presence of rigors, hypotension and shock may point more towards Gram-negative sepsis. The value of combination therapy with a β-lactam antibiotic in combination with an aminoglycoside has been demonstrated in patients with prolonged and severe neutropenia with Gram-negative septicaemia. Gram-negative sepsis is potentially rapidly fatal in the neutropenic patient, but is less common than in the past as the less virulent Gram-positive organisms now predominate. This may be related to several factors including the increased use of permanent indwelling intravenous devices, mucositis, H2 antagonists and the use of quinolones as antibiotic prophylaxis. However, both streptococcal and staphylococcal sepsis can be severe and empiric use of vancomycin in high-risk patients is justified. Staphylococcus epidermidis is an indolent form of sepsis and time is usually available for adjustment of therapy, with most patients recovering.
THE choice of empirical antibiotic therapy
Empirical antibiotic therapy should be aggressive with cover against Gram-negative sepsis as this is potentially lethal for the neutropenic patient.17 Anaerobic cover may need to be included when there is any problem with the gastrointestinal tract (e.g. intra-abdominal or perianal problems). This is not usually necessary when problems are confined to the mouth.
The nature and site of infection is determined by a range of factors including:
Antibiotic regimens
Empirical antimicrobial regimens for neutropenic patients remain a constant changing and controversial issue.17,18 Initial empirical antibiotic therapy should cover Pseudomonas aeruginosa, Escherichia coli, Klebsiella species, Streptococcus viridans, Staphylococci (coagulase negative and coagulase positive) depending on clinical circumstances. Other organisms should be covered after the results of cultures have been assessed or it is known that a specific organism is present in the ward (e.g. Streptococcus viridans) or on surveillance cultures. Coverage against Staphylococcus epidermidis is not necessary until bacteraemia is demonstrated.
Three-drug therapy
With patients on three-drug therapy, antibiotic reassessment at 72 hours is appropriate. If there is no response, further investigation such as a CT chest and bronchoalveolar lavage may be indicated. Depending on the clinical state of the patient the introduction of antifungal therapy should be considered. Fungal infections are an increasing problem in patients with profound prolonged neutropenia.19 There is an increasing range of antifungals available, including voriconazole and the echinocandins (caspofungin, micafungin). Standard amphotericin is now rarely used. Prophylaxis with new azoles such as posaconazole may reduce the incidence of Aspergillus as fluconazole has with Candida infection. The use of potent anti-Aspergillus agents seems to be resulting in the emergence of breakthrough fungal infections with Mucormycosis, Scedosporium and Fusarium. These are uncommon fungal infections which were only rarely seen previously.
Haemopoietic growth factors, immunotherapy and granulocyte transfusions
Genetically engineered haemopoietic growth factors are now widely used to minimise the degree and time of neutropenia. Granulocyte and granulocyte-macrophage colony-stimulating factors are glycoproteins which stimulate the proliferation and maturation of bone marrow progenitor cells, and increase the number and function of these committed cell populations. Treatment reduces the duration and severity of neutropenia in patients receiving chemotherapy and after haemopoietic stem cell transplantation.20 Growth factors do not prevent neutropenia, but do shorten its duration, and their use has been associated with fewer infectious febrile episodes. Intravenous immunoglobulin should be infused in patients with hypogammaglobulinaemia. Granulocyte transfusion may have a role if bacterial-proven sepsis is not resolving or there is evidence of spreading local bacterial infection.
HAEMOPOIETIC STEM CELL TRANSPLANTATION
Haemopoietic stem cell transplantation therapy is increasingly establishing a role in the treatment of a wide range of malignant and non-malignant disorders.21 Allogeneic haemopoietic stem cell transplantation has been used as an adjunct to the treatment of acute leukaemia with an impressive success rate, which is translating into long-term survival and cure. This success is now being extended to the management of a wider range of haematological malignancies and some solid tumours. Haemopoietic stem cells can be obtained from either marrow aspiration or from the peripheral blood by apheresis using a blood cell separator following stimulation of the marrow with haemopoietic growth factors ± cytotoxic chemotherapy. Allogeneic transplantation brings with it a range of potentially serious and potentially fatal complications related to graft-versus-host disease (GVHD).22 It does, however, have the advantage of using normal stem cells which need not be stored and a limited degree of GVHD may have a beneficial anti-tumour effect. Autologous stem cell transplantation is of particular advantage when relatively normal bone marrow can be obtained, in which case GVHD is not a problem.
COMPLICATIONS OF HAEMOPOIETIC CELL TRANSPLANTATION
Respiratory failure in haemopoietic cell transplant patients
Respiratory failure is the commonest cause of death in patients undergoing bone marrow transplantation. Both CMV-induced interstitial pneumonia and the idiopathic pneumonia syndrome rarely occur in the early cytopenic phase post transplantation. Haematological reconstitution with donor-type cells seems to be a prerequisite to the development of these pulmonary complications, suggesting a key role of immunological reactions. While CMV pneumonia can be effectively treated or prevented by ganciclovir, the idiopathic syndrome is usually fatal. Due to improved prophylaxis and therapy, lethal interstitial pneumonia due to Pneumocystis jiroveci (formerly P. carinii), herpes simplex, varicella zoster or Toxoplasma gondii, as well as lethal pneumonia caused by bacteria or Candida species, is generally less common. However, Aspergillus species have emerged as frequent causative pathogens. Prolonged granulocytopenia and prolonged medication with corticosteroids are major risk factors for pulmonary aspergillosis, which is commonly fatal, but prophylaxis may be achieved by sterile air supply during the hospital stay and by prophylactic inhalation of amphotericin B. Pulmonary haemorrhage, diagnosed by bronchoalveolar lavage, may develop due to the toxicity of the conditioning regimen, or may be secondary to infectious pneumonia of various kinds. Congestive heart failure might give rise to the development of pulmonary oedema. Patients with hepatic veno-occlusive disease have a high risk of subsequent pulmonary complications.
