Chapter 44 Gynecologic Laparoscopy
HISTORY
The first experimental laparoscopy (ceolioscopy) was performed by Dr. Georg Kelling in Berlin in 1901; Kelling placed a cystoscope into the abdomen of dogs to evaluate the ability of insufflated air to stop gastrointestinal hemorrhage.1 Dr. Hans Christian Jacobaeus of Sweden published the first description of laparothoroscopy in 1910 as a technique to evaluate patients with peritoneal tuberculosis. However, laparoscopy made little headway into clinical practice until after World War I. It took until the 1960s for laparoscopy to be accepted in the United States and Europe as a safe and valuable surgical procedure.
For many years, gynecologic laparoscopy was performed almost exclusively for diagnostic purposes and for sterilizations. By the 1970s, the role of laparoscopy had expanded to include lysis of adhesions and treatment of endometriosis.2 The technology and equipment advanced over the next three decades such that laparoscopy is now used for a wide variety of procedures ranging from treatment of ectopic pregnancies and ovarian cysts to hysterectomy, incontinence procedures, and management of gynecologic malignancies.
GENERAL TECHNIQUES FOR LAPAROSCOPY
Primary Trocar Placement
Standard Closed Technique: Veress Needle and Primary Trocar Insertion
The standard closed technique was used almost exclusively for decades and continues to be widely used today. Both the Veress needle and primary trocar are blindly placed through a periumbilicar incision into the peritoneal cavity. Using this approach with reusable instruments, the combined risk of injuring retroperitoneal vessels, bladder, or bowel has been found to be less than 1 in 1000 cases.3 This approach has become the gold standard against which all other techniques are judged.
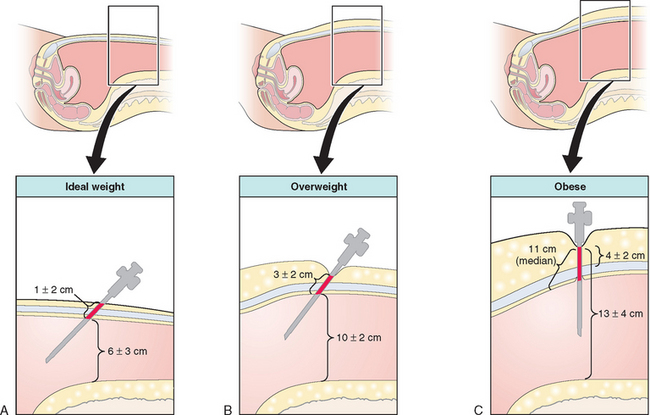
In a woman of ideal weight (body mass index [BMI] <25kg/m2) or only slightly overweight (BMI 25 to 30kg/m2), the lower anterior abdominal wall is grasped and elevated, and the Veress needle is inserted toward the hollow of the sacrum at a 45-degree angle (Fig. 44-1).4 In the thinnest patients in this group, the retroperitoneal vessels are much closer to the abdominal wall and the margin for error is reduced, with as little as 4 cm between the skin and these vessels. In the obese patient (BMI >30kg/m2; weight usually greater than 200 pounds), a more vertical approach, approximately 70 to 80 degrees, is required to enter the peritoneal cavity because of the increased thickness of the abdominal wall.
Verification that the Veress needle tip is in the peritoneal cavity is done by a number of methods, including the hanging drop test, injection and aspiration of fluid through the Veress needle, and close observation of intra-abdominal pressure during carbon dioxide insufflation. After a pneumoperitoneum has been created, the Veress needle is removed and the primary port trocar (most commonly 5 or 10 mm in diameter) is placed at an angle identical to that used for the Veress needle.
Direct Trocar Insertion
Direct trocar insertion is a technique whereby the primary trocar is inserted without the Veress needle being previously inserted and insufflating the abdomen with carbon dioxide.5 The primary trocar is inserted at an angle similar to that described for the closed technique. The peritoneal cavity is then insufflated with carbon dioxide through the umbilical port. This technique decreases the risk of extraperitoneal insufflation by allowing the surgeon to confirm intraperitoneal placement of the primary trocar before insufflation. Although small randomized studies have not demonstrated an increased risk of injuries, some series suggest that this technique might increase the risk of bowel injury.5,6 Further large studies are required.
