Chapter 81 Growth Factors and Other New Methods for Graft-Healing Enhancement
Anterior cruciate ligament (ACL) reconstruction using tendon autograft has been greatly improved over the last 2 decades.1 In ACL reconstruction, however, the strength of the grafted tendon is reduced in the early phase after surgery, and then it gradually increases.2–4 A problem is that this graft remodeling occurs very slowly.5 The slow graft maturation may result in graft failure or elongation during the postoperative rehabilitation period due to unknown causes. In addition, a firm attachment of a tendon graft to the bone is a significant factor for success in ACL reconstruction. In procedures using a hamstring tendon graft, however, the anchoring strength of the soft tissue in a bone tunnel is the weakest in the femur–graft–tibia complex in the early phase after surgery.6 To improve these problems after ACL reconstruction in the near future, we should try to develop a new strategy to accelerate the intraarticular and intraosseous remodeling of the tendon graft. This may enable more aggressive rehabilitation and an earlier return to rigorous sports for patients with ACL reconstruction. In this chapter, the authors will review recent experimental studies that are intended to enhance intraarticular and intraosseous graft healing after ACL reconstruction using growth factors, gene therapy, and cell-based therapy.
Basic Knowledge to Enhance the Graft Remodeling in Anterior Cruciate Ligament Reconstruction
In ACL reconstruction, intrinsic fibroblasts of the tendon graft are necrotized immediately after transplantation, and then numerous extrinsic fibroblasts infiltrate the graft with revascularization.7,8 However, it is possible that the cell infiltration into a core portion of the graft occasionally occurs very slowly. Delay et al5 reported a clinical case in which the core portion of the patellar tendon graft still remained necrotic even at the 18-month period after ligament reconstruction. On the other hand, biomechanically, the mechanical properties of the graft deteriorate in the early phase after transplantation and then are very gradually restored over a long period.2–4
Concerning the graft deterioration mechanism, the fibroblast necrosis itself does not deteriorate the mechanical properties of the tendon matrix, but extrinsic fibroblasts proliferating after the necrosis reduce the strength properties.9 In the extrinsic fibroblasts, type III collagen is overexpressed even under physiological stress in areas where extrinsic fibroblasts infiltrate.10 In the matrix of the autograft after transplantation, ultrastructurally, fibrils having a diameter less than 90 nm predominantly increase in the graft matrix, and these fibrils with small diameters still remain predominant at the 4-year period after surgery.11 Such ultrastructural changes due to type III collagen production are considered to be one of the causes of mechanical deterioration of autografts.
What molecular mechanisms control the autograft remodeling? In a rabbit ACL reconstruction model, vascular endothelial growth factor (VEGF) is overexpressed in the extrinsic fibroblasts at 2 weeks after graft implantation, followed by vascular formation at 3 weeks.12 This fact shows that revascularization in the graft is induced by VEGF produced in the fibroblasts. On the other hand, basic fibroblast growth factor, transforming growth factor (TGF)-β, and platelet-derived growth factor (PDGF) are overexpressed in the autogenous patellar tendon graft used to reconstruct the ACL in the canine model, reaching their greatest expression 3 weeks after implantation.13 This fact suggests that a complex growth factor network controls the fibroblasts, resulting in remodeling of the graft matrix,14,15 and implies that control of the fibroblasts using growth factors is a potential strategy to accelerate the graft remodeling after ACL reconstruction.