Graft-versus-host disease (GVHD)
GVHD is a major complication of allogeneic haemopoietic stem cell transplantation, especially with the increasing use of unrelated and mismatched donors.22 The target of the immune response in GVHD has long been regarded to be histocompatibility antigens possessed by the host, but not the donor. However, it is now recognised that self-antigens have been documented in GVHD, confirming that it is more complex than simple alloreactivity. Cytokines play a central role in mediating many of the manifestations of GVHD.
Veno-occlusive disease of the liver
Veno-occlusive disease can be a major complication of haemopoietic stem cell transplantation, predominantly seen in allogeneic transplants, and is a major contributor to mortality.23 The disorder is due to thrombotic occlusion in the small hepatic vessels, probably as a result of endothelial damage associated with high-dose chemotherapy. The problem manifests as weight gain, oedema, ascites, tender hepatomegaly, jaundice and may proceed to liver failure. Prophylaxis with low-dose heparin or prostaglandins may be useful. Treatment is predominantly supportive with fluid management a critical aspect. Treatment with tissue plasminogen activator and antithrombin III concentrates has been successful.
1 Smith M, Barnett M, Bassan R, et al. Adult acute myeloid leukaemia. Crit Rev Oncol Hematol. 2004;50:197-222.
2 Bassan R, Gatta G, Tondini C, et al. Adult acute lymphoblastic leukaemia. Crit Rev Oncol Hematol. 2004;50:223-261.
3 Kuppers R, Yahalom J, Josting A. Advances in biology, diagnostics, and treatment of Hodgkin’s disease. Biol Blood Marrow Transplant. 2006;12(1 Suppl 1):66-76.
4 Arbuthnot C, Wilde JT. Haemostatic problems in acute promyelocytic leukaemia. Blood Rev. 20, 2006. 289–297
5 Vardiman JW. Hematopathological concepts and controversies in the diagnosis and classification of myelodysplastic syndromes. In: Hematology/the Education Program of the American Society of Hematology. Washington, DC: American Society of Hematology; 2006:199-204.
6 Schiffer CA. Clinical issues in the management of patients with myelodysplasia. In: Hematology/the Education Program of the American Society of Hematology. Washington, DC: American Society of Hematology; 2006:205-210.
7 Filippatos TD, Milionis HJ, Elisaf MS. Alterations in electrolyte equilibrium in patients with acute leukemia. Eur J Haematol. 2005;75:449-460.
8 Jibrin IM, Lawrence GD, Miller CB. Hypercalcemia of malignancy in hospitalized patients. Hosp Physician. 2006;42:29-35.. http://www.turner-white.com/memberfile.php?PubCode=hp_nov06_malig.pdf.
9 Rampello E, Fricia T, Malaguarnera M. The management of tumor lysis syndrome. Nature Clin Pract. 2006;3:438-447.
10 Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
11 Oldfield V, Perry CM. Spotlight on rasburicase in anticancer therapy-induced hyperuricemia. BioDrugs. 2006;20:197-199.
12 Tallman MS, Andersen JW, Schiffer CA, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95:90-95.
13 Price TH. Granulocyte transfusion: current status. Semin Hematol. 2007;44:15-23.
14 Craig M, Cumpston AD, Hobbs GR, et al. The clinical impact of antibacterial prophylaxis and cycling antibiotics for febrile neutropenia in a hematological malignancy and transplantation unit. Bone Marrow Transplant. 2007;39:477-482.
15 Hart S. Review: antibiotic prophylaxis reduces mortality in patients with neutropenia. Evid Based Nurs. 2006;9:50.
16 Rolston KV. Management of infections in the neutropenic patient. Ann Rev Med. 2004;55:519-526.
17 Bal AM, Gould IM. Empirical antimicrobial treatment for chemotherapy-induced febrile neutropenia. Int J Antimicrob Agents. 2007;29:501-509.
18 Paul M, Yahav D, Fraser A, et al. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2006;57:176-189.
19 Bow EJ. Of yeasts and hyphae: a hematologist’s approach to antifungal therapy. In: Hematology/the Education Program of the American Society of Hematology. Washington, DC: American Society of Hematology; 2006:361-367.
20 Crawford J. Update on neutropenia and myeloid growth factors. J Support Oncol. 2007;5(4 Suppl 2):27-46.
21 Ljungman P, Urbano-Ispizua A, Cavazzana-Calvo M, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone Marrow Transplant. 2006;37:439-449.
22 Bacigalupo A. Management of acute graft-versus-host disease. Br J Haematol. 2007;137:87-98.
23 MacQuillan GC, Mutimer D. Fulminant liver failure due to severe veno-occlusive disease after haematopoietic cell transplantation: a depressing experience. QJM. 2004;97:581-589.





