Chapter 8 Graft Options for Anterior Cruciate Ligament Revision Reconstruction
INTRODUCTION
It has been estimated that approximately 100,000 anterior cruciate ligament (ACL) reconstructions are performed annually in the United States.7 This represents a 30% to 40% increase from previous reports.20 The procedure has a successful outcome in 75% to 95% of cases; however, 8% may experience an unsatisfactory result owing to recurrent instability and graft failure.16 It is reasonable to assume that with an increasing number of ACL reconstructions, orthopaedic surgeons are more likely to encounter a patient with a failed reconstruction. Failure has not been universally defined, but in general, an ACL graft is consider failed when there is an increase in anteroposterior (AP) translation of more than 5.5 mm compared with the contralateral knee during instrumented knee arthrometer testing or when a grade II or III pivot shift test is elicited during physical examination. When planning a revision ACL reconstruction, one of the most important and controversial decisions is the choice of graft. In this chapter, the merits of various graft sources (autografts, allografts, and rarely in North America, synthetic grafts) are discussed as well as the advantages and disadvantages of different anatomic graft types. Finally, the authors present their preferred approach.
SYNTHETIC GRAFTS AND AUGMENTATION DEVICES
Gore-Tex Ligament
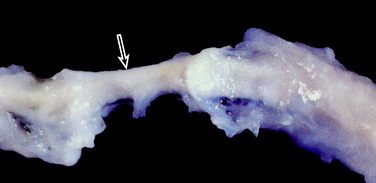
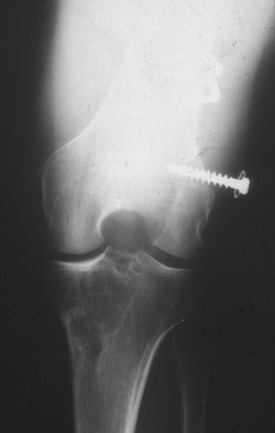
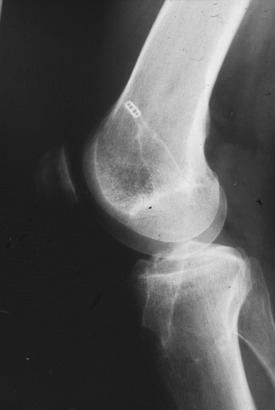
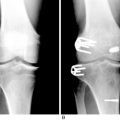
The Gore-Tex synthetic ligament consists of woven strands of polytetrafluoroethylene with molded eyelets at each end that allow fixation of the graft to the bone with screws (Fig. 8-1). This prosthetic device is intended to function as a permanent load-bearing implant with an ultimate tensile strength of 5300 N. In 1986, this device received approval from the U.S. Food and Drug Administration (FDA) for limited clinical use in patients with failed ACL reconstruction. The Gore-Tex ligament is placed in an over-the-top location. Femoral fixation in a bone tunnel is contraindicated because abrasion of the ligament at the entrance of the tunnel can cause premature failure. Long-term outcome studies of patients who received this artificial ligament have shown deterioration of results and high failure rates. Paulos and associates39 reported on 188 patients who received Gore-Tex reconstructions followed an average of 48 months postoperatively. Unacceptable results (fair and poor) were obtained in 56% of the patients. Rupture of the ligament occurred in 12% of the cases (Fig. 8-2). Loosening of greater than 3 mm was observed in 34% of the patients, which led to an unacceptable result in nearly one half. Thirty-four percent of the patients suffered from recurrent effusions. The authors concluded that the use of this ligament in primary as well as revision ACL reconstructions led to unacceptable results in 76% of the cases.
Stryker Dacron Ligament
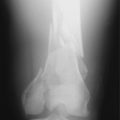
The Stryker Dacron prosthetic ligament (Fig. 8-3) is intended to function as a permanent prosthesis. This device has an ultimate tensile strength of 3600 N and was approved in 1989 for use in failed intra-articular ACL reconstructions. It can be implanted either using an over-the-top technique or through a bone tunnel. Similar to the Gore-Tex ligament, long-term studies have shown high failure rates. Wredmark and Engstrom54 reported 5-year results in a group of 42 patients. During the observation period, the failure rate was nearly 60% based on ligament examination (instrumented knee arthrometer and/or pivot shift test). Eighty percent of the patients had radiographic evidence of ligament rupture. Histologic examination of the failed ligaments during revision surgery revealed an inflammatory or foreign body reaction, and not the expected organized fibrous ingrowth.
