Chapter 56 Graft-Tunnel Healing
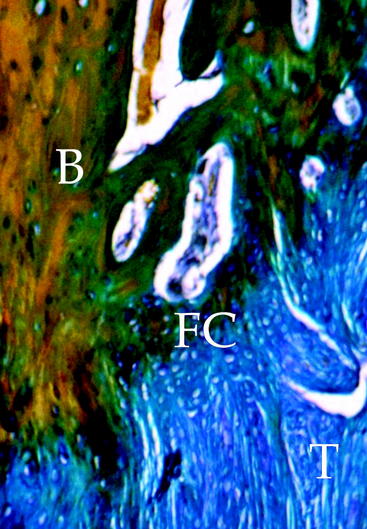
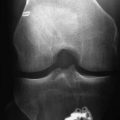
Tendon graft healing within the bone tunnel is one of the most important factors affecting “ligamentization” of the anterior cruciate ligament (ACL) graft, as it contributes to determine the mechanical behavior of the femur–ACL graft–tibia complex. The normal ACL attaches to the bone through a direct-type insertion, which has a highly differentiated morphology. In fact, within 1 mm, four different layers can be recognized: fibrous tissue, fibrocartilage, mineralized fibrocartilage, and bone (Fig. 56-1). This region plays an important mechanical role, as it allows a progressive distribution of the tensile load from a soft tissue (ligament) to a hard tissue (bone).
Human Studies
Despite the large amount of animal studies on bone–tendon graft healing in ACL reconstruction, very few investigations in humans have been reported on this issue. Pinczewski et al1 reported on two biopsies at the bone–graft interface on patients who underwent revision ACL surgery for a traumatic graft failure at 6 and 10 weeks after initial reconstruction with doubled hamstring tendon (HT) graft and fixation with metal interference screws. They described graft integration by way of collagen fibers resembling Sharpey fibers between tendon and bone. Petersen and Laprell2 compared bone–tendon graft healing after ACL reconstruction between the patellar tendon (PT) and HT on biopsy specimens obtained at ACL revision surgery from 14 patients. They observed that the PT graft healed within bone tunnel by bone plug incorporation, maintaining a direct-type insertion at the native bone plug–tendon junction. Tendon–bone healing occurred by formation of a fibrous insertion with no evidence of fibrocartilage. Ishibashi et al3 examined the histological changes in PT autografts at the tibial tunnel in biopsy specimens retrieved during revision surgery after ACL reconstruction in 10 patients. They observed that in the early revisions (less than 1 year from prior reconstruction), the bone–tendon junction was still immature, with presence of granulation tissue between the tendon and the tunnel wall; in the late revisions (more than 1 year), the original bone–tendon junction was not seen, and the tendon continued completely to the tunnel wall with Sharpey-like fibers. Nebelung et al4 obtained biopsies from the femoral tunnel in five patients at 6 to 14 months after ACL reconstruction with HT. Fixation was performed in four patients with a suspension device (Endobutton or TransFix) and in one patient with an interference screw. At histology of the four reconstructions with a suspension device, biopsies resembled granulation tissue without continuity of collagen fibers between the graft and the bony wall. In contrast, in the graft fixed with interference screws, a metaplastic fibrous cartilage between the tendon graft and the lamellar bone was noted. The authors hypothesized that suspensory fixation can produce micromotion at the tendon–bone interface, which can impair graft healing within the bone tunnel. Robert et al5 performed 12 biopsies on patients undergoing an arthroscopy between 3 and 20 months after ACL reconstruction with HT and femoral fixation with a suspension device (TransFix). Histological analysis at 3 months showed a fibrovascular interface and an uncalcified osteoid with very few collagen fibers between the tendon and the bone. At 5 and 6 months, some Sharpey-like fibers and less immature woven bone were seen. Maturity of insertion with numerous Sharpey fibers at the tendon–bone interface was seen by 10 months. After 1 year, the tendon–bone interface was composed of a continuous layer of Sharpey-like fibers. In three cases, no contact was seen at biopsy despite good clinical stability at 1 year. The authors concluded that suspensory femoral fixation of HT graft produces an indirect fixation that reaches maturity 10 to 12 months after reconstruction.
