126 Gynecologic Infections
• Not all gynecologic infections, including pelvic inflammatory disease, are sexually transmitted, although many are. They are often asymptomatic in women and their sex partners.
• Sexually transmitted diseases are common, particularly in young, sexually active women with multiple sex partners.
• A careful history, physical examination, and diagnostic tests are important to differentiate gynecologic infections because modalities of treatments vary.
• Microscopic diagnosis of yeast infections has a sensitivity of only 50% and fails to diagnosis the disorder in a large percentage of patients with symptomatic vulvovaginal candidiasis.
• Most young, sexually active patients with genital ulcers have a genital herpes infection; syphilis or chancroid disease should be considered as well.
• Treatment should be instituted for most gynecologic infections based on the presumed diagnosis because many patients with genital infections will not have a laboratory-confirmed diagnosis.
• Gonorrhea is becoming increasingly resistant to antibiotics; the clinician should check local susceptibilities before treatment.
Epidemiology
Gynecologic infections are often asymptomatic in women, and the majority of these infections are transmitted sexually. In 2009, the Centers for Disease Control and Prevention (CDC) reported approximately 19 million new sexually transmitted infections with an estimated cost of $16.4 billion annually.1 Many cases of sexually transmitted diseases in the United States, such as human papillomavirus (HPV) and genital herpes, are not reported to the CDC.
Diseases Characterized by Genital Ulcers
Chancroid
Epidemiology
The number of reported cases of chancroid in the United States has varied for the past 10 years but remains very low. Only 28 cases were reported domestically in 2009.1
Pathophysiology
Chancroid, a sexually transmitted disease caused by the short gram-negative bacillus Haemophilus ducreyi, is characterized by painful genital ulcers and painful lymphadenopathy.2 The incubation period is 4 to 7 days.
Treatment
Several treatment regimens have been recommended and are listed in Box 126.1.3,4
Granuloma Inguinale (Donovanosis)
Treatment
Herpes Simplex Virus
Epidemiology
HSV is widespread in the United States, with approximately 50 million people infected,5 and it is the most common cause of genital ulcers. The overall domestic seroprevalence of subtype 2 (HSV-2) is 16.2%, but infection rates are considerably higher (39.2%) in African Americans.1 HSV-2 is the most common variant causing genital infections, but subtype 1 (HSV-1) is the causative agent in up to 10% to 15% of all cases.1
Pathophysiology
Two types of HSV may cause genital herpes: HSV-1 and HSV-2. Once acquired, genital herpes becomes a lifelong infection. Herpes simplex is a DNA virus with an incubation period ranging from 2 to 7 days.5 Transmission occurs through exposure to infectious secretions of mucosal surfaces or through skin microabrasions during sexual contact.
Presenting Signs and Symptoms
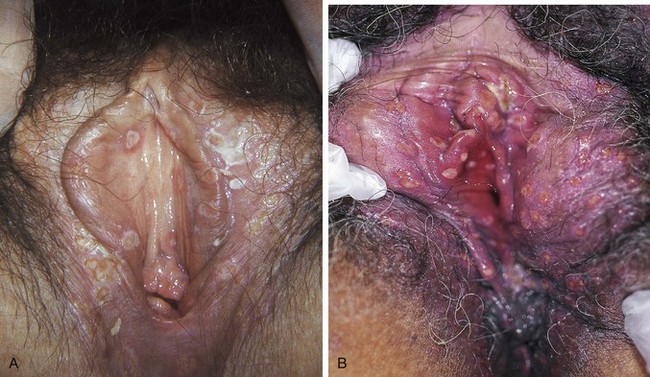
Of all HSV-2 infections studied, 81.1% were reported to be asymptomatic or were not recognized.5 Symptoms usually begin with painful lesions that are often described as burning (Fig. 126.1). These lesions begin as vesicles and then rupture to expose an ulcerated base that persists for 1 to 2 weeks before crusting over and healing without scars. The vesicles and ulcers contain many highly infectious virus particles, and viral shedding occurs until the lesions disappear. Vulvar lesions may last for 3 or more weeks before complete healing. The cervix and vagina may also be involved, with a gray, necrotic cervix and profuse leukorrhea. External dysuria is common, and bilateral inguinal lymphadenopathy is usual.
Treatment
Treatment of HSV infection is dependent on the onset of clinical symptoms (acute versus recurrent) and their frequency, which determines the need for suppressive therapy (Box 126.3).
Box 126.3
Treatment Regimens for Herpes Simplex Virus
A. Initial infection (first episode): Recommended regimens for initial infection should be given orally for 7 to 10 days (may be extended if healing is incomplete).