Open Laparoscopy
Open laparoscopy, first described by Dr. Harrith Hasson in 1971, refers to creating a small incision in the abdomen and placing the port through the incision without using a sharp trocar.7,8 The skin and anterior rectus fascia are incised with a scalpel and the peritoneal cavity is bluntly entered with a Kelly or Crile forceps. A laparoscopic port with a blunt-tipped trocar is then placed into the peritoneal cavity. For the Hasson technique, fascial sutures are used to help maintain a pneumoperitoneum.7 This method almost completely avoids the risk of retroperitoneal vessel injury and is preferred by many laparoscopists for this reason. Although open laparoscopy does not entirely avoid the risk of bowel injury, many laparoscopists use this approach in an effort to decrease this risk in patients with previous abdominal surgery suspected of having adhesions.
Left Upper Quadrant Technique
It is important to know the anatomy of the left upper quadrant before using this technique. The most important organs that are closest to this site are the stomach and left lobe of the liver.9 Although a small series has shown the risk of complications to be small, the relative risk of complications with this technique remains to be demonstrated by a large study.10
Placement of Secondary Ports
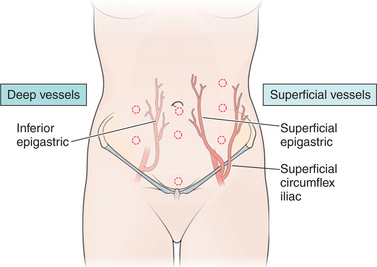
Secondary ports are required to perform most gynecologic laparoscopy procedures today. After identifying the inferior epigastric vessels by visualizing them intra-abdominally through the laparoscope, one to three secondary ports are placed, depending on the procedure.11 A midline port may be placed 3 to 4 cm above the pubic symphysis. Lateral ports are placed approximately 8 cm from the midline and above the pubic symphysis to avoid the inferior epigastric vessels (Fig. 44-2).12 This lateral site corresponds to McBurney’s point in the right lower quadrant, and is approximately one-third the distance from the anterior iliac crest to the pubic symphysis (see Fig. 44-2). An additional lateral port for the principal surgeon is required for most operative laparoscopy cases. The site chosen is typically at the level of the umbilicus lateral to the rectus muscle. This site offers the surgeon comfortable use of both hands and allows access to most areas of the pelvic or abdominal cavity.
Removal of Ports and Port Site Closure
It has become clear that large port sites are at a small but undeniable risk for subsequent bowel herniation if not securely closed.13 Furthermore, patients with ascites or in whom intra-abdominal fluid has been placed for chemotherapy or adhesion prevention are at risk of postoperative leakage through the port site. For these reasons, it is recommended that all extra-umbilical port sites be surgically closed when a trocar greater than 8 mm in diameter was used for port placement or if repeated removal and replacement of the port has enlarged the fascial defect. Although the risk of herniation at the umbilicus appears to be extremely rare, some surgeons recommend closing the fascia at this site as well.
Closure of lateral ports is more of a challenge because the fascia is clearly divided into sheaths. Closure of only one fascial sheath puts the patient at risk of herniation of bowel between the sheaths, often referred to as a Spigelian hernia (see Chapter 45). For this reason, methods have been designed to simultaneously close both layers.
When closing secondary ports, it is imperative to do so under direct laparoscopic visualization to avoid bowel injury. One of several transabdominal suture guides are used to place interrupted absorbable suture through both the anterior and posterior fascial sheaths, usually incorporating the peritoneum as well. However, even this technique does not completely prevent subsequent herniation.14 For this reason, any painful bulge appearing beneath a laparoscopic port site should be evaluated ultrasonographically to detect bowel herniation.
POWER INSTRUMENTS
Power instruments are often used during laparoscopy because suture ligation, the most common hemostatic method used during laparotomy, is difficult to perform laparoscopically. Electrocoagulation was perhaps the first power instrument used during laparoscopy.2 This instrument is heated by passing electrical current through the tip of a grasping instrument, which is then used to coagulate tissue.
In the past 30 years, other methodologies have been developed, most notably electrosurgery. Unipolar electrosurgery passes current through the patient to cut or coagulate tissue. Bipolar electrosurgery was developed in an effort to minimize the risk of inadvertent injury to adjacent tissue, particularly the bowel. Bipolar electrosurgery offers an increased margin of safety because the electrical current is confined to the tip of the instrument, but the cutting ability is reduced. Lasers offer a precise, rapid, and accurate method of thermally destroying the tissue; however, hemostatic effects are lessened and lasers are costly. The ultrasonic scalpel is an ultrasonically activated instrument that moves longitudinally at a rate of 55,000 vibrations per second and is able to cut tissue and coagulate small vessels without heat or electrical energy. Tips available for this instrument include grasper/scissors, a hook blade, and a ball tip. These instruments are discussed in more depth in Chapter 45.