Animal Studies
Third, studies on tendon–bone healing differ in the methods of investigation of the results. Several authors performed a histological examination of the bone–tendon graft interface,6–13 whereas others focused on the mechanical properties of the bone–tendon graft complex.14–19 It has to be considered that most of the biomechanical studies were based on a load-to-failure (LTF) testing of the femur–graft–tibia complex and did not consider the effect of primary fixation on the structural properties of the complex. For this reason, some authors have performed mechanical testing after removal of the fixation devices in order to quantify the mechanical role of bone–tendon graft interface.14,15,17–19 Moreover, almost all the experimental studies performed histological and/or biomechanical evaluations at different time intervals, and although some authors evaluated the long-term fate of tendon graft healing within a bone tunnel,8,10,11,13,16,20 most authors focused on the first 12 weeks after surgery because of the clinical relevance of this period for planning postoperative rehabilitation and return to physical activity.
Type of Graft
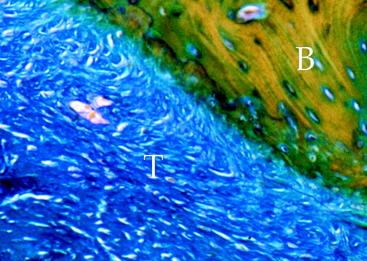
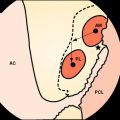
Several experiment studies focused their attention on histological and biomechanical findings of bone–tendon graft healing according to the type of tendon graft. The first reports on tendon graft healing within a bone tunnel showed that it happens through bone apposition at the tunnel wall and formation of fibrous tissue at the bone–graft interface, which matures with time and anchors the graft to the bone.21–23 More recent studies on extraarticular animal models6,9 demonstrated that tendon graft heals within a bone tunnel by formation of an indirect-type junction composed of a fibrous tissue containing perpendicular collagen fibers resembling Sharpey-like fibers that penetrate into the bone, without a transitional fibrocartilaginous layer between tendon and bone (Fig. 56-2). In a biomechanical analysis, Rodeo et al6 showed that until 8 weeks after surgery, the healing tissue at the bone–graft interface was not mechanically competent and the graft failed due to pullout from the tunnel.
Experimental studies performed on intraarticular models of ACL reconstruction with single or doubled semitendinosus tendon graft confirmed that even an autologous ACL graft heals within the bone tunnel by formation of an indirect-type junction at the bone–graft interface, with Sharpey-like fibers perpendicular to the tunnel wall.7,8,11 The newly formed insertion was evident 8 weeks after surgery and completed after 24 weeks. However, biomechanical testing showed that the graft remains weak within 1 year after surgery, with a mean failure load ranging from 25% to 50% of the normal ACL. Regarding the tensile strength of the bone–tendon graft junction, Grana et al,7 using a single-strand semitendinosus tendon graft in a rabbit model, observed that during the first 3 weeks after surgery the graft suffered a rapid and dramatic loss of its mechanical properties and failed mostly at its midsubstance rather than due to pullout from the tunnel. On the contrary, Goradia et al,11 using a doubled semitendinosus tendon graft in a sheep model, observed that up to 12 weeks, graft failure occurred by pullout from the bone tunnel. Therefore they stated that for as long as 3 months after surgery, the graft has not completely healed within the bone tunnel.
Other authors10,12,15 focused their attention on the healing process of grafts with bone plugs, such as the patellar tendon, within a bone tunnel (bone–bone healing). They observed that graft healing occurs differently for the bone plug and the intraosseous tendinous portion of the graft. In fact, bone plug incorporation at the tunnel wall occurs through a progression of necrosis, resorption, and remodeling, and after 3 months it is no longer distinguishable from the surrounding bone. The intraosseous tendinous portion of the graft heals to the bone tunnel by formation of an indirect-type insertion with penetrating collagen fibers that appear well organized by 3 months after surgery, similarly to the healing process observed for free tendon grafts. The native bone–tendon junction of the graft shows degeneration of the fibrocartilaginous layer by 6 weeks, which is during the phase of bone plug remodeling. However, by 6 months it appears to be redifferentiated with four distinct zones.
Comparative studies between tendon–bone and bone–bone healing on intraarticular models of ACL reconstruction confirmed similar histological findings.14,15,24 However, biomechanical testing demonstrated that bone–bone healing occurs more rapidly than tendon–bone healing. In fact, up to 3 weeks, both soft tissue and bone plug tendon grafts fail due to pullout from the bone tunnel. Between 6 and 8 weeks after surgery, the bone–bone interface appears mechanically stronger than the tendon–bone interface, but this difference is no more significant by 12 weeks. These observations led the authors to conclude that soft tissue grafts such as hamstring tendons heal more slowly than PT within the bone tunnel after ACL reconstruction; therefore the fixation device for soft tissue tendon grafts is more important than comparing it to PT graft during the first weeks after surgery.