Enhancement of Graft Healing with Growth Factors
Intraarticular Healing
PDGF-BB
It has been known that PDGF-BB enhances proliferation and migration of ligament fibroblasts in vitro.16,17 In addition, an in vivo rabbit study showed that the strong expression of PDGF correlates with the observed increased cellularity around the wound site in the medial collateral ligament (MCL), suggesting potent mitogenic and chemotactic properties of PDGF in vivo.18 Concerning the in vivo effect of application of PDGF-BB on ligamentous tissues, Woo et al19 and Hildebrand et al20 described that 20-μg PDGF-BB alone is the most effective agent to enhance the extraarticular MCL healing in the rabbit. Regarding intraarticular ACL reconstruction, Weiler et al21 applied PDGF-BB of approximately 60 μg using coated sutures as a vehicle on the flexor tendon autograft in a sheep ACL reconstruction model. They showed that the PDGF-BB application significantly increased the load to failure and vascular density of the graft at 6 weeks after ACL reconstruction, although they found no significant effects at 24 weeks. However, Nagumo et al22 investigated the effect of PDGF-BB using fibrin sealant as a carrier on the in situ frozen-thawed rabbit ACL, an idealized intraarticular autograft model.23–25 They reported that an application of 4-μg PDGF-BB did not significantly affect the mechanical properties of the frozen-thawed ACL at 12 weeks. Hildebrand et al20 suggested that the dose is critical to evaluate the effect of growth factors on ligament healing. Application of a high dose of PDGF-BB appears to be effective for MCL healing. However, the effect of PDGF-BB in ACL reconstruction is controversial.
VEGF
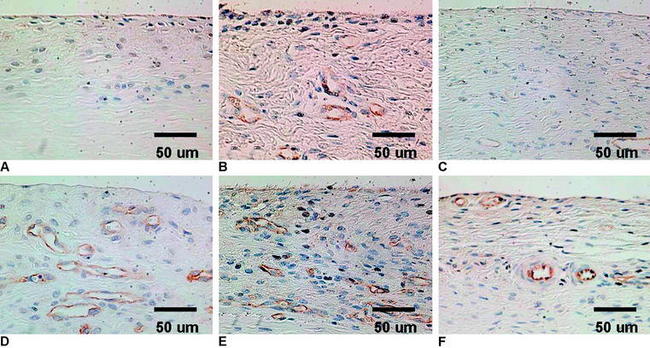
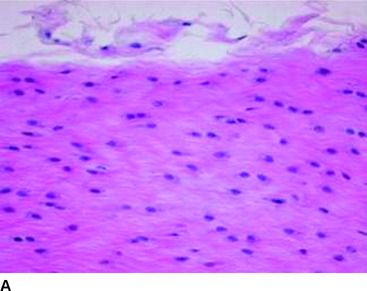
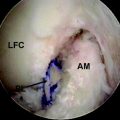
VEGF is a potent mediator of angiogenesis, which involves activation, migration, and proliferation of endothelial cells, in various pathological conditions.26 A rabbit study showed that extrinsic cells newly proliferating in the necrotized tendon graft express VEGF at 3 weeks after surgery, when the revascularization does not occur.12 This finding suggests the high possibility that an application of VEGF to the necrotized tendon graft enhances angiogenesis in the graft and accelerates remodeling of the graft. Ju et al27 histologically and mechanically examined the effect of an application of 30-μg VEGF to the in situ frozen-thawed rabbit ACL. The application of VEGF significantly enhanced vascular endothelial cell infiltration and revascularization in the ACL at 3 and 12 weeks, respectively (Fig. 81-1). On the other hand, the application provided no significant effect on the mechanical properties of the ACL at 12 weeks, although the mechanical properties of the in situ frozen-thawed rabbit ACL were significantly weaker than those of the normal ACL at 12 weeks. Thus VEGF has a potential to be used as a treatment to enhance only revascularization of the autograft after ACL reconstruction surgery.
TGF-β and EGF
A number of in vitro studies have shown that TGF-β enhances collagen and noncollagenous protein synthesis in fibroblasts.28–30 EGF also stimulates fibroblast proliferation in vitro.31 A combined application of these two growth factors enhances these effects.32 Sakai et al25 examined in vivo effects of an application of TGF-β and EGF on the in situ frozen-thawed ACL, using fibrin sealant as a vehicle. They found that a combined application of 4-ng TGF-β and 100-ng EGF significantly inhibited the natural deterioration that occurred in this autograft model with significant reduction of the water content and significant changes of the ultrastructural profile. Azuma et al33 investigated the effect of the timing of this combined application on the same rabbit model. They reported that the effect was significantly greater when 4-ng TGF-β and 100-ng EGF were applied at 3 weeks than when they were applied at 0 and 6 weeks. This study suggested that the timing is critical in application of these growth factors. Recently, Nagumo et al22 distinguished between the effect of 4-ng TGF-β and the effect of 100-ng EGF on the autograft model. According to them, the effect of 100-ng EGF was not significant, but the effect of 4-ng TGF-β was significant. Concerning an intraarticular application of TGF-β and EGF on the bone–patellar tendon–bone (BPTB) autograft in a canine ACL reconstruction model, Yasuda et al34 evaluated the effect on the structural properties of the graft. They stated that a combined application of 4-ng TGF-β and 100-ng EGF significantly inhibited the natural reduction of the structural properties of the autograft at 12 weeks after ACL reconstruction.