Kennedy Ligament Augmentation Device
The ligament augmentation device (LAD) polypropylene graft, in contrast to the grafts described previously, is intended to function as a load-sharing implant to protect a biologic graft while it heals (Fig. 8-4). The FDA released this product in 1986 for use with the Marshall-McIntosh ACL reconstruction, but it has also been used with autogenous bone–patellar tendon–bone (B-PT-B) and hamstring grafts. The device is manufactured in widths of 8 mm and 6 mm, with ultimate tensile strengths of 1730 N and 1500 N, respectively. The Kennedy LAD was perhaps the most widely used prosthetic device in ACL reconstruction. Although complications from its use such as synovitis, breakage, or painful hardware have been reported,5 the device fell out of favor mainly because comparison studies failed to demonstrate any advantage with its use for autograft or allograft augmentation.30,32
ALLOGRAFTS
Graft Options
The wide availability of musculoskeletal allografts in North America has resulted in an ever-increasing utilization of these tissues in orthopaedic surgery. According to data from the American Association of Tissue Banks (AATB), a voluntary accreditation organization that sets standards for tissue banking, approximately 1.5 million bone and soft tissue allografts are distributed each year by AATB-accredited tissue banks in the United States.41 Allografts are appealing during revision ACL reconstruction in which autograft options may be limited or not available. The morbidity associated with autograft harvest is avoided and the operative time is decreased. A variety of allograft tissue is available for ACL reconstruction. The most popular tissues are the Achilles tendon (AT), B-PT-B, quadriceps tendon (QT), hamstring tendons, and the tibialis anterior (TA) tendon. Other less frequently used options are the tibialis posterior tendon, peroneus longus tendon, and fascia lata. These grafts provide a variety of length, width, and strength options and allow for soft tissue or bone fixation. The biomechanical properties of the TA, tibialis posterior (TP), and the peroneus longus (PL) tendon have recently been evaluated. Their ultimate tensile strength when tested as doubled grafts was 3144 N, 3391 N, and 2483 N, respectively. The TA had the greatest stiffness (344 N/mm), followed by the TP (302 N/mm) and the PL (244 N/mm).40 These values compare favorably with historical data of other graft sources.35 In addition, allografts harvested with bone blocks at their end(s) such as the AT, B-PT-B, or QT, may allow greater flexibility during revision reconstructions when bone loss is encountered. In such cases, shaping the bone block(s) of the allograft to match a widened preexisting tunnel or to fill a bone defect from removed hardware can obviate the need for a staged reconstruction. These benefits, however, have to be weighed against certain disadvantages of allografts that are associated with their procurement, processing, and sterilization.
Disease Transmission
One of the major concerns related to the use of allografts is disease transmission. The incidence of infection after allograft knee reconstruction has not been accurately determined. Both bacterial and viral infections have been reported. As of March 11, 2002, the U.S. Centers for Disease Control and Prevention (CDC) had received 26 reports of bacterial infections associated with musculoskeletal tissue allografts. Eighteen of these cases were associated with grafts used for ACL reconstruction. Thirteen (50%) of the 26 patients were infected with Clostridium species (12 with C. septicum, one with C. sordellii). All allografts were processed aseptically, but did not undergo terminal sterilization.3 In response, additional steps were taken to reduce the risk of disease transmission including sterilization methods that kill bacterial spores, swab and destructive cultures, rejection of tissues contaminated by bowel flora, and implementation of time limits for the procurement of the donors (12 hr when the donor is procured at room temperature, 24 hr for refrigerated donors). However, in 2003, an additional case of septic arthritis due to Streptococcus pyogenes after allograft ACL reconstruction was reported, despite adaptation of these measures.2 The risk of viral disease transmission is lower, and most incidents have occurred before the pathogen was known or when the available technology could not identify its presence in the donor owing to a “serologic window.” There have been reports of hepatitis C, and human immunodeficiency virus (HIV) transmission from tendon allografts used in knee reconstruction.1,47 The risk of HIV transmission from musculoskeletal allografts has been estimated to be as low as 1 in 1,667,600.8
Donor Screening and Sterilization Techniques
Currently, allograft donors are initially screened with a detailed medical and social history of the donor and postmortem examination for signs of infectious disease. Blood cultures as well as cultures from the harvested grafts are taken. The AATB requires a serologic examination for HIV type 1 and 2 antibodies, HIV-1 DNA by polymerase chain reaction, hepatitis B surface antigen, hepatitis B core antibody, hepatitis C antibody, human T-cell lymphotropic virus types 1 and 2 antibodies, and syphilis antibody. The introduction of newer testing methods such as nucleic acid testing can decrease the window period associated with conventional antigen and antibody testing and increase the safety of allografts. The Board of Governors of the AATB voted to require AATB-accredited tissue banks to adopt this newer testing method for HIV-1 and hepatitis C by March of 2005.29
Several disinfection and sterilization methods have been used to eliminate bacteria, spores, and viruses from allografts.53 Currently, there is no single standard approach to eliminate pathogens from allografts. Furthermore, some of these methods can alter the biomechanical properties of soft tissue grafts. Aseptic harvesting and processing alone preserve the strength of tendon grafts, but these do not eliminate bacteria, fungi, spores, or viruses. In addition, this method removes blood and lipids from only the surface of the graft. Chemical soaking can eliminate bacteria and fungi, but this process does not eradicate spores or viruses.