Regarding the tendon allograft, experimental studies showed that the bone–graft healing process is similar to that observed for autografts.20,25,26 However, it occurs more slowly and the newly formed bone–tendon junction is evident only after a period varying from 18 weeks to 6 months after surgery. This delayed healing process should be related to the inflammatory response to the allogenic material, which persists for a long time around the graft20 and probably leads to the tunnel-widening phenomenon that mainly occurs during the first weeks after surgery.26
Bone Quality
Another variable that can influence tendon graft healing is the quality of bone where the graft is fixed. It is well known that bone density is different between the distal femur and the proximal tibia; this could affect the quality and rate of incorporation of tendon graft at the cancellous bone surface of the tunnel wall. Some authors11,27 investigated this feature of bone–tendon graft healing on experimental intraarticular and extraarticular models; however, the role of bone quality on bone–tendon graft healing remains unclear. Goradia et al11 performed an ACL reconstruction with doubled semitendinosus tendon graft and did not observe histological differences in tendon–bone healing between the femoral and tibial tunnel at each interval (from 2 to 52 weeks). On the contrary, Grassman et al,27 using the semitendinosus tendon graft for extraarticular reconstruction of the medial collateral ligament in a rabbit model, observed that incorporation and remodeling of the graft within the bone tunnels were much more extensive at the cancellous-filled femoral bone–graft interface than within the marrow-dominated tibial tunnel, thus suggesting that tendon graft healing may depend on the cancellous bone architecture at the bone–graft interface.
Fixation Technique
Most studies performed to evaluate tendon–bone healing did not consider graft fixation technique as a factor affecting the healing process. Particularly, many authors reported the use of periosteal or transosseous sutures for graft fixation.6–928 This fixation technique cannot guarantee high structural properties of the bone–tendon graft complex before biological fixation has occurred. Recently, some authors investigated the role of primary fixation on bone–tendon healing, reproducing in animal models some fixation techniques currently used in humans for ACL tendon grafts. Weiler et al13,16 performed a histological and biomechanical evaluation of healing of a tendon graft fixed within the tibial tunnel with an interference fit screw (1 mm larger than the tunnel) after an ACL reconstruction with autologous Achilles tendon split graft in a sheep model. They observed that bone–tendon healing, under the compressive effect of the interference screw, progressed partially by direct contact without development of a fibrous transition interface, whereas at the articular tunnel aperture site a well-differentiated, direct-type junction was evident by 24 weeks.13 Biomechanical testing16 showed that at 6 and 9 weeks, all grafts failed at the screw insertion site. By 24 weeks, grafts failed by osteocartilaginous avulsion from the tibial attachment site. These findings indicate that interference fit fixation may compromise the mechanical properties of the graft in the early healing phase at the screw insertion site, but the compressive effect of the screw supplies a biological stimulus toward the formation of a physiological, direct–type bone–graft insertion. Singhatat et al17 used an extraarticular reconstruction model in ovine tibiae to evaluate the effect of the fixation technique on the mechanical properties of a bone–tendon graft complex, comparing two different fixation devices: an absorbable interference screw and a spiked screw and washer (WasherLoc). They observed that the strength of biological fixation of tendon to bone increased slower with the interference screw than with the screw and washer. In fact, tensile testing on tendon graft–bone tunnel interface (after removing the fixation device) after 4 weeks of implantation showed that with interference fixation, mean strength and stiffness were respectively 31% and 36% of that observed for the complex at implantation (time zero). With WasherLoc fixation, strength and stiffness were respectively 50% and 143% of the complex at implantation. The authors supposed that interference fixation might impair tendon healing within the bone tunnel as it decreased the contact area between the tendon and the surrounding bone. Furthermore, the compressive effect of the interference screw on the tendon graft may prevent the ingrowth of blood vessels along the entire length of the tendon graft. However, this hypothesis was not confirmed in this study by a histological examination.