Intraosseous Healing
Bone Morphogenetic Proteins
The process of tendon–bone healing involves bone ingrowth into the interface tissue between the tendon and bone.35 Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily and are factors that have a strong osteoinductive and osteogenic capacity.36 They induce endochondral bone formation at extraskeletal sites. They are mostly expressed at sites of epithelial–mesenchymal interaction and serve as signaling molecules during mammalian embryogenesis and morphogenesis, suggesting their capability for differentiation of mesenchymal cells into chondrocytes and osteoblasts. The BMPs have been successfully used to regenerate bone defects, to stimulate bone ingrowth into soft tissues and metal implants, and to regenerate articular cartilage defects in large animals.36 Concerning intraosseous healing of the tendon, Rodeo et al37 showed that tendon healing in a bone tunnel was enhanced by BMP-2 in the canine. Their histological analysis showed that the BMP-2 treatment resulted in earlier and more abundant bone ingrowth into the tendon–bone interface and that it biomechanically increased the anchoring strength. Anderson et al38 described that a bone-derived extract (Bone Protein, Sulzer Biologics, Wheat Ridge, CO) was effective in augmenting healing of a tendon graft within a bone tunnel in a rabbit ACL reconstruction model. Recently, Mihelic et al39 reported that BMP-7 (osteogenic protein-1) induced the new bone formation at the bone–tendon interface, creating a dense trabecular network in a sheep ACL reconstruction model. Their mechanical testing showed greater strength in the knees treated with BMP-7 than in control specimens. Thus BMPs have potential growth factors to enhance intraosseous graft healing.
TGF-β
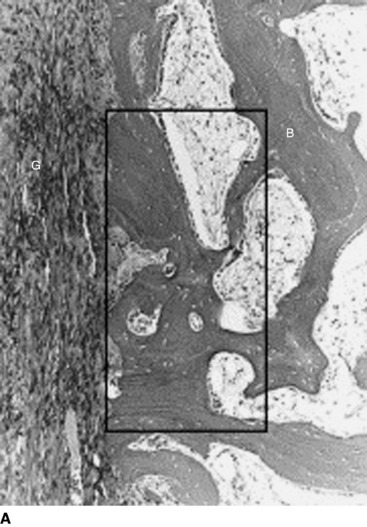
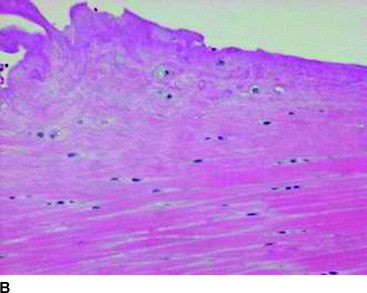
TGF-β is a multifunctional growth factor that induces new matrix synthesis in numerous types of cells. It has been shown that TGF-β can enhance bone ingrowth into biomaterial implants.40,41 Recently, Yamazaki et al42 found that administration of exogenous TGF-β1 significantly increased the bonding strength of the flexor tendon graft to the tunnel wall at 3 weeks in a canine ACL replacement model. This result was accompanied by the histological findings that the administration appeared to enhance not only synthesis or maturation of the perpendicular collagen fibers connecting the graft to the bone, but also new bone formation from the tunnel wall (Fig. 81-2). Thus, TGF-β1 also has the potential to enhance intraosseous graft healing.