Two commonly employed methods for terminal sterilization are gamma irradiation (GI) and ethylene oxide (EO). GI is effective against bacteria at doses of 1.5 to 2.5 Mrad. However, as much as 4.0 Mrad is required to inactivate HIV, and even higher doses may be necessary to kill spores.13 Unfortunately, higher doses of GI result in a substantial decrease of the strength of tendinous grafts. Fideler and colleagues14 studied the effects of GI on B-PT-B allografts. These investigators noted a 15%, 24%, and 46% reduction in all biomechanical properties of the grafts after exposing them to doses of 2.0, 3.0, and 4.0 Mrad of irradiation, respectively. EO has successfully been used to sterilize surgical instruments. However, its use as a method to sterilize ACL allografts has been associated with intra-articular reactions with chronic synovitis, graft failure, and bone dissolution developed from the chemical residues that EO leaves in moist tissues.19
In an attempt to ameliorate the problems associated with each individual method of sterilization, several companies have developed proprietary techniques to process allografts that combine the methods previously discussed or introduce new ones.50 Cryolife, Inc. (Kennisaw, GA) uses slow freezing combined with a dehydrating solvent, such as dimethyl sulfoxide or glycerol, to cryopreserve allografts. After desiccation, the grafts are treated for an extended period of time with an antimicrobial solution. No secondary sterilization technique is used. BioCleanse (Regeneration Technologies, Inc., Alachua, FL) uses a low-temperature chemical sterilization method, which allows better tissue penetration of the chemical and thus eliminates endogenous contamination from the allografts.
Healing of Allografts Postimplantation
Biologic integration and tissue compatibility merit consideration when allografts are used in primary or revision ACL reconstruction. Allografts follow the same healing process observed with autograft reconstruction of an initial period of avascular necrosis, followed by revascularization and cell proliferation.4 However, several studies have documented that this integration occurs at a much slower rate. Jackson and coworkers18 evaluated patellar tendon autografts and fresh frozen allografts 6 months after ACL reconstruction in a goat model. Compared with the allografts, the autografts demonstrated a smaller increase in AP displacement, two-times greater values of maximum force to failure, a significant increase in cross-sectional area, a more rapid loss of large-diameter collagen fibrils, and an increased density and number of small-diameter collagen fibrils. The allografts had a greater decrease in structural properties, a slower rate of biologic incorporation, and a prolonged presence of an inflammatory response. The autografts demonstrated a more robust biologic response, improved stability, and increased strength to failure values.
More recently, Malinin and associates28 studied nine ACL allografts and one autograft, retrieved at autopsy or at the time of revision surgery, to determine the rate and the extent of graft cellular replacement and remodeling. Examination of specimens from 20 days to 10 years after transplantation revealed a pattern of revascularization similar to that reported in previous biopsy studies. However, examination of entire allografts 2 years after transplantation revealed that the central portions of the grafts remained acellular and complete attachment was not present. One specimen retrieved 3.5 years post-transplantation did demonstrate complete attachment. The authors concluded that complete remodeling and cellular replacement of the entire graft may require 3 years or longer.
Similar findings were documented in a study by Beck and coworkers.6 These authors obtained biopsies during arthroscopy from ACL allografts at various times after implantation. Histologic examination of the specimens showed that allografts remain largely avascular and acellular as late as 8 years postimplantation (Fig. 8-5).
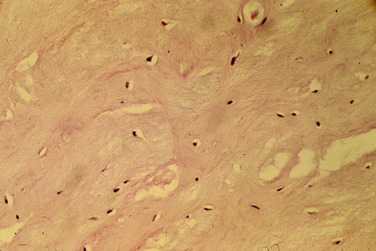
FIGURE 8-5 Histologic examination of specimens from Beck and coworkers6 shows that allografts remain largely avascular and acellular as late as 8 years postimplantation.
ACL allograft antigenicity may be another factor that determines the fate of these tissues. In an animal study, transplantation of a serologically mismatched fresh patellar tendon allograft resulted in a marked inflammatory response and rejection of the graft.4 Bugbee and colleagues9 in a study of blood type in fresh osteochondral allograft transplantation suggested that ABO mismatch may adversely affect the clinical outcome of transplantation through an immune-mediated response. The inflammatory response to allograft tissue has been implicated in osteolysis and tunnel widening observed after ACL reconstruction.11 However, it is difficult to distinguish the contribution of other parameters such as mechanical factors, fixation failure, and exposure to synovial fluid in this phenomenon. It must be noted that the preservation method affects the antigenicity of allografts. Freezing decreases cellular antigenicity, whereas freeze-drying produces grafts considered even less antigenic.
Clinical outcome studies after revision ACL allograft reconstruction have shown satisfactory subjective results, although inferior to primary reconstructions. These investigations also reflect findings from basic science studies that have demonstrated slower biologic incorporation of the allografts and inferior biomechanical properties than those of autografts. Uribe and coworkers52 reported the results of 54 patients who underwent revision ACL reconstruction. A fresh-frozen B-PT-B allograft was used in 35% of the cases and autogenous B-PT-B and hamstring grafts were used in 65%. The authors found no significant difference in the subjective outcomes between the two groups, but autografts provided greater objective stability during KT-1000 testing compared with allografts. In a prospective study of revision ACL reconstruction, Noyes and Barber-Westin34 prospectively evaluated 65 patients who received B-PT-B allografts and 20 patients revised with autogenous B-PT-B grafts. The overall failure rate was 33% for the allografts and 27% for the autografts. Knee examination with KT-2000 showed that 53% of the patients in the allograft group and 67% in the autograft group had less than 3 mm increased displacement, although this difference was not statistically significant. Grossman and associates17 reported on 29 revision ACL reconstructions in which B-PT-B allografts were used in 26 knees, B-PT-B autografts in 6, and AT allograft in 1. The allograft group demonstrated increased laxity during instrumented knee laxity testing compared with autografts (3.21 mm vs. 1.33 mm, respectively). Subjective scores were not significantly different between the two groups.