Gap Size
Another important factor related to bone–tendon healing is the gap between the tendon graft and the walls of the bone tunnel. This is important especially in ACL reconstruction with doubled HT graft and suspensory femoral fixation devices, such as Endobutton or TransFix, as these fixation devices do not produce any compressive effect on the intraosseous portion of the graft, and therefore the thickness of the healing tissue at the bone–tendon interface depends on the size of the gap between the graft and the bone at the time of reconstruction. Tien et al29 evaluated the effect of gap size on the tendon-to-bone healing on ACL reconstruction with autologous semitendinosus tendon graft in a rabbit model and observed that healing tissue at the bone–tendon interface appeared denser and more organized in the specimens with a smaller gap. Tensile testing at 2 weeks confirmed a significantly greater maximal tensile strength for specimens in which the tunnel had the same diameter of the graft than for specimens with a tunnel 25% to 33% larger than the graft. Greis et al,28 using an extraarticular bone–tendon healing model in dogs, reported similar data. They observed that the failure load of tendon–bone complex was significantly greater when the bone tunnel approximated the diameter of the tendon graft (4.2 mm) in comparison with specimens with a 6-mm tunnel. On the contrary, Yamazaki et al30 in an experimental study in dogs observed that a free tendon graft used for ACL reconstruction healed in a bone tunnel that was 2 mm larger than the graft by formation of a connective transitional fibrous layer that was denser and better organized than that observed in specimens with a bone tunnel having the same diameter as the graft. Moreover, mechanical analysis showed that at 3 and 6 weeks, the differences in ultimate failure load and failure mode between the two groups of specimens were not significant.
Mechanical Stresses
Regarding load magnitude, it is important to distinguish between loads applied during healing time and initial tensile load due to graft tensioning at the time of surgery. Sakai et al31 investigated the effect of immobilization on the biological fixation of a tendon graft within the bone tunnel after ACL reconstruction in a rabbit model and showed that no immobilization delays the bone–tendon healing and impairs the mechanical strength of the newly formed bone–graft junction. Abramowitch et al32 evaluated the effect of initial graft tension on the tensile properties of an ACL graft in a goat model and observed that high initial tension of the ACL graft better replicated a normal knee kinematics immediately after reconstruction in comparison with a low graft tension; however, this effect diminished during the early graft healing process.
Some interesting findings regarding the effect of direction of loads applied to the graft on bone–tendon graft healing are reported by Yamakado et al,33 who showed that tendon–bone healing responds to mechanical stress applied to the tendon graft. In an extraarticular tendon graft reconstruction in a rabbit model, they observed that tensile stress enhances the healing process of the bone–tendon junction, compressive stress promotes chondroid formation, and shear load has little or no effect on regeneration of the bone–tendon junction. These forces are differently distributed along the bone–tendon graft interface and according to the direction of tunnel in relation to the axis of the intraarticular portion of the tendon graft.
Authors’ Experience
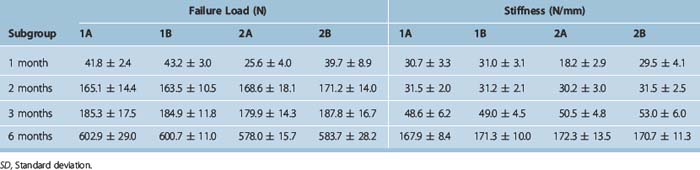
We analyzed in a sheep model the mechanical behavior of the tendon graft–bone interface on ACL reconstruction, comparing patellar tendon (Group 1) and a free tendon graft (Group 2) using the doubled lateral extensor of toes (DLET).18 Femoral fixation was achieved in all specimens with a transverse fixation: a metal setscrew for the PT graft, and a modified TransFix for the DLET graft. On the tibia, the graft was fixed with an absorbable interference screw in both groups. Animals were sacrificed after 1, 2, 3, and 6 months postsurgery, and an LTF mechanical testing on the femur–graft–tibia complex (FGTC) was performed after removing (Subgroup A) or maintaining (Subgroup B) the femoral fixation device in order to evaluate the mechanical properties of the proximal tendon graft–bone tunnel interface and the contribution of the fixation device over time. Specimens were compared with control groups that consisted of normal ACL, normal grafts, and reconstructions at time zero.