Enhancement of Graft Healing with Gene Therapy
Gene therapy approaches may represent a new alternative in delivering these specific growth factors to the grafted tendon after ACL reconstruction.43 Martinek et al44 examined the capacity of BMP-2 gene transfer to improve the integration of semitendinosus tendon grafts at the tendon–bone interface after reconstruction of the ACL in rabbits. They found that in the intraosseous portion of the grafts, the number of infected cells did not decrease until 8 weeks after surgery. Moreover, a number of transduced cells were found in the deeper layers of the tendon in the bone tunnel. In the AdBMP-2–infected ACL graft, the tendon–bone interface in the osseous tunnel was similar to that of a normal ACL insertion. The pullout load and the stiffness were significantly greater in the AdBMP-2–transduced graft than the control graft.
Concerning gene therapy for the intraarticular portion of the graft healing after ACL reconstruction, however, Gerich et al45 evaluated methods for gene delivery to patellar tendons in the rabbits using the lacZ marker gene. They found that after injection of the adenovirus, high-level expression of lacZ was observed only in the portion adjacent to the injection site. Martinek et al44 reported that in the intraarticular portion of the AdLacZ-infected grafts, infected cells were observed only in the surface portion, and the number of the cells decreased between 2 and 8 weeks after ACL reconstruction with the autologous semitendinosus tendon graft in the rabbit. Therefore it seems difficult to directly transfer genes for specific proteins to the core portion of the intraarticular graft and to maintain the number of the cells for a long period. Thus it may be difficult to successfully perform gene therapy by itself to enhance the graft healing of the intraarticular portion in ACL reconstruction. In 1999, Menetrey et al46 reported a myoblast-mediated gene transfer method for a persistent expression of selected growth factors to enhance ACL healing following injury.
Enhancement of Graft Healing with Cell-Based Therapy
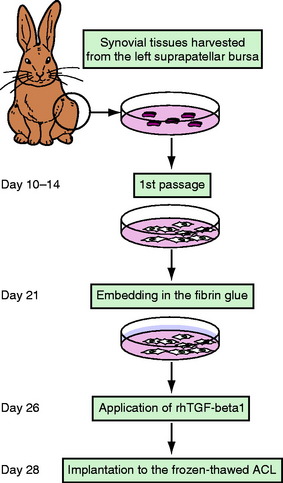
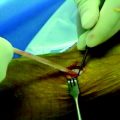
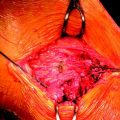
TGF-β1 and EGF significantly inhibited the deterioration of the mechanical properties of the BPTB autograft in ACL reconstruction.34 Nagumo et al22 suggested that only TGF-β1 is a key in this effect on the intraarticular graft. However, Mi et al47 reported that gene transfer of TGF-β induced arthritic changes of the articular cartilage in the knee joint. Thus intraarticular administration of TGF-β is considered to be unsuitable for clinical application with an ACL reconstruction procedure. Therefore Okuizumi et al48 conducted the rabbit study to clarify the effect of cell therapy with autologous synovial tissue–derived fibroblasts activated by TGF-β1 on the necrotized ACL (Fig. 81-3). They wrapped the fibrin glue with autologous synovial tissue–derived fibroblasts after TGF-β stimulation around the necrotized ACL following the freeze-thaw treatment. Histological observation found that implantation of fibroblasts after TGF-β stimulation accelerated cellular infiltration into the ACL following fibroblast necrosis (Fig. 81-4). Biomechanically, the transplantation of synovial tissue–derived autologous fibroblasts activated by TGF-β inhibited deterioration in the tangent modulus of the ACL after the freeze-thaw treatment. Therefore this cell-based therapy using fibroblasts activated by TGF-β may be a potential solution against this problem in order to inhibit the deterioration of the mechanical properties of the graft after ACL reconstruction.