Smith and colleagues48 reviewed retrospectively the results of 32 revision ACL reconstructions using nonirradiated B-PT-B allografts. The authors reported a 28% failure rate based on the presence of a pivot shift and/or greater than 5 mm side-to-side difference on KT-1000 testing. Similar findings were reported by Johnson and coworkers21 in a review of 25 consecutive revision reconstructions with the use of fresh-frozen irradiated B-PT-B (13 patients) or AT (12 patients) allografts. At a mean follow-up of 28 months, 36% of the patients had greater than 5.5 mm side-to-side difference in anterior translation under manual maximum force on KT-1000 testing. Twenty percent of the patients had a grade II pivot shift.
Based on the allograft rupture rate observed in the previously described prospective study,39a the senior author conducted a retrospective review of additional patients with longer follow-up evaluations. One hundred sixteen allograft patients with an average follow-up of 4.4 months were compared with 62 autograft patients with an average follow-up of 44.2 months. Patients in both groups were evenly divided between chronic and acute reconstructions. The results revealed that the allografts had significantly greater AP displacements (P < .001), according to manual maximum knee arthrometer testing (Table 8-1). When only acute reconstructions were compared, this difference was not significant (Table 8-2). However, comparison of chronic reconstruction groups showed significantly greater AP displacements in the allograft group (Table 8-3). In the allograft group, 1 acute reconstruction and 3 chronic reconstructions suffered traumatic ruptures at an average of 3.8 years after surgery. In the autograft group, only 1 patient (chronic) suffered a traumatic rupture.
TABLE 8-1 Results of Manual Maximum Knee Arthrometer Testing in Patients Receiving Allograft and Autograft Anterior Cruciate Ligament Reconstruction
| Difference between Reconstructed and Contralateral Knee (mm) | Allografts (N = 116) | Autografts (N = 62) |
|---|---|---|
| <3 | 67 (58%)* | 44 (71%) |
| 3–5 | 33 (28%) | 16 (26%) |
| >5 | 16 (14%)* | 2 (3%) |
TABLE 8-2 Results of Manual Maximum Knee Arthrometer Testing in Patients Receiving Acute Allograft and Autograft Anterior Cruciate Ligament Reconstruction
| Difference between Reconstructed and Contralateral Knee (mm) | Allografts (%) | Autografts (%) |
|---|---|---|
| <3 | 69 | 75 |
| 3–5 | 19 | 17 |
| >5 | 12 | 8 |
TABLE 8-3 Results of Manual Maximum Knee Arthrometer Testing in Patients Receiving Chronic Allograft and Autograft Anterior Cruciate Ligament Reconstruction
| Difference between Reconstructed and Contralateral Knee (mm) | Allografts (%) | Autografts (%) |
|---|---|---|
| <3 | 48 | 65 |
| 3–5 | 37 | 35 |
| >5 | 15* | 0 |
AUTOGRAFTS
Similar to primary ACL reconstructions, autografts remain the graft of choice of many surgeons in the revision setting. Despite the morbidity associated with graft harvest, autografts carry no risk of disease transmission and there are no concerns for immunologic rejection. Autografts provide more reliable biologic integration, which has great significance in the often-compromised biologic environment of the multiple-operated knee. In addition, the use of an autograft reduces the cost of the procedure. However, because ipsilateral autograft(s) have usually already been used in the primary reconstruction, the surgeon may have to resort to harvesting the necessary graft in the opposite extremity. This tactic may be objectionable to some patients. Shelbourne and associates45,46 studied the use of a contralateral B-PT-B graft in both primary and revision ACL reconstructions. The investigators reported low morbidity rates and expedited recovery in both the donor and the reconstructed knees. In addition, subjective scores were not significantly different from those obtained with primary reconstructions.
Ipsilateral unharvested autografts have been used in other series with good outcomes. In a prospective, nonrandomized study, O’Neil36 reported the results of 48 patients who underwent revision ACL reconstruction using ipsilateral, previously unharvested hamstrings or B-PT-B autografts. At a mean follow-up of 90 months, 73% of the patients had International Knee Documentation Committee (IKDC) scores of normal (A) or nearly normal (B). Sixty-seven percent of the knees had a KT-2000 arthrometer side-to-side difference of 3 mm or less, and an additional 21% had a side-to-side difference of 3 to 5 mm. Therefore, 94% of the grafts were functional or partially functional. Six percent of grafts had more than 5 mm of laxity and were considered failures.