Results of the mechanical testing performed on the control (Table 56-1) and treated groups (Table 56-2) showed a similar trend in the variation of the structural properties of the two groups in comparison with the controls. At 1 month, mean failure load dramatically decreased. After this period, we observed a significant increase, although the variation between the second and the third month was not significant. Mean stiffness showed a dramatic decrease at 1 and 2 months, whereas in the 3-month samples a significant increase occurred. However, at 2 and 3 months, the structural properties of the treated groups remained significantly lower than the control groups. At 6 months, we observed a significant improvement of the structural properties in both groups, much greater than that reported in previous studies.8,11,16 In fact, 6 months after surgery, the PT graft showed a good recovery of its original structural properties and also approximated the strength (about 83%) and stiffness (about 107% to 109%) of a normal ACL. The DLET graft at 6 months showed a severe impairment of its original structural properties. However, strength and stiffness were about 80% and 110% of a normal ACL, respectively. When comparing the structural properties of the femur–PT graft–tibia complex with and without a femoral fixation device, we did not observe significant differences for all the variables considered at every time interval. This would imply that the presence of the fixation device did not influence the mechanical behavior of the FGTC. On the contrary, comparison between the two DLET subgroups, with and without the fixation device, showed a significant difference for mean failure load and stiffness in the 1-month samples. Furthermore, on comparison of the specimens of the two groups in which the femoral fixation devices had been removed before the mechanical test (Subgroup A), we observed that at 1 month the structural properties of the PT group were significantly greater than the DLET group. For the following time intervals, the structural properties of the two types of graft did not significantly differ. Analysis of the failure mode in specimens without the fixation device showed that at 1 month, PT graft failure always occurred at its intraarticular part, whereas the DLET grafts failed by means of pullout from the femoral tunnel. In the following time intervals, the grafts always ruptured at their midsubstance in both groups. This would demonstrate that the bone plug of the PT graft incorporated in the femoral tunnel in an early phase after ACL reconstruction, making the bone–graft junction mechanically competent after 1 month, probably due to the compression effect of transverse fixation that accelerated bone–bone healing. On the contrary, the DLET graft was not yet incorporated in the femoral tunnel 1 month after surgery; therefore, in absence of the fixation device, it slipped out of the tunnel when submitted to traction.
Table 56-1 Results of Load-to-Failure Tests on the Control Groups (Mean ± SD)
| Failure Load (N) | Stiffness (N/mm) | |
|---|---|---|
| Normal ACL | 723.0 ± 12.1 | 156.6 ± 6.1 |
| Normal PT | 830.1 ± 16.6 | 176.8 ± 9.3 |
| Time zero: PT | 607.8 ± 14.4 | 89.8 ± 5.9 |
| Normal DLET | 1139.8 ± 44.5 | 285.7 ± 22.6 |
| Time zero: DLET | 1032.8 ± 43.7 | 210.1 ± 15.5 |
ACL, Anterior cruciate ligament; DLET, doubled lateral extensor of toes; PT, patellar tendon; SD, standard deviation.
Future Directions
Bone Proteins and Growth Factors
Several studies have shown the effect of some polypeptides including bone morphogenetic proteins (BMPs) and growth factors (GFs), such as transforming growth factors (TGFs), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF), on the activation and regulation of proliferation and differentiation of bone and fibrous and cartilaginous tissues.34–36 Based on these experiences, some authors investigated the role of BMPs and GFs in promoting tendon–bone healing through the activation and acceleration of bone ingrowth, collagen fiber synthesis, and fibrocartilaginous differentiation at the bone–tendon graft interface, with the aim of obtaining early formation of bone–tendon insertion similar to that of normal ACL. Rodeo et al37 reported the effect of locally applied BMP-2 on tendon healing in bone tunnel in dogs. They showed that animals treated with recombinant human BMP-2 had radiographic evidence of more extensive formation and closer apposition of new bone around the tendon graft in comparison with controls. Biomechanical analysis confirmed higher pullout strength in BMP-treated specimens. Anderson et al38 reported an experimental study on ACL reconstruction with autologous semitendinosus tendon graft in a rabbit model, using a collagen sponge wrapped around the portion of the graft inside the bone tunnel as a carrier vehicle to release a bone-derived extract that contained several bone morphogenetic proteins (BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, and BMP-7) and growth factors with known osteoinductive activity or that can modulate bone formation, such as TGF-ß1, TGF-ß2, TGF-ß3, and FGF. Although this mixture of bone-derived proteins did not allow the effects of each protein on tendon–bone healing to be discriminated, the authors observed that animals treated with bone-derived proteins had a histological appearance of a more consistent, dense interface tissue and closer apposition of new bone to the graft, with occasional formation of a fibrocartilaginous interface, when compared with control specimens at 2, 4, and 8 weeks after surgery. Biomechanical analysis demonstrated a significant increase in ultimate tensile strength of the treated grafts (from 47% more than controls at 2 weeks to 80% at 8 weeks). Mihelic et al39 reported a study on ACL reconstruction with autologous peroneus tertius tendon graft in a sheep model. They applied a carrier sponge with recombinant human BMP-7 to the bone–tendon graft interface and observed that bone formation and remodeling around the graft at 3 and 6 weeks after surgery were more extensive in knees treated with BMP-7 compared with control knees. Mechanical testing showed a significantly greater tensile strength in grafts treated with BMP-7 than in control specimens. Yamazaki et al19 performed an experimental study in dogs to detect the effect of TGF-ß1 on healing of the flexor tendon autograft in ACL reconstruction. TGF-ß1 was mixed with fibrin sealant and applied in the graft–bone gap of the tibial tunnel. At 3 weeks, histological examination showed that perpendicular collagen fibers connecting the tendon to the bone (resembling Sharpey fibers) were richly generated in knees treated with TGF-ß1 compared with control knees. In mechanical pullout testing after removing tibial graft fixation, the ultimate failure load and stiffness of the graft–tibia complex of knees treated with TGF-ß1 were significantly higher than those of controls.