LAPAROSCOPIC PROCEDURES
Diagnostic Laparoscopy
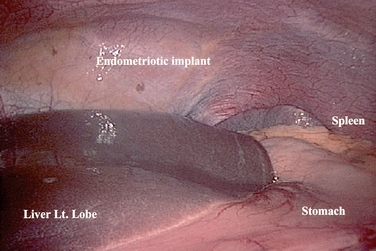
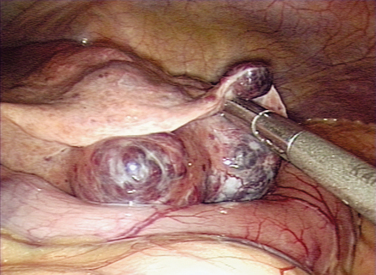
Before initiating any surgery, the peritoneal cavity should be thoroughly inspected using a systematic approach. With the surgeon controlling the movement of the laparoscope, each quadrant of the abdomen and then the pelvis should be carefully inspected. The spleen is usually difficult to see except in thin women (Fig. 44-3). Care should be taken to inspect the appendix, omentum, peritoneal surfaces, stomach, surface of the bowel, diaphragms, and liver (Figs. 44-4 and 44-5).15 If any suspicious lesions are observed, fluid should be obtained for cytology (pelvic washings) before prior to biopsying the lesion for frozen section.
Tubal Sterilization
Laparoscopy is one of the most commonly used techniques for permanent sterilization in the world (see Chapter 28). Original laparoscopic techniques used electrocautery or electrosurgery to coagulate the midportion of the tubes. Other techniques, including clips and Silastic bands, have gained popularity. The pregnancy rates vary by age of the patient, ranging from 1% to 3% after 10 years.16
Lysis of Adhesion and Tubal Reconstructive Surgery
Unfortunately, adhesions often reform after lysis. Multiple techniques have been used in an effort to decrease reformation (see Chapter 52). Gentle tissue handling and good hemostasis also appear to be important. Barrier methods have been shown in clinical trials to decrease adhesions, but have yet to be proven to improve pain relief or future fertility.
Fulgarization of Endometriosis
Laparoscopy is the primary surgical approach used to treat endometriosis. Endometriosis lesions may be resected or ablated, using scissors or any of the power instruments. These treatment approaches have been shown to improve fertility and decrease pelvic pain (see Chapter 49).
Ectopic Pregnancy Treatment
Laparoscopy has become the surgical approach of choice for most ectopic pregnancies17 (see Chapter 48). The embryo and gestational sac are removed either through a longitudinal incision (linear salpingostomy) or by removing a part of the tube (salpingectomy). Even a ruptured tubal pregnancy can be treated laparoscopically, as long as the patient is hemodynamically stable.
Ovarian Cystectomy and Oophorectomy
Ovarian pathologic conditions, including cysts, commonly result in a gynecologic complaint such as pain. The underlying pathology ranges from physiologic and self-limiting functional cysts to ovarian torsion and other benign conditions, to ovarian malignancy. Ovarian cysts are usually characterized ultrasonographically and treated when necessary by laparoscopy or laparotomy, depending on the size of the cyst and the level of suspicion for malignancy (see Chapter 50).18 The most important concept in adnexal surgery is to avoid spilling the cyst contents whenever possible.
Myomectomy
Many women with a symptomatic fibroid uterus prefer a myomectomy over hysterectomy to preserve fertility or the uterus (see Chapter 46).19 In some cases, myomectomy can be performed laparoscopically. The challenges in the case of intramural myomas are related to hemostasis, effective closure of the resulting myometrial defect, and removal of the specimen from the abdomen. Vasopressin can be injected into the uterus to help maintain hemostasis. The excised fibroid can be removed by morcellation or culpotomy. Power morcellators are available to expedite the process. Barrier techniques may be used to decrease subsequent adhesion formation. Some early case series have reported increased risk of subsequent uterine rupture during pregnancy after laparoscopic myomectomy compared to those performed by laparotomy.20 However, several randomized clinical trials have shown no increased risk in expert hands.21 A totally laparoscopic approach should be attempted only by gynecologists skilled in laparoscopic suturing.