The ipsilateral QT has been used for revision ACL reconstruction.33 In one study, the indications to use the QT included prior harvest of the patellar tendon in the ipsilateral knee, inability to harvest the contralateral patellar tendon (prior harvest, patient refusal), inability to consider the semitendinosus gracilis (STG) tendons because of expanded tunnels, and patient request for autogenous rather than allograft tissues. Persistent patellar bony defect from prior patellar tendon harvest was considered a contraindication to harvest the QT. Using IKDC rating, 17 of the 21 knees included in the study were rated as normal or nearly normal, 3 were graded as abnormal, and 1 was graded as severely abnormal at a mean follow-up of 49 months. During instrumented knee laxity testing, 8 knees had less than 3 mm, 7 knees had 3 to 5 mm, and 4 knees had more than 5 mm increased AP displacement. On physical examination, 10 knees had a grade 0 pivot shift; 7 knees, grade I; 3 knees, grade II; and 1 knee, grade III.
Hamstring autografts have been successfully used in revision ACL reconstruction.15,44 Salmon and colleagues44 reported on 57 consecutive revision ACL reconstructions with a four-strand hamstring tendon graft and interference screw fixation. Assessment included the IKDC knee ligament evaluation, instrumented laxity testing, and radiologic examination. The average length of follow-up was 89 months. Of the 50 knees reviewed, 5 (10%) had objective failure of the revision ACL reconstruction. Of the 45 patients with functional grafts, knee function was normal or nearly normal in 33 patients (73%). Thirty-four patients were evaluated with KT-1000. The mean side-to-side difference on manual maximum testing was 2.5 mm. Fifty percent of the tested patients had translation of less than 3 mm, and 50% had between 3 and 5 mm. On pivot shift testing, 31 knees (69%) had grade 0 and 14 knees grade I test results.
Reharvested B-PT-B autografts have also been used in revision ACL reconstruction. Nixon and coworkers31 evaluated the donor site in the patellar tendon at various time periods after a graft from the central third of the patellar tendon was harvested. Two groups of patients were evaluated, one with magnetic resonance imaging (MRI) of the knee and the other with an open biopsy from the donor site in the patellar tendon. On MRI, the size of the defect and the intensity of the signal in the central third of the tendon decreased with time from surgery. At 2 years, the defect was indistinguishable from normal tendon. Histologically, the scar in the defect progressively matured with time, becoming nearly identical to normal tendon at 2 years. However, Proctor and associates42 showed in the goat model that even with restoration of the signal intensity, the biomechanical and histologic properties of the reconstituted tendon had not returned to normal. In this study, the maximum force to failure and ultimate stress of the reharvested tendons had decreased by 51% and 65%, respectively, compared with the contralateral side. Using a canine model, LaPrade and colleagues25 demonstrated with biomechanical testing that average load to failure of the reharvested central third patellar tendon was 54% of that of the contralateral control tendons after 1 year.
Clinical studies on revision ACL reconstruction with reharvested B-PT-B grafts reflect the controversy surrounding this graft choice. Colosimo and coworkers10 reported on 13 patients undergoing revision ACL reconstruction with a reharvested patellar tendon. At an average follow-up of 39.4 months, 11 patients had good or excellent results and 2 patients had fair results. Postoperative KT-1000 arthrometer results showed an average side-to-side difference of 1.92 mm. No patient demonstrated loss of range of knee motion and only 1 reported patellofemoral problems, which were moderate. The authors concluded that reharvesting the patellar tendon was a viable option in revision ACL reconstruction but advised against the use of this graft in any patient with patellofemoral disease or patella baja. Good subjective and objective results were also reported by O’Shea and Shelbourne37 in a group of 11 patients with a mean objective follow-up of 49 months. In these two studies, the average length of time between primary B-PT-B harvest and reharvest of the tendon was 82.5 and 71 months, respectively. In contrast, Kartus and associates22 found a higher rate of complications with the use of reharvested central-third patellar tendons, as well as poorer functional scores, when compared with the use of the contralateral patellar tendon. Of the 12 patients who had reconstruction with a reharvested tendon, 1 sustained a displaced patellar fracture 2 weeks after the index procedure, and another, a patellar tendon rupture 6 months postoperatively. In a more recent 10-year follow-up study, Liden and colleagues27 reported on the clinical and radiographic results of 14 patients who had revision ACL reconstruction with reharvested patellar tendon. The average time between the primary and the revision reconstruction was 64 months. The patellar tendon at the donor site had not normalized 10 years after the reharvesting procedure, as evaluated on MRI. Clinical results in terms of the Lysholm score, IKDC evaluation system, single-leg hop test, KT-1000 test, and knee-walking test revealed no significant differences between the 2- and the 10-year assessments. In overall terms, the clinical results were considered to be poor on both occasions. Two major complications, 1 patellar fracture and 1 patellar tendon rupture, occurred in this series as well.
AUTHORS’ APPROACH
Aside from knowledge of the performance of various grafts, the choice of revision graft material is predicated on a number of clinical factors that are inextricably related (Table 8-4). Each case must be evaluated and decisions made based on the unique presentation of the patient.