Gene Therapy
Some limits in the intraarticular use of bone proteins and growth factors are represented by their short half-life and removal by synovial fluid that can affect the maintenance of therapeutic local concentrations. For this reason, some researchers have attempted to develop techniques of gene transfer, consisting of the injection of transduced cells that express genes for synthesis of desired proteins (i.e., growth factors) at high local concentration for prolonged time periods. These techniques have been applied to enhance tendon–bone healing. Martinek et al40 reported a study on genetically engineered semitendinosus tendon grafts used for ACL reconstruction in rabbits to evaluate the capacity of BMP-2 gene transfer to improve healing of a tendon graft in the bone tunnel. They observed that at 6 weeks, grafts infected with adenovirus-BMP-2 (AdBMP-2) had broad zones of a newly formed transition layer at the bone–graft interface, resembling a direct-type insertion. Mechanical testing showed that ultimate failure load and stiffness of grafts infected with AdBMP-2 were significantly greater than controls. This study was highly remarkable, as it showed that sustained and prolonged local BMP-2 delivery using gene transfer modality created a bone–tendon graft insertion similar to that of a normal ACL. However, many questions need to be answered regarding safety and regulatory issues before gene transfer is suggested as a therapeutic method in orthopaedics.41
Inhibitors of Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) such as collagenases and stromelysins are zinc-dependent enzymes that have a catalytic effect on connective tissues by degrading different types of collagen. These enzymes are increased in the intraarticular environment in the acute phase following ACL rupture and are presumably increased following ACL reconstruction. Therefore it seems likely that MMPs have an adverse effect on tendon–bone healing that occurs by formation of collagen fibers at the bone–graft interface. For these reasons, some authors42,43 have hypothesized that tissue inhibitors of metalloproteinases (TIMPs) could have a potential positive effect on the healing process of a tendon graft within a bone tunnel. Demirag et al43 tested the potential enhancement effect of α-2 macroglobuline on bone–tendon healing of ACL graft by blockage of synovial MMP activity in a rabbit model. They injected α-2 macroglobuline into the knee joint after ACL reconstruction with semitendinosus tendon autograft to block the MMPs in the synovial fluid and observed after 2 and 5 weeks a significantly decreased concentration of type I collagenase (MMP-8) in the synovial fluid compared with the control group. Histological examination showed that the bone–tendon interface within the tunnel in the treated specimens had more areas of denser connective tissue ingrowth compared with the controls. Mechanical testing showed that the mean ultimate failure load of treated specimens was significantly greater than control specimens at both 2 and 5 weeks.
Cell Therapy
Some authors have investigated the effects of mesenchymal stem cells (MSCs) on the quality and rate of a tendon graft osteointegration.44,45 MSCs are pluripotent cells that can be harvested from bone marrow of the iliac crest, isolated and cultured in vitro, included in a fibrin gel as carrier, and applied along the bone–tendon graft interface. Ouyang et al,44 in an extraarticular rabbit model, showed the presence of discontinuous areas of fibrocartilage-like tissue containing type II collagen at the bone–graft interface by 4 weeks after surgery. Lim et al45 reported similar results on an experimental model of ACL reconstruction. By 2 weeks, they observed large areas of cartilage at the newly formed bone–graft junction with histological features similar to those of the normal ACL by 8 weeks. Mechanical testing showed that the tensile strength of the grafts treated with stem cells was significantly greater than controls by 8 weeks.