Laparoscopic Uterine Nerve Ablation for Pelvic Pain
Many women have severe dysmenorrhea that is unresolved despite medical management, but wish to maintain future childbearing potential. In these patients, two laparoscopic approaches have been attempted with some success. Laparoscopic uterosacral nerve ablation (LUNA) is performed by stretching and dividing each uterosacral ligament using electrosurgery or laser alone or in combination with scissors. Care must be taken to avoid injuring the ureters. This procedure has been shown to have some temporary success, but a recent Cochrane review has questioned the validity of this procedure.22
Laparoscopic presacral neurectomy is a second approach for central pain. This technically challenging procedure is performed by careful retroperitoneal dissection between the common iliac artery on the right and the inferior mesenteric artery where it crosses over both left common iliac artery and vein on the left. The superior hypogastric plexus, which includes the presacral nerves, is dissected from the left common iliac vein and periosteum of sacral promontory and a 2- to 3-cm segment is resected. Surgical risks include vascular complications, and long-term risks, such as constipation, are more common than with LUNA. Although both LUNA and laparoscopic presacral neurectomy appear to give some patients at least temporary relief from central pain, many clinicians believe that there is insufficient evidence to recommend the use of nerve interruption in the management of dysmenorrhea, regardless of cause.22
Hysterectomy
Laparoscopic hysterectomy, first described by Dr. Harry Reich in 1992, is commonly performed today.23 The three basic laparoscopic approaches for hysterectomy are laparoscopic-assisted vaginal hysterectomy (LAVH), laparoscopic hysterectomy, and laparoscopic supracervical hysterectomy. Although the basic techniques for each of these approaches are fairly standardized, controversy exists over the risks, benefits, and most appropriate indication of each.
Supracervical Hysterectomy
The supracervical hysterectomy is a third common laparoscopic approach to hysterectomy for benign indications.24 The technique begins in a manner identical to LAVH and laparascopic hysterectomy. However, before dissecting the cervix, the fundus is transected at the uterocervical junction. To minimize residual cyclic vaginal bleeding and decrease the risk of developing cervical dysplasia or cancer, the glandular tissue endocervix is cored out or cauterized. The uterine specimen is removed through a 12-mm abdominal port using a power morcellator.
This approach eliminates both the vaginal and abdominal incision, thus decreasing the risk of infection. The risk of ureteral injury is also decreased, because the procedure stops above the level of the internal os. However, a risk of subsequently developing cervical dysplasia or cancer remains due to the presence of the cervical stump. For this reason, routine Pap smears are required, and some patients will require additional surgery related to cervical abnormalities. Furthermore, at least two randomized clinical trials have failed to show superior results in bladder function or sexual function.25,26 These studies did show a higher reoperation rate for bleeding and prolapse.
Although small trials have tried to assess the value of laparoscopic hysterectomy, a large multicenter, randomized trial that compared laparoscopic with abdominal hysterectomy and laparoscopic with vaginal hysterectomy has provided insight into the role of this procedure.27 The study confirmed that the laparoscopic approach offers no advantage over the vaginal approach. It also confirmed that the laparoscopic approach is associated with less postoperative pain, shorter hospital stay, and faster convalescence compared with the abdominal approach. It demonstrated that the laparoscopic approach was associated with a slightly higher risk of urinary tract injury. The shorter length of hospitalization with laparoscopic hysterectomy offset some of the additional costs incurred by longer operating room times and the expense of disposable instruments.28
Risks of Laparoscopy
Laparoscopy appears to decrease the traditional risks of surgery such as infection and generalized bleeding compared to laparotomy. However, laparoscopy is associated with several unique complications. These include venous gas embolism related to carbon dioxide insufflation and trocar injuries to bladder, bowel, and blood vessels. During hysterectomy, laparoscopy also appears to increase the risk of bladder and ureter injury compared to hysterectomies performed via laparotomy or transvaginally.29 The methods for avoiding, diagnosing, and treating laparoscopic complications are presented in Chapter 45.
CONCLUSION
The role of laparoscopy in gynecologic surgery continues to expand as experience and more sophisticated techniques and instrumentation allow a greater variety of procedures to be safely performed. Many laparoscopic procedures commonly done today would have required laparotomy in the recent past. Laparoscopy has become the primary surgical approach for many gynecologic procedures, including tubal ligation, treatment of endometriosis and adhesions, and removal of ectopic pregnancies and adnexal structures. Ongoing studies are needed to determine the ultimate utility of laparoscopy for the performance of more complex procedures, including the treatment of malignancies.