B. Recurrent infection (episodic treatment): Recommended regimens are given orally.
C. Suppressive therapy: Given for frequent recurrent infections and includes several recommended oral regimens.
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Lymphogranuloma Venereum
Epidemiology
LGV is rarely reported in developed countries—perhaps in part because of the lack of a standardized diagnostic test or surveillance requirements. However, outbreaks have been reported in populations of homosexual men in Western Europe and North America since 2003, with the largest cluster of case reports occurring in the United Kingdom and New York City.6
Treatment
Box 126.4 details the treatment regimen for LGV.
Box 126.4
Treatment Regimens for Lymphogranuloma Venereum
A. Recommended regimen: It is the preferred treatment.
B. Alternative regimen: Recommended regimens are given orally.
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Syphilis
Epidemiology
Rates in women increased from 0.8 case per 100,000 in 2004 to 1.4 cases per 100,000 in 2009 despite a previously decreasing incidence.1
Pathophysiology
Syphilis is a systemic, sexually transmitted disease caused by the spirochete Treponema pallidum. It can also be acquired congenitally. The risk for transmission following sexual exposure depends on many factors and is estimated to be about 30%.7 T. pallidum causes infection after traversing mucous membrane surfaces or denuded skin and then extending systemically through hematogenous and lymphatic routes.
Presenting Signs and Symptoms
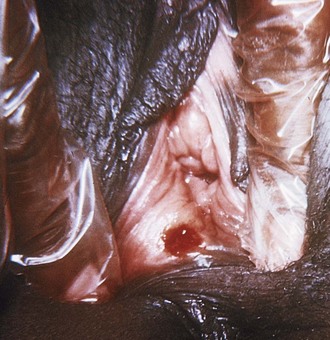
Syphilis can be seen in the ED in any of the four stages through which untreated syphilis can pass. Primary infection is manifested as a single, painless ulcer, usually within 3 weeks but sometimes 2 to 3 months after infection. The labia and vaginal walls are most often affected; however, lesions can also occur on the cervix (Fig. 126.2). Secondary syphilis is associated with a characteristic maculopapular generalized rash on the palms and soles and may include components of arthralgia, pharyngitis, and lymphadenopathy. This stage is typically seen 4 to 10 weeks after the initial appearance of a chancre. Infectivity can occur in the first two stages (up to 2 to 4 years following infection).
The fourth stage—tertiary syphilis—has numerous neurologic, cardiovascular, and other systemic effects and develops in about 25% of untreated patients.6
Treatment
Penicillin remains the mainstay of treatment across the board (Box 126.5). According to the CDC guidelines, parenteral penicillin is considered to be the only treatment documented to be efficacious in pregnancy.3 Treatment with penicillin is the same as for the corresponding stage of syphilis in nonpregnant women. For pregnant patients who are allergic to penicillin, tetracycline and doxycycline are contraindicated. Pregnant patients who are allergic to penicillin should be skin-tested and desensitized.
Box 126.5
Treatment Regimens for Syphilis
A. Primary and secondary infections:
C. Tertiary syphilis (with no evidence of neurosyphilis): For example, patients with gumma and cardiovascular syphilis.
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Diseases Characterized by Vaginal Discharge
Bacterial Vaginosis
Epidemiology
Although the range is wide, depending on the population, prevalence rates for bacterial vaginosis (BV) have been estimated to be 4% to 40%. However, because Gardnerella vaginalis is present in 50% to 70% of asymptomatic women, the exact incidence of BV is difficult to estimate.8
Differential Diagnosis and Medical Decision Making
Clinical diagnosis requires three of the following four criteria:
• Homogeneous, thin vaginal fluid that adheres to the vaginal walls
• Presence of vaginal epithelial cells with borders obscured by adherent small bacteria (clue cells)
• Vaginal fluid pH higher than 4.5
• Release of an amine “fishy” odor with alkalinization (adding 10% KOH) of the vaginal fluid (“whiff test”)
Treatment
Only women with symptoms require treatment (Box 126.6).
Box 126.6
Treatment Regimens for Bacterial Vaginosis
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Trichomoniasis
Epidemiology
Trichomoniasis affects 2 to 3 million women in the United States per year. A total of 216,000 visits to physician offices were reported in women 15 to 44 years of age for this issue in 2009 alone.1
Differential Diagnosis and Medical Decision Making
Although candidiasis and BV are included in the differential diagnosis of vaginitis, trichomonal infections are less typically associated with pruritus or malodor.1 The diagnosis is made by identification of motile trichomonads on a saline wet preparation but are seen in only 60% to 70% of confirmed cases when cultures are performed.