Biological Scaffolds
Some authors have focused their attention on the use of periosteal grafts to enhance tendon graft healing within a bone tunnel.46–49 Periosteum is an osteogenic tissue that modulates bone formation and remodeling at the cortical bone surface. Therefore it was hypothesized that periosteum should behave as a biological scaffold to promote and accelerate bone–tendon graft healing. Experimental studies on extraarticular models in rabbits evaluated histological and biomechanical features of tendon grafts wrapped in periosteum in the intraosseous part. They showed that periosteum promotes a more extensive and closer apposition of new bone around the tendon graft.48 Moreover, a bone–tendon fibrocartilaginous insertion has been observed by 4 to 12 weeks after surgery.46,47 It was reported that a biological effect of periosteum on bone–tendon graft healing is more evident when the cambial layer of the scaffold faced the tunnel wall49 and that fresh periosteal graft is more effective than fresh-frozen graft.46 Biomechanical testing showed controversial results regarding the mechanical strength of biological fixation in the early phase after surgery. In fact, Kyung et al48 reported that the mean pullout strength of periosteum-treated grafts was significantly greater than controls at 3 and 6 weeks. On the contrary, Chen et al47 observed that at 4 weeks the difference in tensile strength between periosteum-treated grafts and controls was not significant and graft failure at that period always occurred due to pullout from the bone tunnel. The difference in mechanical properties between treated specimens and controls was significant by 8 weeks after surgery.
Another technique of biological scaffolding to promote tendon graft healing within a bone tunnel consists of filling the gap between the tendon and the bone with a bone substitute. Tien et al50 performed an ACL reconstruction with semitendinosus tendon graft in a rabbit model and filled the gap between the tendon graft and the femoral bone tunnel with a paste of calcium-phosphate cement (CPC) obtained from mixing tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA) powders. Histological examination showed that CPC produced early, diffuse, and massive bone ingrowth within the tunnel in comparison with control specimens. Mechanical testing showed that the mean maximal tensile strength for treated grafts was significantly greater than controls at 1 and 2 weeks after reconstruction.
1 Pinczewski LA, Clingeleffer AJ, Otto DD, et al. Integration of hamstring tendon graft with bone in reconstruction of the anterior cruciate ligament. Arthroscopy. 1997;13:641-643.
2 Petersen W, Laprell H. Insertion of autologous tendon grafts to the bone: a histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc. 2000;8:26-31.
3 Ishibashi Y, Toh S, Okamura Y, et al. Graft incorporation within the tibial bone tunnel after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft. Am J Sports Med. 2001;29:473-479.
4 Nebelung W, Becker R, Urbach D, et al. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003;123:158-163.
5 Robert H, Es-Sayeh J, Heymann D, et al. Hamstring insertion site healing after anterior cruciate ligament reconstruction in patients with symptomatic hardware or repeat rupture: a histologic study in 12 patients. Arthroscopy. 2003;19:948-954.
6 Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg. 1993;75A:1795-1803.
7 Grana WA, Egle DM, Mahnken R, et al. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344-351.
8 Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25:554-559.
9 Liu SH, Panossian V, Al-Shaikh R, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997;339:253-260.
10 Schiavone Panni A, Milano G, Lucania L, et al. Graft healing after anterior cruciate ligament reconstruction in rabbits. Clin Orthop Relat Res. 1997;343:203-212.
11 Goradia VK, Rochat MC, Grana WA, et al. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13:143-151.
12 Yoshiya S, Nagano M, Kurosaka M, et al. Graft healing in the bone tunnel in anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2000;376:278-286.
13 Weiler A, Hoffmann RFG, Bail HJ, et al. Tendon healing in a bone tunnel. Part II: histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:124-135.
14 Papageorgiou CD, Ma CB, Abramowith SD, et al. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sports Med. 2001;29:620-626.
15 Tomita F, Yasuda K, Mikami S, et al. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:461-476.
16 Weiler A, Peine R, Pashmineh-Azar A, et al. Tendon healing in a bone tunnel. Part I: biomechanical results after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:113-123.
17 Singhatat W, Lawhorn KW, Howell SM, et al. How four weeks of implantation affect the strength and stiffness of a tendon graft in a bone tunnel: a study of two fixation devices in an extraarticular model in ovine. Am J Sports Med. 2002;30:506-513.
18 Milano G, Mulas PD, Sanna-Passino E, et al. Evaluation of bone plug and soft tissue anterior cruciate ligament graft fixation over time using transverse femoral fixation in a sheep model. Arthroscopy. 2005;21:532-539.
19 Yamazaki S, Yasuda K, Tomita F, et al. The effect of transforming growth factor-beta1 on intraosseous healing of flexor tendon autograft replacement of anterior cruciate ligament in dogs. Arthroscopy. 2005;21:1034-1041.
20 Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176-185.