Mesenchymal progenitor cells (MPCs) or mesenchymal stem cells (MSCs) also have a potential for cell therapy. For example, an autologous MSC–collagen graft could improve the quality as well as accelerate the rate of healing after the defect of the patellar tendon in rabbits.49 Recently, Ouyang et al50 reported the efficacy of using a large number of MSCs to enhance tendon–bone healing in a rabbit model. They found that introduction of a large number of MSCs to the bone tunnel improves the insertion healing of tendon to bone in a rabbit model through formation of fibrocartilaginous attachment at early time points. However, there are no reports on application of the cell-based therapy with MPCs or MSCs for ACL reconstruction at present.
1 Fu FH, Bennett CH, Ma CB, et al. Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am J Sports Med. 2000;28:124-130.
2 Clancy WGJr, Narechania RG, Rosenberg TD, et al. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg. 1981;63A:1270-1284.
3 Butler DL, Grood ES, Noyes FR, et al. Mechanical properties of primate vascularized vs. nonvascularized patellar tendon grafts; changes over time. J Orthop Res. 1989;7:68-79.
4 Ballock RT, Woo SL, Lyon RM, et al. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7:474-485.
5 Delay BS, McGrath BE, Mindell ER. Observation on a retrieved patellar tendon autograft used to reconstruct the anterior cruciate ligament. A case report. J Bone Joint Surg. 2002;84A:1433-1438.
6 Tomita F, Yasuda K, Mikami S, et al. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:461-476.
7 Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg. 1982;64A:217-224.
8 Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14:449-462.
9 Tohyama H, Yasuda K. Extrinsic cell infiltration and revascularization accelerate mechanical deterioration of the patellar tendon after fibroblast necrosis. J Biomech Eng. 2000;122:594-599.
10 Tohyama H, Yasuda K, Uchida H, et al. Is the increase in type III collagen of the patellar tendon graft after ligament reconstruction really caused by “ligamentization” of the graft? Knee Surg Sports Traumatol Arthrosc. 2006;14:1270-1277.
11 Oakes BW. Collagen ultrastructure in the normal ACL and in ACL graft. In: Jackson DW, editor. The anterior cruciate ligament. Current and future concepts. New York: Raven Press; 1993:209-217.
12 Yoshikawa T, Tohyama H, Enomoto H, et al. Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14:804-810.
13 Kuroda R, Kurosaka M, Yoshiya S, et al. Localization of growth factors in the reconstructed anterior cruciate ligament: immunohistological study in dogs. Knee Surg Sports Traumatol Arthrosc. 2000;8:120-126.
14 Koski JA, Ibarra C, Rodeo SA. Tissue-engineered ligament: cells, matrix, and growth factors. Orthop Clin North Am. 2000;31:437-452.
15 Woo SL, Hildebrand K, Watanabe N, et al. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999;367:S312-S323.
16 Schmidt CC, Georgescu HI, Kwoh CK, et al. Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments. J Orthop Res. 1995;13:184-190.
17 Kobayashi K, Healey RM, Sah RL, et al. Novel method for the quantitative assessment of cell migration: a study on the motility of rabbit anterior cruciate (ACL) and medial collateral ligament (MCL) cells. Tissue Eng. 2000;6:29-38.
18 Lee J, Harwood FL, Akeson WH, et al. Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments. Iowa Orthop J. 1998;18:19-25.
19 Woo SL, Smith DW, Hildebrand KA, et al. Engineering the healing of the rabbit medial collateral ligament. Med Biol Eng Comput. 1998;36:359-364.
20 Hildebrand KA, Woo SL, Smith DW, et al. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med. 1998;26:549-554.
21 Weiler A, Forster C, Hunt P, et al. The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:881-891.
22 Nagumo A, Yasuda K, Numazaki H, et al. Effects of separate application of three growth factors (TGF-β1, EGF, and PDGF-BB) on mechanical properties of the in situ frozen-thawed anterior cruciate ligament. Clin Biomech (Bristol, Avon). 2005;20:283-290.
23 Jackson DW, Grood ES, Cohn BT, et al. The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg. 1991;73A:201-213.
24 Katsuragi R, Yasuda K, Tsujino J, et al. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47-56.
25 Sakai T, Yasuda K, Tohyama H, et al. Effects of combined administration of transforming growth factor-beta1 and epidermal growth factor on properties of the in situ frozen anterior cruciate ligament in rabbits. J Orthop Res. 2002;20:1345-1351.