Treatment
Treatment regimens are listed in Box 126.7. Patients with allergy to a nitroimidazole may undergo metronidazole desensitization. Because of adverse pregnancy outcomes with Trichomonas infection, such as premature rupture of membranes, preterm delivery, and low birth weight, women can be treated with 2 g metronidazole in a single dose at any stage of pregnancy.
Box 126.7
Treatment Regimens for Trichomoniasis
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Vulvovaginal Candidiasis
Epidemiology
Vulvovaginal candidiasis (VVC) is exceedingly common, with 75% of women expected to have at least one episode during their lifetime. However, only 10% to 20% of women will have symptoms to the extent that diagnostic and therapeutic considerations are warranted.2
Differential Diagnosis and Medical Decision Making
Several infections may mimic VVC; however, the diagnosis is based on the characteristic clinical findings along with microscopy. Microscopic evaluation reveals a normal pH (4 to 4.5) with hyphae, pseudohyphae, or budding yeast on a saline wet preparation or 10% potassium hydroxide preparation. Fifty percent of women with candidiasis have a negative wet mount but a positive Candida culture. Recurrent VVC, defined as four or more episodes of symptomatic VVC in 1 year, affects 5% of women. Vaginal cultures should be performed for patients with recurrent VVC to confirm the clinical diagnosis and to identify unusual species (including non-albicans species), particularly Candida glabrata.9
Treatment
Treatment regimens are listed in Box 126.8. For recurrent VVC caused by C. albicans, some specialists recommend a longer duration of initial therapy (e.g., 7 to 14 days of topical therapy or a 100-mg, 150-mg, or 200-mg oral dose of fluconazole every third day for a total of three doses [days 1, 4, and 7]) before initiating a maintenance antifungal regimen. Maintenance regimens include oral fluconazole (i.e., 100-mg, 150-mg, or 200-mg dose) weekly for 6 months, which is the first line of treatment. If this regimen is not feasible, topical treatments used intermittently as a maintenance regimen can be considered. For severe VVC (i.e., extensive vulvar erythema, edema, excoriation, and fissure formation), either 7 to 14 days of topical azole or 150 mg of fluconazole in two sequential doses (second dose 72 hours after the initial dose) should be used.3
Box 126.8
Treatment Regimens for Uncomplicated Vulvovaginal Candidiasis
Over-the-Counter Intravaginal Agents
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Options for the treatment of non-albicans VVC include a longer duration of therapy (7 to 14 days) with an azole drug other than fluconazole (oral or topical) as first-line therapy. If VVC recurs, 600 mg of boric acid in a gelatin capsule administered vaginally once daily for 2 weeks is recommended.2
Diseases Characterized by Cervicitis and Urethritis
Chlamydia
Epidemiology
C. trachomatis infection is the most commonly reported notifiable disease in the United States. C. trachomatis and Neisseria gonorrhoeae are isolated in combination in about 20% to 40% of women with purulent cervicitis,10 but accurate disease rates are limited by the fact that 80% of women infected with these pathogens are asymptomatic. During the period 2005 to 2009, the chlamydial infection rate in women increased by 20.3% (from 492.2 to 592.2 cases per 100,000 females).1
Treatment
Treatment regimens are listed in Box 126.9. In pregnant women, treatment with doxycycline, ofloxacin, and levofloxacin are contraindicated. Therefore, the recommended regimen is azithromycin, 1 g orally in a single dose, or amoxicillin, 500 mg orally three times per day for 7 days, with erythromycins being the alternative regimen.
Box 126.9
Treatment Regimens for Uncomplicated Genital Chlamydia Infections
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Gonorrhea
Epidemiology
The highest incidence of infection is found in the 15- to 24-year-old age group, and transmission occurs via sexual contact. In 2009, the gonorrhea rate was 105.5 cases per 100,000 population in women.1
Treatment
Therapy is typically administered before antimicrobial susceptibilities are known (Box 126.10). The decision to treat can be based on laboratory confirmation; however, in patients with high clinical suspicion for the disease or in those likely to be lost to follow-up, empiric therapy should not be delayed. Specific antibiotic regimens are recommended for patients with complicated gonococcal infections such as bacteremia, endocarditis, arthritis, meningitis, and conjunctivitis. Because antibiotic resistance is becoming an increasing problem, the physician should check local sensitivities before treating.