21 Kernwein GA. A study of tendon implantation into bone. Surg Gynecol Obstet. 1942;75:794-796.
22 Whinston TB, Walmsley R. Some observation of reaction of bone and tendon after tunneling of bone and insertion of tendon. J Bone Joint Surg. 1960;42B:337-386.
23 Forward AD, Cowan RJ. Tendon suture to bone: an experimental investigation in rabbit. J Bone Joint Surg. 1963;45:807-823.
24 Park MJ, Lee MC, Seong SC. A comparative study of the healing of tendon autograft and tendon-bone autograft using patellar tendon in rabbits. Int Orthop. 2001;25:35-39.
25 Shino K, Kawasaki T, Hirose H, et al. Replacement of the anterior cruciate ligament by an allogeneic tendon graft. An experimental study in the dog. J Bone Joint Surg. 1984;66B:672-681.
26 Harris NL, Indelicato PA, Bloomberg MS, et al. Radiographic and histologic analysis of the tibial tunnel after allograft anterior cruciate ligament reconstruction in goats. Am J Sports Med. 2002;30:368-373.
27 Grassman SR, McDonald DB, Thornton GM, et al. Early healing processes of free tendon grafts within bone tunnels is bone-specific: a morphological study in a rabbit model. Knee. 2002;9:21-26.
28 Greis PE, Burks RT, Bachus K, et al. The influence of tendon length and fit on the strength of a tendon-bone tunnel complex. A biomechanical and histologic study in the dog. Am J Sports Med. 2001;29:493-497.
29 Tien YC, Chih HW, Cheng YM, et al. The influence of the gap size on the interfacial union between the bone and the tendon. Kaohsiung J Med Sci. 1999;15:581-588.
30 Yamazaki S, Yasuda K, Tomita F, et al. The effect of graft-tunnel diameter disparity on intraosseous healing of the flexor tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:498-505.
31 Sakai H, Fukui N, Kawakami A, et al. Biological fixation of the graft within bone after anterior cruciate ligament reconstruction in rabbits: effects of the duration of postoperative immobilization. J Orthop Sci. 2000;5:43-51.
32 Abramowitch SD, Papageorgiou CD, Withrow JD, et al. The effect of initial graft tension on the biomechanical properties of a healing ACL replacement graft: a study in goats. J Orthop Res. 2003;21:708-715.
33 Yamakado K, Kitaoka K, Yamada H, et al. The influence of mechanical stress on graft healing in a bone tunnel. Arthroscopy. 2002;18:82-90.
34 Yasko AW, Lane JM, Fellinger EJ, et al. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg. 1992;74A:659-670.
35 Des Rosiers EA, Yahia L, Rivard C-H. Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J Orthop Res. 1996;14:200-208.
36 Jelic M, Pecina M, Haspl M, et al. Regeneration of articular chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19:101-113.
37 Rodeo SA, Suzuki K, Deng XH, et al. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476-488.
38 Anderson K, Seneviratne AM, Izawa K, et al. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29:689-698.
39 Mihelic R, Pecina M, Jelic M, et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619-1625.
40 Martinek V, Latterman C, Usas A, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg. 2002;84A:1123-1131.
41 Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg. 1995;77A:1103-1114.
42 Deehan DJ, Cawston TE. The biology of integration of the anterior cruciate ligament. J Bone Joint Surg. 2005;87B:889-895.
43 Demirag B, Sarisozen B, Ozer O, et al. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J Bone Joint Surg. 2005;87A:2401-2410.
44 Ouyang HW, Goh JC, Lee EH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32:321-327.
45 Lim JK, Hui J, Li L, et al. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899-910.
46 Ohtera K, Yamada Y, Aoki M, et al. Effects of periosteum wrapped around tendon in a bone tunnel: a biomechanical and histological study in rabbits. Crit Rev Biomed Eng. 2000;28:115-118.
47 Chen C-H, Chen W-J, Shih C-H, et al. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: a biomechanical and histologic study in rabbits. Arthroscopy. 2003;19:290-296.
48 Kyung HS, Kim SY, Oh CW, et al. Tendon-to-bone tunnel healing in a rabbit model: the effect of periosteum augmentation at the tendon-to-bone interface. Knee Surg Sports Traumatol Arthrosc. 2003;11:9-15.
49 Youn I, Jones DG, Andrews PJ, et al. Periosteal augmentation of a tendon graft improves tendon healing in the bone tunnel. Clin Orthop Relat Res. 2004;419:223-231.
50 Tien YC, Chih TT, Lin J-HC, et al. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J Bone Joint Surg. 2004;86B:1072-1076.