26 Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4-25.
27 Ju YJ, Tohyama H, Kondo E, et al. Effects of local administration of vascular endothelial growth factor on properties of the in situ frozen-thawed anterior cruciate ligament in rabbits. Am J Sports Med. 2006;34:84-91.
28 DesRosiers EA, Yahia L, Rivard CH. Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J Orthop Res. 1996;14:200-208.
29 Deie M, Marui T, Allen CR, et al. The effects of age on rabbit MCL fibroblast matrix synthesis in response to TGF-beta 1 or EGF. Mech Aging Dev. 1997;97:121-130.
30 Marui T, Niyibizi C, Georgescu HI, et al. Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res. 1997;15:18-23.
31 Scherping SCJr, Schmidt CC, Georgescu HI, et al. Effect of growth factors on the proliferation of ligament fibroblasts from skeletally mature rabbits. Connect Tissue Res. 1997;36:1-8.
32 Assoian RK, Frolik CA, Roberts AB, et al. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984;36:35-41.
33 Azuma H, Yasuda K, Tohyama H, et al. Timing of administration of transforming growth factor-beta and epidermal growth factor influences the effect on material properties of the in situ frozen-thawed anterior cruciate ligament. J Biomech. 2003;6:373-381.
34 Yasuda K, Tomita F, Yamazaki S, et al. The effect of growth factors on biomechanical properties of the bone-patellar tendon-bone graft after anterior cruciate ligament reconstruction: a canine model study. Am J Sports Med. 2004;32:870-880.
35 Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg. 1993;75:1795-1803.
36 Termaat MF, Den Boer FC, Bakker FC, et al. Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg. 2005;87:1367-1378.
37 Rodeo SA, Suzuki K, Deng XH, et al. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476-488.
38 Anderson K, Seneviratne AM, Izawa K, et al. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29:689-698.
39 Mihelic R, Pecina M, Jelic M, et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619-1625.
40 Lind M, Overgaard S, Soballe K, et al. Transforming growth factor-beta 1 enhances bone healing to unloaded tricalcium phosphate coated implants: an experimental study in dogs. J Orthop Res. 1996;14:343-350.
41 Sumner DR, Turner TM, Purchio AF, et al. Enhancement of bone ingrowth by transforming growth factor-beta. J Bone Joint Surg. 1995;77A:1135-1147.
42 Yamazaki S, Yasuda K, Tomita F, et al. The effect of transforming growth factor-beta1 on intraosseous healing of flexor tendon autograft replacement of anterior cruciate ligament in dogs. Arthroscopy. 2005;21:1034-1041.
43 Evans CH, Robbins PD. Genetically augmented tissue engineering of the musculoskeletal system. Clin Orthop Relat Res. 1999;367:S410-S418.
44 Martinek V, Latterman C, Usas A, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg. 2002;84A:1123-1131.
45 Gerich TG, Kang R, Fu FH, et al. Gene transfer to the rabbit patellar tendon: potential for genetic enhancement of tendon and ligament healing. Gene Ther. 1996;3:1089-1093.
46 Menetrey J, Kasemkijwattana C, Day CS, et al. Direct-, fibroblast- and myoblast-mediated gene transfer to the anterior cruciate ligament. Tissue Eng. 1999;5:435-442.
47 Mi Z, Ghivizzani SC, Lechman E, et al. Adverse effects of adenovirus-mediated gene transfer of human transforming growth factor beta 1 into rabbit knees. Arthritis Res Ther. 2003;5:R132-R139.
48 Okuizumi T, Tohyama H, Kondo E, et al. The effect of cell-based therapy with autologous synovial fibroblasts activated by exogenous TGF-beta1 on the in situ frozen-thawed anterior cruciate ligament. J Orthop Sci. 2004;9:488-494.
49 Awad HA, Butler DL, Boivin GP, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267-277.
50 Ouyang HW, Goh JC, Lee EH. Viability of allogeneic bone marrow stromal cells following local delivery into patella tendon in rabbit model. Cell Transplant. 2004;13:649-657.