Box 126.10
Treatment Regimens for Uncomplicated Genital Gonorrhea Infections*
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Urethritis
Epidemiology
Causative agents of nongonococcal urethritis include Ureaplasma urealyticum (40% to 60% of cases), C. trachomatis (15% to 55% of cases), M. hominis (5% to 10% of cases), and T. vaginalis (<5% of cases).1
Differential Diagnosis and Medical Decision Making
Urethritis can be documented on the basis of any of the following signs or laboratory tests:
• Mucopurulent or purulent discharge on examination
• Microscopy of urethral secretions demonstrating five or more white blood cells (WBCs) per oil immersion field
• Positive leukocyte esterase test on first-void urine or microscopic examination of first-void urine sediment demonstrating 10 or more WBCs per high-power field
Treatment
For gonococcal urethritis, refer to the treatment of uncomplicated gonorrhea infections. Because of possibility of concomitant infection, Chlamydia infection should be presumed and also treated unless it is ruled out. See Box 126.11 for the treatment of nongonococcal urethritis.
Box 126.11
Treatment Regimens for Nongonococcal Urethritis
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Diseases Characterized by Abdominal or Pelvic Pain
Pelvic Inflammatory Disease
Epidemiology
PID is diagnosed in more than 1 million women each year.1 Infertility as a result of tubal involvement after PID is the second most common cause of female infertility in the United States. Following PID, infertility occurs in 12% of women; the risk for ectopic pregnancy increases 7- to 10-fold, and chronic pelvic pain develops in approximately 20% of women.11
Differential Diagnosis and Medical Decision Making
Diagnosis of PID is usually based on the clinical findings of lower abdominal tenderness, cervical motion tenderness, or adnexal tenderness for which another cause is not likely (Box 126.12). Women with PID may have subtle or mild symptoms, thus complicating the diagnosis.
Box 126.12
CDC Suggested Criteria for Diagnosis, Empiric Treatment, and Hospitalization for Patients with PID
A. Minimum diagnostic criteria (for diagnosis and empiric treatment of PID in sexually active young women experiencing pelvic or lower abdominal pain if no other cause is identified):
B. Additional diagnostic criteria (used to enhance specificity of the diagnosis of PID once any of the minimum criteria listed above is met):
C. Additional diagnosis criteria (most specific criteria for the diagnosis of PID):
D. Criteria for hospitalization (recommended criteria for hospitalization of patients with PID):
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
The differential diagnosis includes appendicitis, ectopic pregnancy, septic abortion, hemorrhagic or ruptured ovarian cysts or tumors, degeneration of a myoma, and acute enteritis (Fig. 126.3).
Treatment
Antimicrobial coverage should include N. gonorrhoeae, C. trachomatis, gram-negative facultative bacteria, anaerobes, and streptococci. No single therapeutic regimen has been established (Box 126.13). Outpatient management is appropriate for most patients with mild to moderate PID who do not meet the criteria for admission.
Box 126.13
Treatment Regimens for Pelvic Inflammatory Disease
Outpatient Treatment Regimens
Inpatient Treatment Regimens (Parenteral)
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
As a result of the emergence of quinolone-resistant N. gonorrhoeae, regimens that include a quinolone agent are no longer recommended for the treatment of PID.13
Tuboovarian Abscess
Pathophysiology
TOA is usually a complication of PID, although cases can infrequently occur without the preexisting presence of PID. TOAs typically result from salpingitis that progresses to oophoritis.14 Ovarian infection occurs after contamination with purulent material from the fallopian tube. Adherence of tubal fimbriae to the ovary forms a large, cylinder-shaped TOA. Most TOAs consist of polymicrobic anaerobic bacteria.
Fitz-Hugh–Curtis Syndrome
Epidemiology
The incidence of FHCS is lower in adult women (4% to 14%) than in adolescent girls (27%).15
Differential Diagnosis and Medical Decision Making
Laparoscopy is useful to identify unclear cases and, in conjunction with positive cervical or abdominal cultures, represents the standard criterion for diagnosis. Negative cervical cultures alone, however, do not rule out the diagnosis. “Violin string” adhesions may be seen on ultrasound or computed tomography scans (Fig. 126.4).