TABLE 8-4 Clinical Factors That Influence the Choice of Graft Material
| Cause of failure of the previous reconstruction(s) |
| Previous graft selection |
| Type and location of hardware used in previous reconstruction(s) |
| Bone quality |
| Unrecognized concomitant ligament instability |
| Condition of the patellofemoral joint |
| Condition of the contralateral knee |
| Skin condition |
| Is staged reconstruction necessary? |
| Individual patient factors |
| Age |
| Activity level |
| Personal preferences, religious beliefs |
Cause of Failure
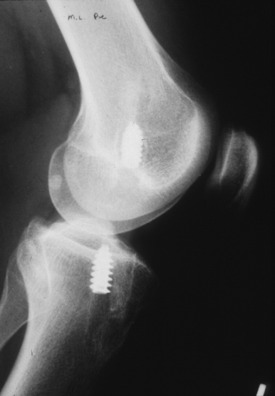
Determining the cause of failure is frequently very difficult because rarely there is just one factor; more often, several factors exist. Erroneous tunnel placement is the most common cause and includes both tibial and femoral malposition (Fig. 8-6). If the old tunnel position infringes on normal tunnel placement, graft/tunnel size mismatch will occur. This limits the choice of graft source, and if the surgeon’s and the patient’s desire is to avoid allografts, a two-stage procedure is required. This approach begins with arthroscopic bone grafting and ends approximately 12 weeks later with ACL reconstruction using ipsilateral or contralateral autograft material. In the authors’ experience, which is supported by recent publications, a two-staged procedure results in more stable knees more frequently than a single-stage procedure, which compromises optimal tunnel placement or size and employs slower healing allograft tissues.51
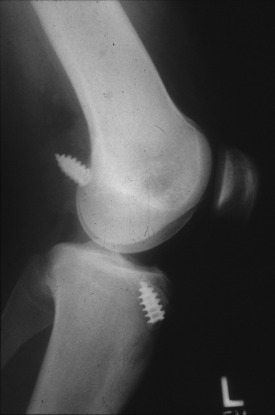
Conversely, a poor surgical technique with grossly malpositioned tunnels, inadequate fixation (Fig. 8-7), failure to recognize and address complex instabilities, or prior traumatic rupture of the primary ACL graft generally allows the surgeon more latitude as to graft choice.
Critical Points AUTHORS’ APPROACH
Type and Location of Hardware from Previous Reconstruction
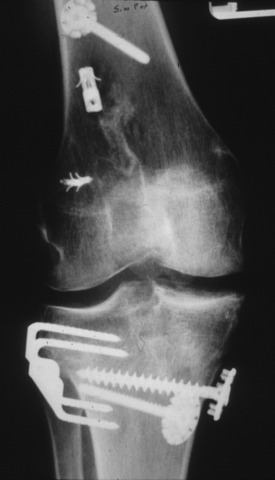
The location of existing hardware, whether it will be retained or removed, may have a bearing on the fixation method of the revision graft and, therefore, affect the type and source of tissue that will be used. Occasionally, the surgeon has to navigate the reconstruction through a number of fixation devices or complex bone defects (Fig. 8-8). The versatility of an allograft in such cases can be invaluable, especially in cases in which the patient desires a single-stage reconstruction.
Bone Quality
ACL reconstruction results in a decrease of the bone mineral density around the knee. This decrease can be substantial, reaching values of 21% for the distal femur, 17% for the patella, and 14% for the proximal tibia.26 It has been hypothesized that this bone loss may be related to the use of autografts, which results in a decrease of the mechanical properties of the donor structures (e.g., patellar tendon) and their ability to transmit mechanical forces to the adjacent bony structures.43 In patients in whom severe periarticular osteopenia is observed in the preoperative radiographs, the preference is to use an ipsilateral quadruple STG autograft, if available, which appears to be less disruptive for the knee. Alternatively, an autograft from the opposite knee can be used. In the presence of excessive tunnel widening in the preoperative radiographs, some surgeons will not consider STG grafts. However, tunnel enlargement after ACL reconstruction is a multifactorial phenomenon in which the fixation method may be more important than the type of graft used12,38 (Fig. 8-9). In these patients, the authors prefer to stage the reconstruction, bone graft the enlarged tunnels, and use an autograft, even a tendinous one, employing a high-stiffness aperture fixation device.
Condition of the Ipsilateral Patellofemoral Joint and Contralateral Knee
The patellofemoral joint of the ipsilateral knee should be carefully evaluated preoperatively and during the revision procedure. A B-PT-B or QT autograft should not be used in the presence of significant patellofemoral degenerative joint disease. The inevitable postoperative extensor mechanism weakness may create or aggravate patellofemoral symptoms that, at times, can be very difficult to cope with and can compromise rehabilitation efforts. Patellofemoral symptoms are one of the major determinants of patient satisfaction after ACL reconstruction.23 If other autograft sources are not available, an allograft can be used. Likewise, an allograft is a reasonable option in cases in which the contralateral knee has pathology that prohibits the harvest of an autograft.
Single versus Two-Stage Reconstruction
Allograft tissue will more likely be necessary for complex revisions attempted in a single stage. A two-staged procedure must be seriously entertained when the original bone tunnels are significantly enlarged owing to allograft or synthetic material reaction, hardware removal that leave sizable bone defects, or previous infection (Fig. 8-10). The advantage of such approach is that the second procedure generally has fewer technical challenges and an autograft can easily be used.