1 Litynski GS. Laparoscopy—the early attempts: Spotlighting Georg Kelling and Hans Christian Jacobaeus. JSLS. 1997;1:83-85.
2 Semm K. Endocoagulation: A new and completely safe medical current for sterilization. Int J Fertil. 1977;22:238-242.
3 Yuzpe AA. Pneumoperitoneum needle and trocar injuries in laparoscopy. A survey on possible contributing factors and prevention. J Reprod Med. 1990;355:485-490.
4 Hurd WW, Bude RO, DeLancey JOL, et al. Abdominal wall characterization by magnetic resonance imaging and computed tomography: The effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473-476.
5 Kaali SG, Barad DH. Incidence of bowel injury due to dense adhesions at the sight of direct trocar insertion. J Reprod Med. 1992;37:617-618.
6 Mintz M. Risks and prophylaxis in laparoscopy: A survey of 100,000 cases. J Reprod Med. 1977;18:269-272.
7 Hasson HM. Open laparoscopy: A report of 150 cases. J Reprod Med. 1974;12:234-238.
8 Hurd WW, Ohl DA. Blunt trocar laparoscopy. Fertil Steril. 1994;61:1177-1180.
9 Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for trocar insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
10 Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
11 Hurd WW, Amesse LS, Gruber JS, et al. Visualization of the bladder and epigastric vessels prior to trocar placement in diagnostic and operative laparoscopy. Fertil Steril. 2003;80:209-212.
12 Hurd WW, Bude RO, DeLancey JOL, Newman JS. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol. 1994;171:642-646.
13 Montz FJ, Holschneider CH, Munro M. Incisional hernia following laparoscopy: A survey of the American Association of Gynecologic Laparoscopists. J Am Assoc Gynecol Laparosc. 1994;1:S23-S24.
14 Cottam DR, Gorecki PJ, Curvelo M, et al. Preperitoneal herniation into a laparoscopic port site without a fascial defect. Obes Surg. 2002;12:121-123.
15 Tulandi T, Falcone T. Incidental liver abnormalities at laparoscopy for benign gynecologic conditions. J Am Assoc Gynecol Laparosc. 1998;5:403-406.
16 Westhoff C, Davis A. Tubal sterilization: Focus on the U.S. experience. Fertil Steril. 2000;73:913-922.
17 Tulandi T, Saleh A. Surgical management of ectopic pregnancy. Clin Obstet Gynecol. 1999;42:31-38.
18 Mettler L, Semm K, Shive K. Endoscopic management of adnexal masses. JSLS. 1997;1:103-112.
19 Miller CE. Myomectomy. Comparison of open and laparoscopic techniques. Obstet Gynecol Clin North Am. 2000;27:407-420.
20 Hockstein S. Spontaneous uterine rupture in the early third trimester after laparoscopically assisted myomectomy. A case report. J Reprod Med. 2000;45:139-141.
21 Falcone T, Bedaiwy MA. Minimally invasive management of uterine fibroids. Curr Opin Obstet Gynecol. 2002;14:401-408.
22 Proctor ML, Farquhar CM, Sinclair OJ, Johnson NP. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhea. Cochrane Database Syst Rev. 2003. CD001895.
23 Reich H. Laparoscopic hysterectomy. Surg Laparosc Endosc. 1992;2:85-88.
24 Johns A. Supracervical versus total hysterectomy. Clin Obstet Gynecol. 1997;40:903-913.
25 Kuppermann M, Summitt R, Varner E, et al. Sexual function after total compared with supracervical hysterectomy: A randomized trial. Obstet Gynecol. 2005;105:1309-1318.
26 Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. NEJM. 2002;347:1318-1325.
27 Garry R, Fountain J, Mason S, et al. The eVALuate study: Two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:129-135.
28 Falcone T, Paraiso MF, Mascha E. Prospective randomized clinical trial of laparoscopic assisted vaginal hysterectomy versus total abdominal hysterectomy. Am J Obstet Gynecol. 1999;180:955-962.
29 Johnson N, Barlow D, Lethaby A, et al. Methods of hysterectomy: Systematic review and meta-analysis of randomized controlled trials. BMJ. 2005;330:1478.