Treatment
Although no formal antibiotic recommendations exist, patients with FHCS usually require admission for observation and parenteral antibiotic therapy. Initial options are the same as those listed for inpatient treatment of PID. Like TOA, continued outpatient oral therapy should include clindamycin (or metronidazole) for 14 days in addition to doxycycline.2
Diseases Characterized by Genital Warts or Mucosal Abscess
Human Papillomavirus
Epidemiology
HPV is the most common sexually transmitted virus in the United States. Its prevalence corresponds inversely with age (highest in younger age groups, 50% in females 20 to 24 years of age) and declines substantially after the age of 24 years.16 However, the gross clinical prevalence of HPV is less than 1%.11
Pathophysiology
HPV is a double-stranded DNA virus that infects the epithelial cells of skin and mucosa and can cause cellular changes leading to formation of warts. HPV infection is a sexually transmitted illness that is most commonly asymptomatic but has been associated with various disease processes such as benign anogenital warts, as well as with invasive cancer. Of the greater than 100 types of HPV, more than 40 can infect the genital area. A quadrivalent HPV vaccine that provides protection against types 6, 11, 16, and 18 was licensed for use in the United States in June 2006. HPV subtypes 6 and 11 most commonly cause genital warts (condylomata acuminata),3 which can occur on the vulva, perianal area, urethra, vaginal walls, or cervix. These subtypes have also been identified as the causative agent of laryngeal or respiratory papillomatosis in infants and children, but the route of transmission is not completely understood.
Presenting Signs and Symptoms
The average incubation period for visible warts is about 3 months. Genital warts may be asymptomatic but can be pruritic. On clinical examination the lesions are generally papillary, verrucous (wartlike), or macular in character (Fig. 126.5).

Fig. 126.5 Vulvovaginal human papillomavirus infection.
(From Cohen J, Powderly WG. Infectious diseases. 2nd ed. Philadelphia: Mosby; 2004.)
Lesions usually first appear individually, but large confluent growths can develop.
Treatment
Subclinical genital HPV infection usually clears spontaneously. Treatment is indicated for those with genital warts or precancerous lesions and not for subclinical HPV infection (Box 126.14).
Box 126.14
Treatment Regimens Recommended for External Genital Warts Caused by Human Papillomavirus
From Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. Accessed May 30, 2012.
Bartholin Gland Abscess
Treatment
Management of the abscess consists of simple incision and drainage. After sterile preparation of the area, a scalpel stab incision, ideally no longer than 1.5 cm (longer incisions will make it difficult to keep the Word catheter in place), should be made deep into the abscess from the inside of the labium. An outside incision can cause permanent fistula formation.17 Loculations should be broken manually followed by placement of a Word catheter with its balloon tip inserted into the abscess before inflation with water or lubricating gel (Fig. 126.6). Simple incision and drainage without Word catheter placement may be inadequate and result in recurrence.
Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2009. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/STD/stats09/surv2009-Complete.pdf Accessed January 18, 2011
Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm Accessed January 18, 2011
Ramin SM, Wendell JD, Hemsell DL. Sexually transmitted diseases and pelvic infections. DeCherney AH, Pernoll ML. Current obstetric and gynecologic diagnosis and treatment, 10th ed, New York: McGraw-Hill, 2007.
1 Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2009. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/STD/stats09/surv2009-Complete.pdf Accessed January 18, 2011
2 Lewis DA. Chancroid: clinical manifestations, diagnosis, and management. Sex Transm Infect. 2003;79:68–71.
3 Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. Atlanta: U.S. Department of Health and Human Services; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm Accessed on January 18, 2011
4 Rosen T, Vandergriff T, Harting M. Antibiotic use in sexually transmissible diseases. Dermatol Clin. 2009;27:49–61.
5 Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973.
6 Pathela P, Blank S, Schillinger JA. Lymphogranuloma venereum: old pathogen, new story. Curr Infect Dis Rep. 2007;9:143–150.
7 Kent ME, Romanelli F. Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother. 2008;42:226–236.
8 Tabrizi SN, Fairley CK, Bradshaw CS, et al. Prevalence of Gardnerella vaginalis and Atopobium vaginae in virginal women. Sex Transm Dis. 2006;33:663–665.
9 ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, Number 72, May 2006: vaginitis. Obstet Gynecol. 2006;107:1195–1206.
10 Datta SD, Sternberg M, Johnson RE, et al. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med. 2007;147:89–96.
11 Ramin SM, Wendell JD, Hemsell DL. Sexually transmitted diseases and pelvic infections. DeCherney AH, Pernoll ML. Current obstetric and gynecologic diagnosis and treatment, 10th ed, New York: McGraw-Hill, 2007.
12 Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22:1327–1334.
13 Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56(14):332–336.
14 Halperin R, Svirsky R, Vaknin Z, et al. Predictors of tuboovarian abscess in acute pelvic inflammatory disease. J Reprod Med. 2008;53:40–44.
15 Risser WL, Risser JM, Benjamins LJ, et al. Incidence of Fitz-Hugh–Curtis syndrome in adolescents who have pelvic inflammatory disease. J Pediatr Adolesc Gynecol. 2007;20:179–180.
16 Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819.
17 Wechter ME, Wu JM, Marzano D, et al. Management of Bartholin duct cysts and abscesses: a systematic review. Obstet Gynecol Surv. 2009;64:395–404.