Other Factors
Allografts have been advocated for ACL reconstruction in older patients or those with a decreased activity level.24,49 However, the authors are not aware of any study documenting superiority of the allografts over autografts in patients of advanced age or lower physical demands. The lower perioperative morbidity associated with allograft use should be weighed against the potential decreased capacity of an older patient to integrate a slower healing graft in an already compromised biologic environment. The personal preferences of the patient should be respected, but the graft selection must be a reasonable option not only for the patient but for the surgeon as well.
1 Hepatitis C virus transmission from an antibody-negative organ and tissue donor—United States, 2000–2002. MMWR Morb Mortal Wkly Rep. 2003;52:273-274. 276
2 Invasive Streptococcus pyogenes after allograft implantation—Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1174-1176.
3 Update: Allograft associated bacterial infections—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:207-210.
4 Arnoczky S.P., Warren R.F., Ashlock M.A. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68:376-385.
5 Barrett G.R., Field L.D. Comparison of patella tendon versus patella tendon/Kennedy ligament augmentation device for anterior cruciate ligament reconstruction: study of results, morbidity, and complications. Arthroscopy. 1993;9:624-632.
6 Beck C.L., Paulos L.E., Rosenberg T.D. Post-implantation histologic examination of anterior cruciate allografts. Op Tech Orthop. 1992;2:2.
7 Brown C.H.Jr., Carson E.W. Revision anterior cruciate ligament surgery. Clin Sports Med. 1999;18:109-171.
8 Buck B.E., Malinin T.I., Brown M.D. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res. 1989;240:129-136.
9 Bugbee W.D., Kwak C., Lebeck L. The effect of ABO blood type on outcome of fresh osteochondral allograft transplantation. In: AAOS Annual Meeting. San Francisco: AAOS; 2004:572.
10 Colosimo A.J., Heidt R.S., Traub J.A., Carlonas R.L. Revision anterior cruciate ligament reconstruction with a reharvested ipsilateral patellar tendon. Am J Sports Med. 2001;29:746-750.
11 Fahey M., Indelicato P.A. Bone tunnel enlargement after anterior cruciate ligament replacement. Am J Sports Med. 1994;22:410-414.
12 Fauno P., Kaalund S. Tunnel widening after hamstring anterior cruciate ligament reconstruction is influenced by the type of graft fixation used: a prospective randomized study. Arthroscopy. 2005;21:1337-1341.
13 Fideler B.M., Vangsness C.T.Jr., Moore T., et al. Effects of gamma irradiation on the human immunodeficiency virus. A study in frozen human bone–patellar ligament–bone grafts obtained from infected cadavera. J Bone Joint Surg Am. 1994;76:1032-1035.
14 Fideler B.M., Vangsness T., Lu B., et al. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23:643-646.
15 Fules P.J., Madhav R.T., Goddard R.K., Mowbray M.A. Revision anterior cruciate ligament reconstruction using autografts with a polyester fixation device. Knee. 2003;10:335-340.
16 Getelman M.H., Friedman M.J. Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg. 1999;7:189-198.
17 Grossman M.G., ElAttrache N.S., Shields C.L., Glousman R.E. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005;21:418-423.
18 Jackson D.W., Grood E.S., Goldstein J.D., et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176-185.
19 Jackson D.W., Windler G.E., Simon T.M. Intra-articular reaction associated with the use of freeze-dried, ethylene oxide–sterilized bone–patella tendon–bone allografts in the reconstruction of the anterior cruciate ligament. Am J Sports Med. 1990;18:1-11.
20 Johnson D.L., Coen M.J. Revision ACL surgery. Etiology, indications, techniques, and results. Am J Knee Surg. 1995;8:155-167.
21 Johnson D.L., Swenson T.M., Irrgang J.J., et al. Revision anterior cruciate ligament surgery: experience from Pittsburgh. Clin Orthop. 1996;325:100-109.
22 Kartus J., Stener S., Lindahl S., et al. Ipsi- or contralateral patellar tendon graft in anterior cruciate ligament revision surgery. A comparison of two methods. Am J Sports Med. 1998;26:499-504.
23 Kocher M.S., Steadman J.R., Briggs K., et al. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84:1560-1572.
24 Kuechle D.K., Pearson S.E., Beach W.R., et al. Allograft anterior cruciate ligament reconstruction in patients over 40 years of age. Arthroscopy. 2002;18:845-853.
25 LaPrade R.F., Hamilton C.D., Montgomery R.D., et al. The reharvested central third of the patellar tendon. A histologic and biomechanical analysis. Am J Sports Med. 1997;25:779-785.
26 Leppala J., Kannus P., Natri A., et al. Effect of anterior cruciate ligament injury of the knee on bone mineral density of the spine and affected lower extremity: a prospective one-year follow-up study. Calcif Tissue Int. 1999;64:357-363.
27 Liden M., Ejerhed L., Sernert N., et al. The course of the patellar tendon after reharvesting its central third for ACL revision surgery: a long-term clinical and radiographic study. Knee Surg Sports Traumatol Arthrosc. 2006;14:1130-1138.
28 Malinin T.I., Levitt R.L., Bashore C., et al. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18:163-170.
29 Miller B.S., Wojtys E.M. Basic science aspects of the use of allografts in revision anterior cruciate ligament surgery. Sports Med Arthrosc Rev. 2005;13:3-7.
30 Moyen B.J.L., Jenny M.-Y., Mandrino A.H., Lerat L.-L. Comparison of reconstruction of the anterior cruciate ligament with and without a Kennedy ligament-augmentation device. A randomized, prospective study. J Bone Joint Surg Am. 1992;74:1313-1319.
31 Nixon R.G., SeGall G.K., Sax S.L., et al. Reconstitution of the patellar tendon donor site after graft harvest. Clin Orthop Relat Res. 1995;317:162-171.
32 Noyes F.R., Barber S.D. The effect of a ligament-augmentation device on allograft reconstructions for chronic ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 1992;74:960-973.
33 Noyes F.R., Barber-Westin S.D. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon-patellar bone autograft. Am J Sports Med. 2006;34:553-564.
34 Noyes F.R., Barber-Westin S.D. Revision anterior cruciate ligament surgery: experience from Cincinnati. Clin Orthop. 1996;325:116-129.
35 Noyes F.R., Butler D.L., Grood E.S., et al. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66:344-352.
36 O’Neill D.B. Revision arthroscopically assisted anterior cruciate ligament reconstruction with previously unharvested ipsilateral autografts. Am J Sports Med. 2004;32:1833-1841.
37 O’Shea J.J., Shelbourne K.D. Anterior cruciate ligament reconstruction with a reharvested bone–patellar tendon–bone graft. Am J Sports Med. 2002;30:208-213.
38 Otsuka H., Ishibashi Y., Tsuda E., et al. Comparison of three techniques of anterior cruciate ligament reconstruction with bone–patellar tendon–bone graft. Differences in anterior tibial translation and tunnel enlargement with each technique. Am J Sports Med. 2003;31:282-288.
39 Paulos L.E., Rosenberg T.D., Grewe S.R., et al. The Gore-Tex anterior cruciate ligament prosthesis. A long-term follow-up. Am J Sports Med. 1992;20:246-252.
39a Paulos L.E., Jackson D.W. Clinical data of allografts. In Southern Cal Orthopedic and Sports Medicine Group, 4th Annual Symposium: Prosthetic Ligament Reconstruction of the Knee. Palm Springs, CA, March. 1987: 26-28.
40 Pearsall A.W.T., Hollis J.M., Russell G.V.Jr., Scheer Z. A biomechanical comparison of three lower extremity tendons for ligamentous reconstruction about the knee. Arthroscopy. 2003;19:1091-1096.
41 Prevention, C. f. D. C. a. Frequently asked questions about tissue transplants. CDC. 2006.
42 Proctor C.S., Jackson D.W., Simon T.M. Characterization of the repair tissue after removal of the central one-third of the patellar ligament. An experimental study in a goat model. J Bone Joint Surg Am. 1997;79:997-1006.
43 Rittweger J., Maffulli N., Maganaris C.N., Narici M.V. Reconstruction of the anterior cruciate ligament with a patella-tendon-bone graft may lead to a permanent loss of bone mineral content due to decreased patellar tendon stiffness. Med Hypotheses. 2005;64:1166-1169.
44 Salmon L.J., Pinczewski L.A., Russell V.J., Refshauge K. Revision anterior cruciate ligament reconstruction with hamstring tendon autograft: 5- to 9-year follow-up. Am J Sports Med. 2006;34:1604-1614.
45 Shelbourne K.D., Thomas J.A. Contralateral patellar tendon and the Shelbourne experience. Part 2. Results of revision anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev. 2005;13:69-72.
46 Shelbourne K.D., Urch S.E. Primary anterior cruciate ligament reconstruction using the contralateral autogenous patellar tendon. Am J Sports Med. 2000;28:651-658.
47 Simonds R.J., et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326:726-732.
48 Smith A.H., Bach B.R., Bush-Joseph C.A. Allograft for revision ACL reconstruction: The RUSH experience. Sports Med Arthrosc Rev. 2005;13:86-92.
49 Stein D.A., Brown H., Bartolozzi A.R. Age and ACL reconstruction revisited. Orthopedics. 2006;29:533-536.
50 Suarez L.S., Richmond J.C. Overview of procurement, processing, and sterilization of soft tissue allografts for sports medicine. Sports Med Arthrosc Rev. 2007;15:106-113.
51 Thomas N.P., Kankate R., Wandless F., Pandit H. Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med. 2005;3:1701-1709.
52 Uribe J.W., Hechtman K.S., Zvifac J.E., Tjin-A-Tsoi E.W. Revision anterior cruciate ligament surgery: experience from Miami. Clin Orthop Relat Res. 1996;325:91-99.
53 Vangsness C.T.Jr., Wagner P.P., Moore T.M., Roberts M.R. Overview of safety issues concerning the preparation and processing of soft-tissue allografts. Arthroscopy. 2006;22:1351-1358.
54 Wredmark T., Engstrom B. Five-year results of anterior cruciate ligament reconstruction with the Stryker Dacron high-strength ligament. Knee Surg Sports Traumatol Arthrosc. 1993;1:71-75.